Abstract
Background
Inflammation and neuroinflammation are integral to the progression and severity of many diseases and are strongly associated with cardiovascular disease, cancer, autoimmune disorders, neurodegenerative disease, and neuropsychiatric disorders. These diseases can be difficult to treat without addressing the underlying inflammation, and, as such, a growing need has arisen for pharmaceutical treatments that target inflammatory mediators and signaling pathways. Our lab has investigated the therapeutic potential of the irreversible µ-opioid antagonist β-funaltrexamine (β-FNA) and discovered that acute treatment ameliorates inflammation in astrocytes in vitro and inhibits central and peripheral inflammation and reduces anxiety- and sickness-like behavior in male C57BL/6J mice. Now, our investigation has expanded to investigate the chronic pre-treatment effects of β-FNA on lipopolysaccharide (LPS)-induced inflammation and behavior in male C57BL/6J mice.
Results
Micro-osmotic drug pumps were surgically inserted into the subcutaneous intrascapular space of male C57BL/6J mice. β-FNA or saline vehicle was continuously administered for seven days. On the sixth day, mice were given intraperitoneal injections of LPS or saline. An elevated plus maze test, followed by a forced swim test, were administered 24 h post-injection to measure sickness-, anxiety- and depressive-like behavior. Immediately after testing, frontal cortex, hippocampus, spleen, and plasma were collected. Levels of inflammatory chemokines C–C motif chemokine ligand 2 (CCL2) and C-X-C motif chemokine ligand 10 (CXCL10) were measured in tissues by enzyme-linked immunosorbent assay (ELISA). Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was used to assess expression of the enzyme indoleamine 2, 3-dioxygenase 1 (IDO1) and the NLR family pyrin domain-containing protein 3 (NRLP3) inflammasome in frontal cortex and spleen tissues. Chronic pre-treatment robustly decreased inflammation in the hippocampus, frontal cortex, and spleen and reduced or abolished anxiety- and sickness-like behavior (e.g., increased time spent motionless, increased time spent in a contracted position, and reduced distance moved). However, treatment with β-FNA alone increased both inflammation in the frontal cortex and anxiety-like behavior.
Conclusion
These findings provide novel insights into the anti-inflammatory and behavior-modifying effects of chronic β-FNA pre-treatment and continue to support the therapeutic potential of β-FNA under inflammatory conditions.
Keywords: β-funaltrexamine, Neuroinflammation, Inflammation, Anxiety, Depression, CCL2, CXCL10, IDO1, NLRP3
Background
Chronic inflammation is associated with pathological states, including cardiovascular disease, diabetes, cancer, autoimmune disorders [1–7]. Additionally, persistent neuroinflammation has been linked to a variety of neurodegenerative and neuropsychiatric conditions and, in some cases, has been associated with disease progression, severity, and treatment-resistance [2, 8–14]. Consequently, developing pharmaceuticals that target inflammatory pathways has become increasingly important.
Our lab has a longstanding interest in the anti-inflammatory effects of β-FNA, a well-tolerated and non-toxic irreversible µ-opioid antagonist and reversible κ-opioid agonist. We have substantial evidence that β-FNA has in vitro anti-inflammatory effects on human astrocytes, and, in vivo,
β-FNA inhibits LPS-induced inflammation and anxiety- and sickness-like behavior in male C57BL/6J mice [15–20]. Peripheral LPS administration is an established pre-clinical model of neuroinflammation in mice [21–23]. LPS activates toll-like receptor 4 (TLR4) on immune cells, microglia, and astrocytes leading to upregulation of pro-inflammatory cytokines/chemokines, enzymes, and inflammatory metabolites/products that disrupt synaptic and neuronal function [21, 22]. Sickness behavior (as indicated by increased time spent motionless, increased time spent in a contracted position, and/or reduced distance moved) typically peaks around 6 h post LPS, followed by anxiety- and depressive-like behavior at 24 h [15, 23]. Both in vitro and in vivo, these β-FNA-mediated effects appear to be driven, in part, through inhibition of nuclear factor kappa B (NF-κB) and p38 mitogen-activated protein kinase (MAPK) activation [17–20]. Here, we provide the first report of the anti-inflammatory and behavioral effects of chronic, continuous β-FNA pre-treatment in a pre-clinical model of LPS-induced inflammation. As a result, this study advances our understanding of the neuroprotective potential of β-FNA, particularly in the context of inflammation-associated psychiatric disorders.
Methods
Animals
The protocol for all experimental procedures and animal manipulations was approved by the Oklahoma State University Center for Health Sciences Institutional Animal Care and Use Committee. Male C57BL/6J mice (4–6 weeks; Jackson Laboratories, Bar Harbor, ME) were housed in USDA-approved facilities at the Oklahoma State UniversityCenter for Health Sciences and were acclimated for at least 7 days prior to initiation of experiments. Each plastic cage housed two to three mice and contained pine chip bedding, ad libitum food and water, and a plastic igloo and cardboard tube for environmental enrichment. The room was maintained at an ambient temperature of 21 °C and programmed with a 12 h light/12 h dark cycle.
Experimental protocol
Seven-week-old male C57BL/6J mice (n = 16/group) were administered 150 mg meloxicam then anesthetized using continuous-flow isoflurane (5L/min, 2.5%) prior to surgically inserting a drug/vehicle-loaded micro-osmotic pump (Alzet 1007D) into the intrascapular subcutaneous space. Meloxicam (150 mg) was administered again 6 h post-surgery to minimize discomfort. The pumps delivered either β-FNA (National Institute on Drug Abuse reagent supply program; 42 μg/d, 0.5μL/h for 7d) or saline vehicle. To induce neuroinflammation and behavioral deficits, a well-established pre-clinical model [24] was used wherein six days post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25μL LPS (Escherichia coli O55:B5; Sigma L2880; 0.83 mg/kg, as previously reported [15]) or saline. Doses of LPS and β-FNA were based upon previous research [15, 16, 19]. At 24 h post-injection, each mouse underwent an elevated plus maze test (EMT), followed by a forced swim test (FST).
Elevated plus maze
Anxiety- and sickness-like behavior were measured using an EPM. The testing apparatus was an elevated, white plexiglass structure with two open arms (25 cm × 5 cm × 0.5 cm), two enclosed arms (25 cm × 5 cm × 16 cm), and a center region (5 cm × 5 cm × 0.5 cm). Each mouse was placed in the center of the maze, and Noldus EthoVision XT 16.0 Software was used to record and analyze various behavioral measures for five minutes. Anxiety-like behavior was indicated by an increase in closed arm activity, whereas sickness-like behavior was denoted by a decrease in locomotor activity and greater time spent in a contracted body position [25, 26].
Forced swim test
Depressive-like behavior was measured using an FST. Each mouse was placed in a 4-L glass beaker containing approximately 2700 mL of fresh water at a temperature of 28 ± 1 °C. Three beakers, separated by blinding dividers, were used to administer the test to three mice simultaneously. Activity was digitally recorded with a Microsoft Surface Book 2 Windows 11 camera for six minutes. Each video was converted to a standard frame rate of 30 frames per second and then automatically scored using the DBscorer 1.0 software [27]. The perimeter of the scoring area was manually defined using the software’s guidelines and scored using the recommended ∆ area threshold of 1.6%. Scoring occurred during the initial 4 min. period after placement in the water. Each analysis was performed three times per mouse to ensure scoring area accuracy, and the average value for each mouse was used for statistical analysis. Depressive-like behavior was defined as an increase in floating time and/or decreased latency to immobility relative to control mice.
Tissue collection
Immediately after behavioral testing, animals were euthanized via CO2 inhalation and subsequent decapitation. Trunk blood was collected in sodium heparin tubes and placed on ice. Frontal cortex, hippocampus, and spleen were removed and bisected on a chilled surface, flash frozen with liquid nitrogen, and then placed on ice. Plasma was isolated from blood specimens via cold centrifugation (5000 × rpm, 10 min, 4 °C). All tissues were then stored at -80 °C until assays were performed.
Measurement of cytokine/chemokines
Standard dual-antibody solid phase immunoassays (ELISA Development Kit; Peprotech, Rocky Hill, NJ) were used according to manufacturer’s instructions for quantitation of secreted CXCL10 (cat#900-K153 and CCL2 (cat#900-K126) in spleen, frontal cortex, and hippocampus tissue homogenates. Tissues were homogenized in ice-cold triple detergent lysis buffer containing HALT Protease/Phosphatase Inhibitor Cocktail (cat#1,861,282, Thermo Scientific) using a pellet pestle cordless motor, cold centrifuged (20,000 × g, 20 m, 4 °C), and the supernatant collected and stored at -80 °C. Absorbance was read at 405 nm (650 nm wavelength correction) using a BIOTEK Synergy 2 Multi-Detection Microplate Reader and quantified using BioTek Gen5 software. Levels were normalized to total cell protein as determined by bicinchoninic assay (BCA).
Measurement of IDO and NLRP3 expression
RT-qPCR was used to quantify relative expression of NLRP3 and IDO1 in RNA extracts from tissue homogenates. Tissues were homogenized in TRIzol reagent using a pellet pestle cordless motor. Total RNA was extracted using a phenol–chloroform process as previously described [28], quantified using a NanoDrop spectrophotometer, and frozen at − 80 °C until used. RNA was reverse transcribed using a combination of Moloney murine leukemia virus (M-MLV) reverse transcriptase (cat#M170B, ProMega), 10 mM dNTP Mix (cat#100,004,893, Invitrogen) and random hexamer primers (cat#SO142, Thermo Scientific). For qPCR, 160 ng cDNA was used to quantify the relative expression of NLRP3 and IDO. Sense and antisense oligonucleotide primers were designed for RT-qPCR using DNA sequence information obtained from the Genome Database (National Center for Biotechnology Information) and were synthesized by Integrated DNA Technologies. The following specific primers were used for RT-qPCR:
IDO1 sense (5′-CTAGAAATCTGCCTGTGCTGATTGAG-3′);
IDO1 antisense (5′-GCTCGCAGTAGGGAACAGCAATATTG 3′);
NLRP3 sense (5′-GGGAAAAAGCTAAGAAGGACCAGCC-3′);
NLRP3 antisense (5′-TCCTTGATAGAGTAGAACCTGCTTCTCACATG-3′);
18S rRNA sense (5′-GTATATTAAAGTTGCTGCAGTTAAAAAGCTCGTAGTTGG-3′);
18S rRNA antisense (5′-CAACAAAATAGAACCGCGGTCCTATTCCATTATTC-3′).
Quantitative analysis was performed using PowerUp SYBR Green Master Mix (cat#A25742, Applied Biosystems) in an Applied Biosystems QuantStudio 5 Real-Time PCR system. Primers for 18S rRNA were used as internal controls. The results were analyzed using the ΔcT-ΔcT method and were expressed as fold change relative to the control group.
Statistical analysis
Details of the analyses are presented with each experiment. While power analysis was not performed for this specific study, we previously observed relatively large effect sizes for β-FNA treatment on LPS-induced changes in CXCL10 (Ƞ2p = 0.35), and our recent study used a similar design and yielded significant effects with n = 5–6 [16]. In this study, data were analyzed using two-way ANOVA (β-FNA × LPS), Fisher’s Least Significant Difference (LSD) for pairwise comparisons, and linear regression. Data are presented as mean ± SEM, and p-values < 0.05 are considered statistically significant. GraphPad Prism 10.0.1 software (GraphPad Inc, San Diego, CA) was used for data analysis and figure preparation.
Results
Effects of β-FNA on anxiety- and sickness-like behavior
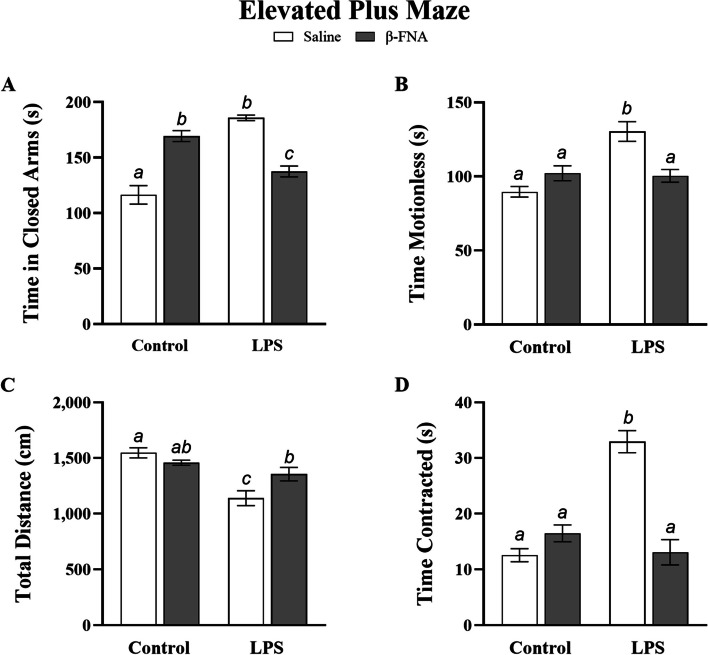
Multiple behavioral traits were recorded and analyzed during the elevated plus maze test. Specifically, anxiety-like behavior was evaluated by examining time spent in closed arms, while sickness-like behavior was examined by evaluating time spent motionless, total distance moved, and time spent in a contracted body position. Two-way ANOVA results indicated a significant main effect of LPS (F1, 17 = 9.96, p < 0.01), no significant main effect of β-FNA (F1, 17 = 0.14, p = 0.71), and a significant interaction of main effects (F1, 17 = 72.42, p < 0.0001) on time spent in closed arms (Fig. 1A). Pairwise comparisons revealed that LPS mice spent significantly more time in the closed arms than saline (p < 0.0001) or β-FNA + LPS mice (p < 0.0001), and β-FNA + LPS mice spent significantly more time in closed arms than saline mice (p < 0.05). Notably, the β-FNA mice spent significantly more time in closed arms than saline mice (p < 0.0001) or β-FNA + LPS mice (p < 0.005). While β-FNA mice tended to spend less time in closed arms than LPS mice, the reduction in time was not significant (p = 0.07).
Fig. 1.
Chronic effects of β-FNA on LPS-induced anxiety- and sickness-like behavior in male C57BL/6J mice. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. Data are presented as mean ± SEM. A Time in closed arms: Two-way ANOVA indicated significant main effect of LPS (p < 0.01), no significant main effect of β-FNA (p = 0.71), and a significant interaction of main effects (p < 0.0001) on time spent in the closed arms of the EPM (n = 5–6/group). Pairwise comparisons were assessed using Fisher's LSD test; bars with letters in common indicate data are not significantly different (p > 0.05). B Time spent motionless: Two-way ANOVA indicated significant main effect of LPS (p < 0.005), no significant main effect of β-FNA (p = 0.12), and a significant interaction of main effects (p < 0.001) on time spent motionless in EPM (n = 5–8/group). C Total distance moved: Two-way ANOVA indicated significant main effect of LPS (p < 0.001), no significant main effect of β-FNA (p = 0.26), and a significant interaction of main effects (p < 0.05) on total distance moved in EPM (n = 6–8/group). D Time spent in a contracted position: Two-way ANOVA indicated significant main effects of LPS (p < 0.0001) and β-FNA (p < 0.0005), as well as a significant interaction of main effects (p < 0.0001) on time spent in a contracted position (n = 6–7/group). Pairwise comparisons were assessed using Fisher's LSD test; bars with letters in common indicate data are not significantly different (p > 0.05)
Two-way ANOVA revealed a significant main effect of LPS (F1, 22 = 13.10, p < 0.005), no significant main effect of β-FNA (F1, 22 = 2.61, p = 0.12), and a significant interaction of main effects (F1, 22 = 15.57, p < 0.001) on time spent motionless in the elevated plus maze (Fig. 1B). Pairwise comparisons revealed that LPS mice spent significantly more time motionless than saline (p < 0.0001), β-FNA (p < 0.001), or β-FNA + LPS mice (p < 0.01), while time spent motionless between those three groups was similar (p = 0.10, p = 082, p = 0.20, respectively).
Two-way ANOVA also revealed a significant main effect of LPS (F1, 22 = 22.02, p < 0.001), no significant main effect of β-FNA (F1, 22 = 1.36, p = 0.26), and a significant interaction of main effects (F1, 22 = 7.90, p < 0.05) on total distance moved (Fig. 1C). Pairwise comparisons indicated that LPS mice covered significantly less distance than saline (p < 0.0001), β-FNA (p < 0.0005), or β-FNA + LPS (p < 0.05) mice. While distance covered was similar between saline and β-FNA mice (p = 0.25) and β-FNA and β-FNA + LPS mice (p = 0.21), β-FNA + LPS mice covered significantly less distance than saline mice (p < 0.05).
Finally, two-way ANOVA showed significant main effects of LPS (F1, 20 = 22.55, p < 0.001) and β-FNA (F1, 20 = 19.77, p < 0.0005), as well as a significant interaction of main effects (F1, 20 = 43.94, p < 0.0001) on time spent in a contracted position (Fig. 1D). LPS mice spent significantly more time in a contracted position than saline, β-FNA, or β-FNA + LPS mice (p < 0.0001). Additionally, time spent in a contracted position was similar between saline and β-FNA mice (p = 0.14), saline and β-FNA + LPS mice (p = 0.84), and β-FNA and β-FNA + LPS mice (p = 0.22).
In summary, chronic, continuous β-FNA treatment ameliorated LPS-induced anxiety- and sickness-like behaviors, as indicated by an increase in total distance moved, less time spent in closed arms, and less time spent motionless and in a contracted body position. β-FNA alone did not significantly affect sickness-like behaviors under non-inflammatory conditions; but it did increase anxiety-like behavior, as measured by increased time spent in closed arms.
Effects of β-FNA on depressive-like behavior
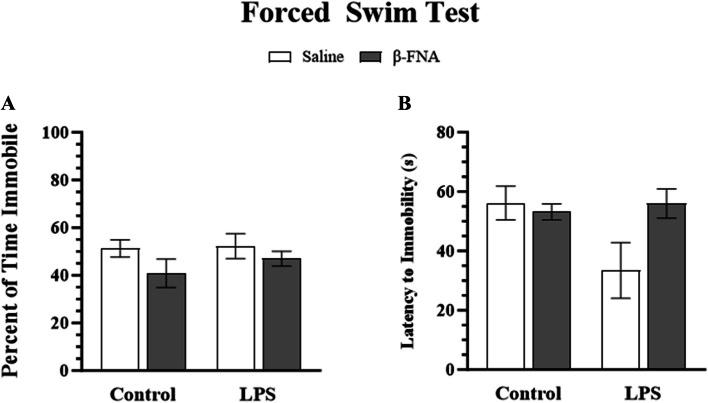
DBscorer software was used to automatically score key depressive-like traits in the FST, including percentage of time spent immobile and latency to immobility. Two-way ANOVA showed no significant main effect of LPS (F1, 19 = 0.55, p = 0.46), β-FNA (F1, 19 = 1.75, p = 0.20), or interaction of main effects (F1, 19 = 0.12, p = 0.74) on percentage of time spent immobile (Fig. 2A). Similarly, two-way ANOVA did not show a significant main effect of LPS (F1, 19 = 2.00, p = 0.17, β-FNA (F1, 19 = 1.94, p = 0.18), or interaction of main effects (F1, 19 = 3.31, p = 0.08) on latency to immobility (Fig. 2B). While β-FNA did not significantly reduce depressive-like behavior, control and β-FNA treatment groups tended to have increased latency to immobility compared to LPS mice.
Fig. 2.
Chronic effects of β-FNA on LPS-induced depressive-like behavior in male C57BL/J6 mice. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. Endpoints measured included A percentage of time spent immobile and (B) latency to immobility. Data are presented as mean ± SEM. A Two-way ANOVA indicated no significant main effect of LPS (p = 0.46), β-FNA (p = 0.20), or interaction of main effects (p = 0.74) on percentage of time immobile (n = 5–7/group). B Two-way ANOVA indicated no significant main effect of LPS (p = 0.17), β-FNA (p = 0.18) or interaction of main effects (p = 0.08) on latency to immobility (n = 4–7/group). Pairwise comparisons were assessed using Fisher's LSD test; bars with letters in common indicate data are not significantly different (p > 0.05)
Effects of β-FNA on CCL2 in the brain and spleen
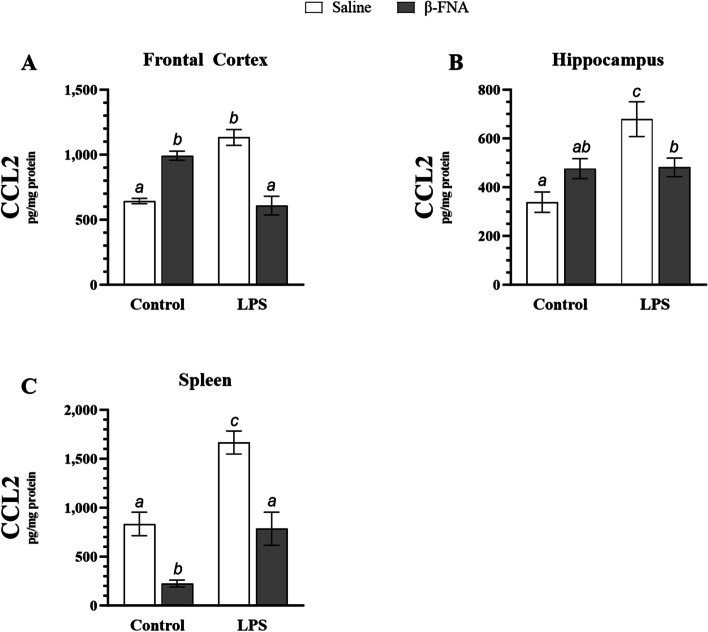
CCL2 expression was quantified in frontal cortex, hippocampus, and spleen tissues (Fig. 3). Two-way ANOVA indicated that there was no significant main effect of LPS (F1, 19 = 0.98, p = 0.33) or β-FNA (F1, 19 = 2.74, p = 0.11) on CCL2 levels in the frontal cortex, but there was a significant interaction of main effects (F1, 19 = 67.23, p < 0.0001) (Fig. 3A). LPS mice had significantly higher levels of CCL2 in the frontal cortex than either saline (p < 0.0001) or β-FNA + LPS (p < 0.0001) mice, which were similar (p = 0.66). Additionally, β-FNA mice tended to have lower CCL2 levels in the frontal cortex than LPS mice (p = 0.06). Interestingly, β-FNA mice had significantly higher levels of CCL2 in the frontal cortex than either saline (p < 0.0005) or β-FNA + LPS mice (p < 0.0001).
Fig. 3.
Chronic effects of β-FNA on LPS-induced elevations of CCL2 in male C57BL/J6 mice. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. CCL2 was measured via ELISA in (A) frontal cortex, (B) hippocampus, and (C) spleen tissues. Data are presented as mean ± SEM. A Two-ANOVA (n = 5–7/group) indicated that there was no significant main effect for LPS (p = 0.33) or β-FNA (p = 0.11) on CCL2 levels in the frontal cortex, but there was a significant interaction of main effects (p < 0.0001). B Two-way ANOVA revealed a significant main effect of LPS (p < 0.005), no significant main effect of β-FNA (p = 0.55), and a significant interaction of main effects (p < 0.005) on CCL2 levels in the hippocampus (n = 6–7/group). C Two-way ANOVA (n = 5–8/group) suggested a main effect for LPS (p < 0.0001) and β-FNA (p < 0.0001) on CCL2 levels in the spleen, but no interaction of main effects (p = 0.24). Pairwise comparisons were assessed using Fisher's LSD test; bars with letters in common indicate data are not significantly different (p > 0.05)
Two-way ANOVA revealed a significant main effect of LPS (F1, 21 = 12.24, p < 0.005), no significant main effect of β-FNA (F1, 21 = 0.36, p = 0.55), and a significant interaction of main effects (F1, 21 = 11.50, p < 0.005) on CCL2 levels in the hippocampus (Fig. 3B). LPS mice had significantly higher levels of CCL2 in the hippocampus than saline (p < 0.0001), β-FNA (p < 0.01), or βFNA + LPS mice (p < 0.05). CCL2 levels in the hippocampus were similar between β-FNA and β-FNA + LPS mice (p = 0.94), but β-FNA + LPS mice had significantly higher CCL2 levels than saline mice (p < 0.05). While β-FNA mice tended towards higher levels of CCL2 in the hippocampus than saline mice, it fell short of significance (p = 0.06).
Two-way ANOVA indicated a main effect of LPS (F1, 21 = 38.03, p < 0.0001) and β-FNA (F1, 21 = 43.70, p < 0.0001) on CCL2 levels in the spleen, but no interaction of main effects (F1, 21 = 1.44, p = 0.24) (Fig. 3C). Pairwise comparisons revealed that LPS mice had significantly higher levels of CCL2 in the spleen than saline, β-FNA, or β-FNA + LPS mice (p < 0.0001). While saline and β-FNA + LPS mice had similar levels of CCL2 (p = 0.77), it is notable than β-FNA mice had significantly lower levels of CCL2 in the spleen than either saline (p < 0.001) or β-FNA + LPS mice (p < 0.005).
In summary, β-FNA abolished LPS-induced CCL2 elevations in the frontal cortex and spleen, while also significantly reducing CCL2 in the hippocampus. Under non-inflammatory conditions, β-FNA differentially affected select tissues by raising CCL2 levels in the frontal cortex; yet, reducing CCL2 levels in the spleen.
Effects of β-FNA on CXCL10 in the brain and spleen
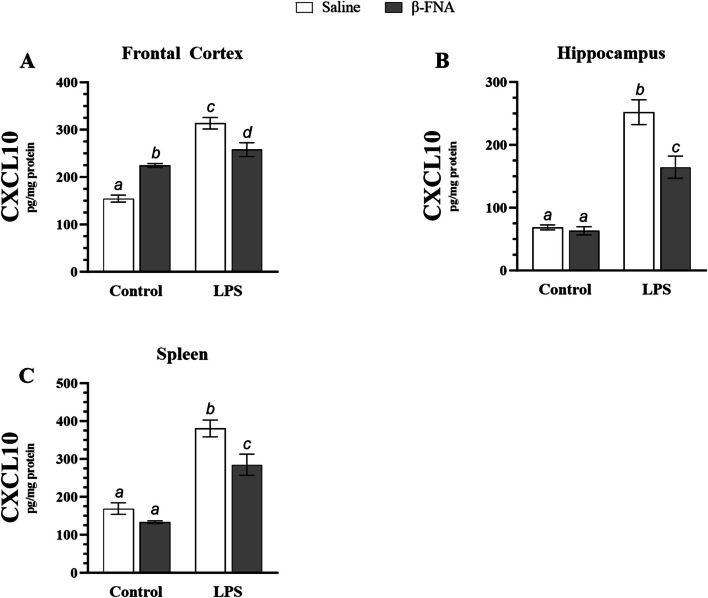
Levels of the chemokine CXLC10 were also quantified in frontal cortex, hippocampus, and spleen (Fig. 4). Two-way ANOVA revealed a significant main effect of LPS (F1, 18 = 96.94, p < 0.0001), no significant main effect of β-FNA (F1, 18 = 0.51, p = 0.48), and a significant interaction of main effects (F1, 18 = 41.26, p < 0.0001) on CXCL10 levels in the frontal cortex (Fig. 4A). Pairwise comparisons revealed that LPS mice had significantly higher levels of CXCL10 in the frontal cortex than saline (p < 0.0001), β-FNA (p < 0.0001) or β-FNA + LPS (p < 0.005) mice. While saline (p < 0.0001) and β-FNA (p < 0.05) mice had significantly lower levels of CXCL10 in the frontal cortex than β-FNA + LPS mice, it is notable that β-FNA mice had significantly higher levels of CXCL10 than saline mice (p < 0.0001).
Fig. 4.
Chronic effects of β-FNA on LPS-induced elevations in CXCL10 in male C57BL/6J mice. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. CXCL10 was measured via ELISA in (A) frontal cortex, (B) hippocampus, and (C) spleen tissues. Data are presented as mean ± SEM. A Two-way ANOVA revealed a significant main effect of LPS (p < 0.0001), no significant main effect of β-FNA (p = 0.48), and a significant interaction of main effects (p < 0.0001) on CXCL10 levels in the frontal cortex (n = 5–6/group). B Two-way ANOVA indicated that there were significant main effects of LPS (p < 0.0001) and β-FNA (p < 0.001), as well as an interaction of main effects (p < 0.005) on CXCL10 levels in the hippocampus (n = 5–8/group). C Two-way ANOVA (n = 6–7/group) revealed that there was a significant main effect for LPS (p < 0.0001) and β-FNA (p < 0.005) on CXCL10 levels in the spleen, but there was no significant interaction of main effects (p = 0.13). Pairwise comparisons were assessed using Fisher's LSD test; bars with letters in common indicate data are not significantly different (p > 0.05)
Two-way ANOVA indicated that there were significant main effects of LPS (F1, 23 = 142.30, p < 0.001) and β-FNA (F1, 23 = 15.29, p < 0.001), as well as an interaction of main effects (F1, 23 = 11.90, p < 0.005) on CXCL10 levels in the hippocampus (Fig. 4B). LPS mice had significantly elevated levels of CXCL10 when compared to saline, β-FNA, and β-FNA + LPS mice (p < 0.0001). β-FNA + LPS mice had significantly higher CXCL10 levels than saline or β-FNA mice (p < 0.0001), while levels of CXCL10 were similar between saline and β-FNA mice (p = 0.72).
Two-way ANOVA revealed that there was a significant main effect for LPS (F1, 22 = 91.15, p < 0.0001) and β-FNA (F1, 22 = 12.06, p < 0.005) on CXCL10 levels in the spleen, but there was no significant interaction of main effects (F1, 22 = 2.49, p = 0.13) (Fig. 4C). LPS mice had significantly higher levels of CXCL10 than saline (p < 0.0001), β-FNA (p < 0.0001) or β-FNA + LPS mice (p < 0.005). CXCL10 levels were similar between saline and β-FNA mice (p = 0.19), and both saline (p < 0.0005) and β-FNA mice (p < 0.0001) had significantly lower levels of CXCL10 than β-FNA + LPS mice.
In conclusion, β-FNA attenuated LPS-induced elevations of CXCL10 in the frontal cortex, hippocampus, and spleen. Also, β-FNA prevented LPS-induced elevations of CCL2 in the brain and spleen. However, under non-inflammatory conditions, β-FNA raised CCL2 and CXCL10 levels in the frontal cortex and inhibited CCL2 expression in the spleen.
Correlation of inflammatory mediators in the brain and spleen with anxiety-like behavior
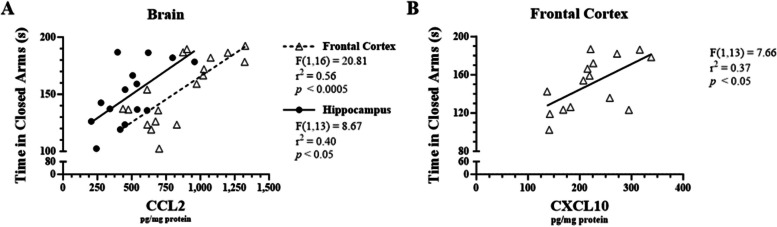
Linear regression analysis was performed to determine whether there were significant correlations between pro-inflammatory chemokine expression and anxiety-like behavior. CCL2 levels in the frontal cortex and hippocampus were positively correlated with time spent in closed arms (r2 = 0.56, F1, 16 = 20.81, p < 0.0005 and r2 = 040, F1, 13 = 8.67, p < 0.05, respectively; Fig. 5A). Furthermore, there was a significant positive correlation with CXCL10 expression in the frontal cortex and time spent in closed arms (r2 = 0.37, F1, 13 = 7.66, p < 0.05; Fig. 5B).
Fig. 5.
Correlations between LPS-induced CXCL10 levels in male C57BL/6J mouse brains and anxiety-like behavior. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and delivered at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. A CCL2 and (B) CXCL10 were measured via ELISA in brain region and spleen homogenates. Data are presented as mean ± SEM. Linear regression analysis was used to assess frontal cortex, hippocampus, and spleen CCL2 and CXCL10 levels with EPM-time spent in closed arms. Linear regression statistics and symbols are provided in the figure, and only significant results are shown
Correlation of inflammatory mediators in the brain and spleen with sickness-like behavior
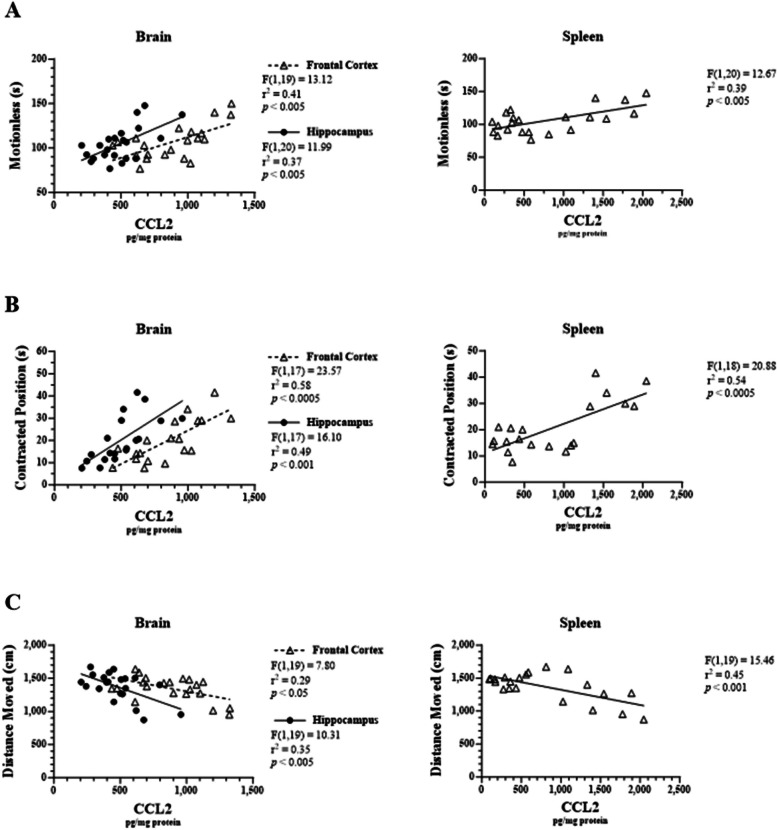
Linear regression analysis demonstrated that there were significant correlations between tissue-specific chemokine levels and sickness-like behaviors. Specifically, CCL2 levels in the frontal cortex, hippocampus, and spleen were each positively correlated with time spent motionless in the elevated plus maze (r2 = 0.41, F1, 19 = 13.12, p < 0.005; r2 = 0.37, F1, 20 = 11.99, p < 0.005; r2 = 0.39, F1, 20 = 12.67, p < 0.005, respectively; Fig. 6A). Similarly, CCL2 levels were correlated with time spent in a contracted body position (r2 = 0.58, F1, 17 = 23.57, p < 0.0005; r2 = 0.49, F1, 17 = 16.10, p < 0.001; r2 = 0.54, F1, 18 = 20.88, p < 0.0005, respectively; Fig. 6B). Lastly, CCL2 levels in the frontal cortex, hippocampus, and spleen were each negatively correlated with total distance moved (r2 = 0.29, F1, 19 = 7.80, p < 0.05; r2 = 0.35, F1, 19 = 10.31, p < 0.005; r2 = 0.45, F1, 19 = 15.46, p < 0.001, respectively; Fig. 6C).
Fig. 6.
Correlations between LPS-induced CCL2 levels in male C57BL/6J mouse tissues and sickness-like behavior. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. CCL2 was measured via ELISA in brain region and spleen homogenates. Behavioral endpoints that were measured included (A) time spent motionless, (B) time spent in contracted position, and (C) total distance moved. Data are presented as mean ± SEM. Linear regression analysis was used to assess frontal cortex, hippocampus, and spleen CCL2 levels with EPM behavioral endpoints. Linear regression statistics and symbols are provided in figure, and only significant results are shown
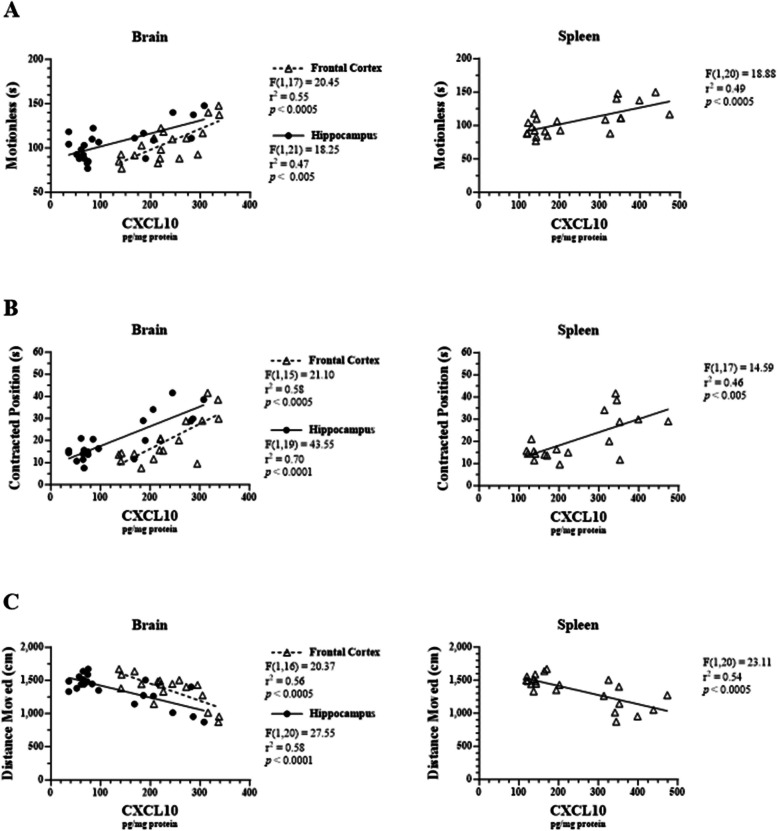
CXCL10 levels in the frontal cortex, hippocampus, and spleen were also positively correlated with time spent motionless (r2 = 0.55, F1, 17 = 20.45, p < 0.0005; r2 = 0.47, F1, 21 = 18.25, p < 0.005; r2 = 0.49, F1, 20 = 18.88, p < 0.0005, respectively; Fig. 7A). CXCL10 levels in the frontal cortex, hippocampus, and spleen were also positively correlated with time spent in a contracted body position (r2 = 0.58, F1, 15 = 21.10, p < 0.0005; r2 = 0.70, F1, 19 = 43.55, p < 0.0001; r2 = 0.46, F1, 17 = 14.59, p < 0.005, respectively; Fig. 7B). Finally, CXCL10 levels in the frontal cortex, hippocampus, and spleen were negatively correlated with total distance moved (r2 = 0.56, F1, 16 = 20.37, p < 0.0005; r2 = 0.58, F1, 20 = 27.55, p < 0.0001; r2 = 0.54, F1, 20 = 23.11, p < 0.0005, respectively; Fig. 7C).
Fig. 7.
Correlations between LPS-induced CXCL10 levels in male C57BL/6J mouse tissues and sickness-like behavior. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. CXCL10 was measured via ELISA in brain region and spleen homogenates. Behavioral endpoints that were measured included (A) time spent motionless, (B) time spent in contracted position, and (C) total distance moved. Data are presented as mean ± SEM. Linear regression analysis was used to assess frontal cortex, hippocampus, and spleen CXCL10 levels with EPM behavioral endpoints. Linear regression statistics and symbols are provided in figure, and only significant results are shown
Correlation of inflammatory mediators in the brain and spleen with depressive-like behavior
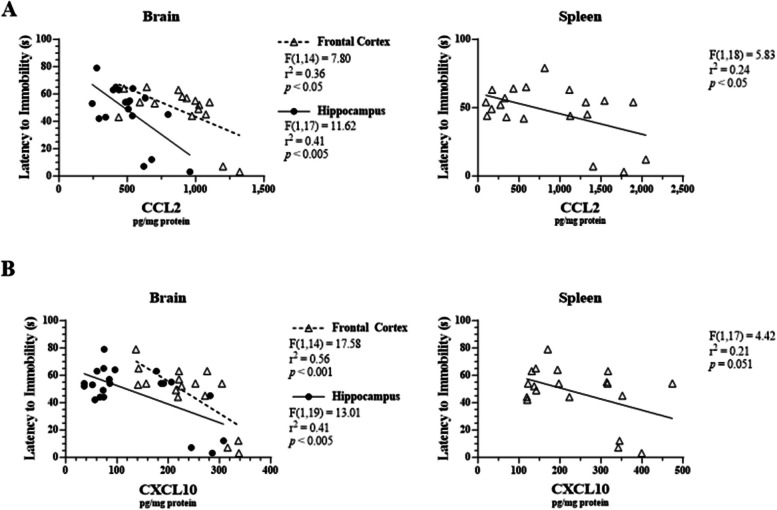
Linear regression analysis revealed significant correlations between chemokine levels in various tissues and depressive-like behavior. Specifically, CCL2 levels in the frontal cortex, hippocampus, and spleen were negatively correlated with latency to immobility in the FST (r2 = 0.36, F1, 14 = 7.80, p < 0.05; r2 = 0.41, F1, 17 = 11.62, p < 0.005; r2 = 0.24, F1, 18 = 5.83, p < 0.05, respectively; Fig. 8A). Additionally, CXCL10 levels in the frontal cortex (r2 = 0.56, F1, 14 = 17.58, p < 0.001) and hippocampus (r2 = 0.41, F1, 19 = 13.01, p < 0.005) were negatively correlated with latency to immobility (Fig. 8B). Notably, CXCL10 levels in the spleen trended towards correlation with latency to immobility (p = 0.051, Fig. 8B).
Fig. 8.
Correlations between LPS-induced chemokines in male C57BL/6J tissues and latency to immobility in the FST. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. A CCL2 and (B) CXCL10 were measured via ELISA in brain region and spleen homogenates. Data are presented as mean ± SEM. Linear regression analysis was used to assess frontal cortex, hippocampus, and spleen CCL2 and CXCL10 levels with percentage of time spent immobile and latency to immobility in the FST. Linear regression statistics and symbols are provided in the figure, and only significant results are shown
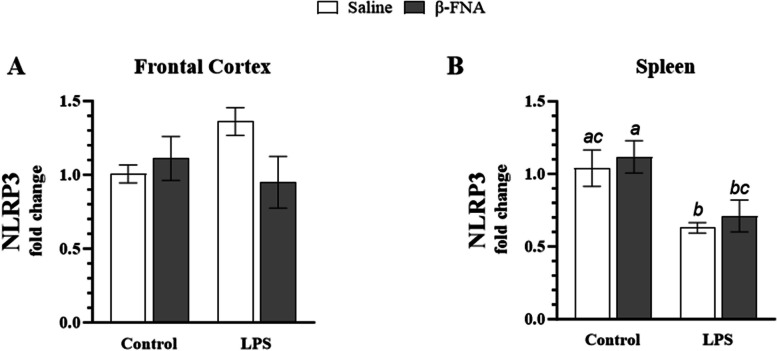
Effects of β-FNA on NLRP3 in the frontal cortex and spleen
Two-way ANOVA indicated no significant main effect of LPS (F1, 13 = 0.60, p = 0.45) or β-FNA (F1, 13 = 1.49, p = 0.24), as well as no significant interaction of main effects (F1, 13 = 4.24, p = 0.06) on NLRP3 expression in the frontal cortex (Fig. 9A). However, β-FNA + LPS mice tended to have lower levels of NLRP3 than LPS mice.
Fig. 9.
Chronic β-FNA effects on LPS-induced NLRP3 expression in male C57BL/6J frontal cortex and spleen tissues. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. RNA was extracted from (A) frontal cortex and (B) spleen tissues, and NLRP3 expression was measured via RT-qPCR. Statistical analysis was performed using the ∆CT-∆CT method and reported as fold change relative to the control group. Data are presented as mean ± SEM. A Two-way ANOVA (n = 3–6/group) indicated no significant main effect for LPS (p = 0.45) or β-FNA (p = 0.24), as well as no significant interaction of main effects (p = 0.06) on NLRP3 expression in the frontal cortex. (B) Two-way ANOVA (n = 4–7/group) revealed a significant main effect for LPS (p < 0.005), no significant main effect for β-FNA (p = 0.50), and no significant interaction of main effects (p = 0.98) on NLRP3 expression in the spleen. Pairwise comparisons were assessed using Fisher's LSD test; bars with letters in common indicate data are not significantly different (p > 0.05)
Two-way ANOVA revealed a significant main effect of LPS (F1, 19 = 12.71, p < 0.005), no significant main effect for β-FNA (F1, 19 = 0.48, p = 0.50), and no significant interaction of main effects (F1, 19 = 0.0004, p = 0.98) on NLRP3 expression in the spleen (Fig. 9B). Pairwise comparisons revealed that expression was similar between saline and β-FNA mice (p = 0.60) and LPS and β-FNA + LPS mice (p = 0.65). However, LPS mice had significantly lower NLRP3 expression in the spleen than saline (p < 0.05) or β-FNA mice (p < 0.01). Additionally, β-FNA + LPS mice had significantly lower NLRP3 expression than β-FNA mice (p < 0.05) and tended to have lower NLRP3 expression than saline mice (p = 0.06).
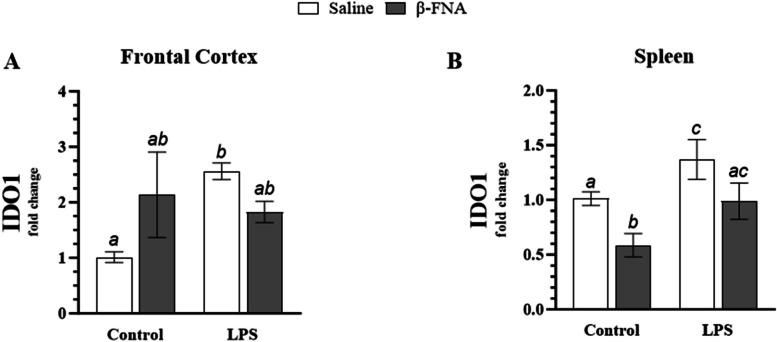
Effects of β-FNA on IDO1 in the frontal cortex and spleen
Two-way ANOVA revealed a significant interaction of main effects (F1, 13 = 5.81, p < 0.05), but no significant main effect for LPS (F1, 13 = 2.59, p = 0.13) or β-FNA (F1, 13 = 0.25, p = 0.62) on IDO1 expression in the frontal cortex (Fig. 10A). Pairwise comparisons revealed that LPS mice had significantly higher levels of IDO1 expression in the frontal cortex than saline mice (p < 0.05). There was no significant difference between saline and β-FNA mice (p = 0.07), saline and β-FNA + LPS mice (p = 0.17), β-FNA and LPS mice (p = 0.44), β-FNA and β-FNA + LPS mice (p = 0.59), or LPS and β-FNA + LPS mice (p = 0.19).
Fig. 10.
Chronic β-FNA effects on LPS-induced IDO1 expression in male C57BL/6J frontal cortex and spleen tissues. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. RNA was extracted from (A) frontal cortex and (B) spleen tissues, and NLRP3 expression was measured via RT-qPCR. Statistical analysis was performed using the ∆CT-∆CT method and reported as fold change relative to the control group. Data are presented as mean ± SEM. A Two-way ANOVA (n = 4–5/group) revealed a significant interaction of main effects (p < 0.05) and no significant main effect for LPS (p = 0.13) or β-FNA (p 0.62) on IDO1 expression in the frontal cortex. B Two-way ANOVA (n = 4–7/group) indicated a significant main effect for LPS (p < 0.01) and β-FNA (p < 0.005), but no significant interaction of main effects (p = 0.87) on IDO1 expression in the spleen. Pairwise comparisons were assessed using Fisher's LSD test; bars with letters in common indicate data are not significantly different (p > 0.05)
Two-way ANOVA indicated a significant main effect for LPS (F1, 18 = 9.14, p < 0.01) and β-FNA (F1, 18 = 10.29, p < 0.005), but no significant interaction of main effects (F1, 18 = 0.03, p = 0.87) on IDO1 expression in the spleen (Fig. 10B). Pairwise comparisons revealed that LPS mice had significantly higher IDO1 expression than saline (p < 0.05) or β-FNA mice (p < 0.0005). While β-FNA + LPS mice tended to have lower IDO1 expression than LPS mice, it fell short of significance (p = 0.06). Pairwise comparisons revealed that β-FNA mice had significantly lower IDO1 expression than saline (p < 0.05) or β-FNA + LPS mice (p < 0.05). IDO1 expression was similar between saline and β-FNA + LPS mice (p = 0.90).
To conclude, while trends showed that β-FNA + LPS mice had lower expressions of IDO1 in the frontal cortex and spleen than LPS mice, it was not significant. Additionally, it appeared that β-FNA treatment suppressed splenic IDO1 expression under control conditions.
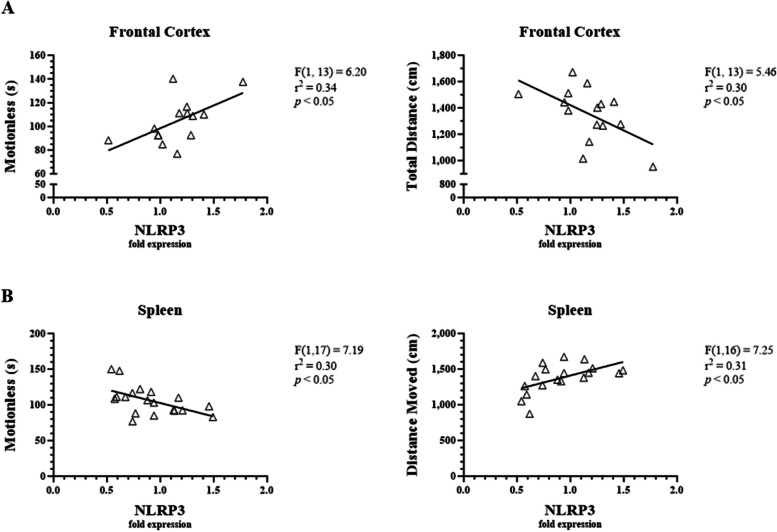
Correlation of NLRP3 in the frontal cortex and spleen with behavioral test measures
Linear regression analysis was used to determine whether NLRP3 expression in the frontal cortex and spleen were significantly correlated with anxiety-, sickness-, and depressive-like behavioral measures. Behavioral endpoints analyzed included time in closed arms (EPM), time spent motionless (EPM), time spent in a contracted position (EPM), percentage of time immobile (FST), and latency to immobility (FST). NRLP3 expression in the frontal cortex was not correlated with anxiety-like behavior (F1,10 = 2.64, p = 0.14). However, NLRP3 expression in the frontal cortex increased with sickness-like behavior (as determine by increased time spent motionless and decreased total distance moved; p < 0.05, Fig. 11A). Conversely, linear regression analysis demonstrated that NLRP3 expression in the spleen was negatively correlated with time spent motionless and positively correlated with total distance moved (p < 0.05, Fig. 11B). These findings are consistent with the significantly lower levels of NLRP3 in the spleen of LPS-treated mice compared to the saline-treated mice (Fig. 9B). Overall, NLRP3 expression in the frontal cortex was positively correlated with sickness-like behaviors, while NLRP3 expression in the spleen was inversely related to sickness-like behaviors.
Fig. 11.
Correlations between LPS-induced NLRP3 expression and measures of sickness-like behaviors in male C57BL/6J mice. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. NLRP3 was measured via RT-qPCR using frontal cortex and spleen RNA extracts. Linear regression analysis was used to assess (A) frontal cortex and (B) spleen NLRP3 expression with various anxiety-, sickness-, and depressive-like behavioral endpoints. Data are presented as mean ± SEM. Linear regression statistics and symbols are provided in figure, and only significant results are shown
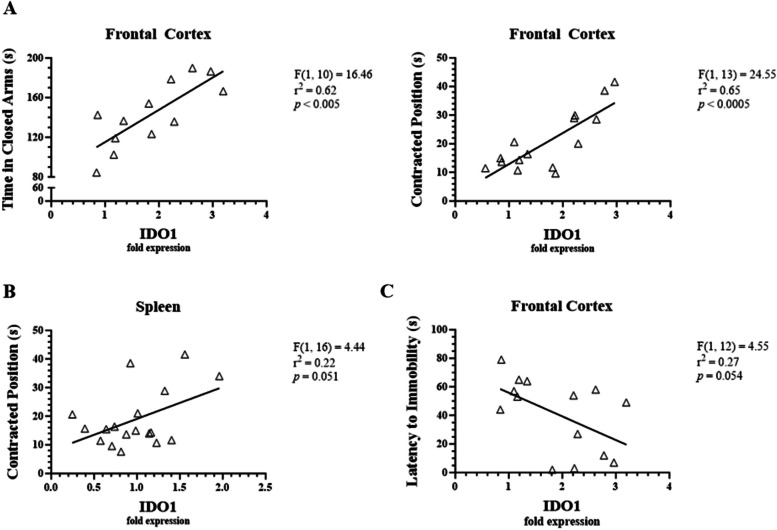
Correlation of IDO1 in the frontal cortex and spleen with behavioral test measures
Linear regression analysis revealed that IDO1 expression in the frontal cortex was significantly correlated with measures of anxiety- and sickness-like behaviors. Specifically, IDO1 in the frontal cortex was correlated with time spent in the closed arms (p < 0.005) and time spent in a contracted position (p < 0.0005, Fig. 12A). Time spent in a contracted position also tended to increase with IDO1 expression in the spleen (p = 0.051, Fig. 12B). While IDO1 levels did not significantly correlate with depressive-like behaviors, latency to immobility in the FST tended to decrease as IDO1 expression increased in the frontal cortex (p = 0.054, Fig. 12C). There were no other significant correlations between IDO1 expression in the frontal cortex or spleen and measures of anxiety-, sickness-, or depressive-like behaviors. Consequently, only IDO1 expression in the frontal cortex significantly correlated with anxiety- and sickness-like behaviors.
Fig. 12.
Correlations between LPS-induced IDO1 expression and behavioral measures in male C57BL/6J mice. Micro-osmotic pumps containing saline or β-FNA (42 μg/d) were surgically implanted and dispensed at a flow rate of 0.5 μL/h for 7d. 6d post-surgery, mice (n = 7–8/group) were injected (i.p.) with either 25 μL saline control or LPS (0.83 mg/kg). Behavioral tests were administered 24 h later, and termination followed immediately after. IDO1 was measured via RT-qPCR using frontal cortex and spleen RNA extracts. Linear regression analysis was used to assess frontal cortex and spleen NLRP3 expression with various anxiety-, sickness-, and depressive-like behavioral endpoints. A IDO1 in the frontal cortex correlated with anxiety- and sickness-like behavior. B IDO1 in the spleen trended with sickness-like behavior but fell short of significance. C IDO1 in the frontal cortex trended with depressive-like behavior but fell short of significance. Data are presented as mean ± SEM. Linear regression statistics and symbols are provided in figure
Discussion
To our knowledge, this is the first examination of the effects of chronic, continuous β-FNA pre-treatment on inflammation and behavior. We found that β-FNA greatly reduced anxiety- and sickness-like behavior in male C57BL/6J mice, while simultaneously inhibiting or even abolishing LPS-driven elevations in CCL2 and CXCL10 in the hippocampus, frontal cortex, and spleen. The spleen is integral to innate immunity and accumulating evidence suggests that communications between the brain and spleen (brain-spleen axis) are important for well-being, and disruptions in this axis play an integral role in the underlying pathophysiology of numerous diseases, such as traumatic brain injury, autoimmune diseases, and depression [29–32]. Furthermore, we established that anxiety-like behaviors were predominantly correlated with CNS levels of CCL2 and CXCL10, whereas sickness-like behaviors were correlated with systemic levels of CCL2 and CXCL10. Moreover, we demonstrated that sickness-like behavior was correlated with NLRP3 expression in the frontal cortex and inversely related to NLRP3 expression in the spleen, and IDO1 expression in the frontal cortex was correlated with anxiety- and sickness-like behavior.
Previous work in our lab has shown that acute (6–24 h) β-FNA treatment reduces LPS-stimulated pro-inflammatory chemokine expression in C57BL/6J mice [15–17, 19]. Acute β-FNA treatment reduced CXCL10 levels across brain regions, but those changes were not always detected in whole brain tissue [15–17, 19]. Likewise, β-FNA appeared to inconsistently reduce CCL2 levels in whole brain tissue and plasma, and region-specific reductions have only been found in the cerebellum and brainstem [15–17]. Additionally, acute β-FNA effects appear to be sex-specific, as LPS-induced CXCL10 in the hippocampus, prefrontal cortex, cerebellum, and brainstem and CCL2 in the cerebellum and brainstem were decreased by β-FNA in males, but not in females [17].
The apparent, differential effects of acute versus chronic, continuous β-FNA pretreatment on LPS-induced CXCL10 and CCL2 may be attributed to pre-exposure and cumulative dose. Studies with rats have shown that μ-opioid receptor (MOR) turnover becomes slower and less efficient with increased β-FNA pre-exposure [33]. Therefore, it is possible that chronic β-FNA pre-treatment could reduce MOR turnover and efficiency and effectively decrease the ability of endogenous opioids to surmount β-FNA antagonism [34, 35]. Additionally, the extent of irreversible MOR inhibition is dose-dependent, and, as such, the mice in this study were exposed to higher doses due to the continuous administration of the drug via micro-osmotic pump [36–38]. Importantly though, it remains unclear the extent to which the anti-inflammatory and/or behavior-modifying effects of β-FNA in this preclinical model are related to actions at MOR. In fact, the in vitro findings indicate that the anti-inflammatory effects of β-FNA in astrocytes involve a MOR-independent mechanism [15, 18, 19, 39]. Briefly, these investigations suggest that β-FNA inhibits cytokine/chemokine and inducible nitric oxide synthase activation through disruption of upstream, pre-transcriptional events including NF-κB and p38 MAPK activation [18, 19, 40]. Our group also demonstrated that β-FNA blocks TLR4 signaling by LPS in TLR4-HEK reporter cells [39]. Our investigation into the mechanism by which β-FNA modulates inflammatory signaling at the cellular and molecular level is ongoing.
Our previous studies found that LPS-induced chemokine expression in the brain was positively correlated with anxiety- and sickness-like behaviors [15–17]. Furthermore, we previously found that LPS-induced sickness-like behavior in male mice was inhibited by acute β-FNA treatment [15, 16]. Consistent with these earlier reports, the current study revealed that LPS-induced sickness-like behavior is also inhibited by chronic, continuous β-FNA pretreatment. Interestingly, previous findings in this model showed that acute β-FNA decreased LPS-induced anxiety-like behavior predominantly in a female-specific manner [17]. While only male mice were used in the present study, chronic, continuous β-FNA pre-treatment effectively inhibited anxiety-like behavior.
We did not observe pronounced LPS-induced depressive-like behavior in the present study, which was unexpected given the extensive literature showing that LPS is frequently and effectively used to induce depressive-like behavior in C57BL/6J mice utilizing the LPS strain, dose, and administration method used in our study [41]. Furthermore, it has been shown that depressive-like behaviors peak at 24 h post-injection and can still be observed 48 h after LPS administration [41]. One explanation for the lack of measurable depressive-like activity in the present study is disruption of swimming mechanics due to displacement of micro-osmotic drug pumps. Over the course of the experiment, in numerous cases the mini-pump shifted laterally from the original placement between the scapulae, potentially hindering balance and swimming mechanics. Furthermore, most of the mice atypically became immobile within seconds of entering the water, hence the elimination of the standard 2-min delay in FST scoring. In future experiments, daily, i.p. injections of β-FNA are warranted when utilizing the FST; alternatively, a tail suspension test may be more effective in mice implanted with a mini-pump.
NLRP3 expression in the frontal cortex correlated with sickness-like behaviors. Since the NLRP3 inflammasome is responsible for processing and releasing bioactive IL-1β, this trend aligns with evidence that inflammasome signaling and IL-1β in the brain is a key mediator of depressive-, anxiety-, and sickness-like behaviors [25, 42–45]. Interestingly, NLRP3 expression was lower in LPS-stimulated mice compared to saline counterparts and was negatively correlated with sickness-like behavior. The suppression of NLRP3 in the spleen under LPS stimulation may be due to the prolonged LPS stimulation, as it has been shown that acute LPS (4 h) robustly induces NLRP3 inflammasome activation in bone marrow-derived macrophages, but chronic LPS (12 h-24 h) attenuates it [46]. This has been attributed to the release of interleukin-10 (IL-10) as a protective mechanism against excessive inflammation, as macrophages and bone marrow-derived macrophages only release IL-10 under chronic and not acute LPS stimulation [46]. This assertion was strengthened by a recent study that demonstrated that TLR4, NF-κB, and interleukin-6 (IL-6) were significantly increased, while IL-1β was significantly decreased, in the spleens of ICR mice 12 h after LPS injection, whereas IL-1β was significantly elevated in the brain [47].
IDO1 is a pivotal mediator of LPS-induced depression- and anxiety-like behavior in C57BL/6J mice [48]. Likewise, this study found that LPS raised IDO1 levels in the frontal cortex, and IDO1 in the frontal cortex was positively correlated with anxiety- and sickness-like behavior. However, β-FNA treatment did not significantly alter LPS-induced IDO1 levels in the frontal cortex. The lack of significance may simply reflect a relatively low sample size and warrants further investigation.
Surprisingly, we determined that β-FNA treatment alone increased CCL2 and CXCL10 levels in the frontal cortex and seemed to be anxiogenic. These are the only seemingly adverse effects of β-FNA that we have observed and are presumably due to the chronic, continuous administration. Indeed, acute β-FNA, per se, has not impacted inflammatory factors or behaviors in our previous studies or those of others using mixed neuron/glia cultures from Sprague–Dawley rats [49]. It has been well documented that β-FNA inhibits NF-κB activation, which results in the subsequent downregulation of pro-inflammatory cytokines, such as CCL2 and CXCL10 [16, 17, 19, 20, 49]. While seen as predominantly pro-inflammatory, NF-κB regulates a wide expression of genes involved in cell survival, growth, stress responses, and immune system activity [50]. Additionally, NF-κB is constitutively active in region-specific neurons, especially the cortex and hippocampus, and plays an integral role in synaptic plasticity, learning and memory, and synapse-to-nucleus communication under normal physiological conditions [50–52]. Moreover, it has been shown that inhibiting or blocking NF-κB expression induces death in cortical neurons and causes loss of neuroprotection and defects in learning and memory [53–55]. As such, it is possible that continuous administration of β-FNA may inhibit constitutive NF-κB to the detriment of neurons in the frontal cortex [56–62].
Conclusion
This study builds upon our previous findings that acute β-FNA treatment inhibits inflammatory signaling in human astrocytes and ameliorates LPS-induced neuroinflammation and anxiety- and sickness-like behavior in a pre-clinical mouse model. We now provide evidence that chronic β-FNA pre-treatment inhibits LPS-driven elevations in CCL2 and CXCL10, as well as limits or abolishes anxiety- and sickness-like behaviors. In particular, anxiety-like behaviors were predominantly correlated with CCL2 and CXCL10 in the brain, whereas sickness-like behaviors were correlated with CCL2 and CXCL10 in the spleen. Moreover, we demonstrated that sickness-like behavior was positively correlated with NLRP3 expression in the frontal cortex and inversely related to NLRP3 expression in the spleen. Additionally, IDO1 expression in the frontal cortex was correlated with both anxiety- and sickness-like behavior. However, β-FNA did not significantly inhibit LPS-induced NLRP3 or IDO1 expression in the frontal cortex or spleen. Further investigation is needed to fully understand the differential effects of acute and chronic, pre-treatment in terms of the anti-inflammatory and behavior-modifying effects of β-FNA. Similarly, additional investigation is needed to define the cellular and molecular events governing the anti-inflammatory effects of β-FNA.
Acknowledgements
Not applicable.
Abbreviations
- β-FNA
Beta-funaltrexamine
- BCA
Bicinchoninic assay
- cAMP
Cyclic adenosine monophosphate
- CCL2 (MCP-1)
C–C motif chemokine ligand 2 (monocyte chemoattractant protein-1)
- CXCL10 (IP-10)
C-X-C motif chemokine 10 (interferon γ-induced protein 10 kDa)
- dNTP
Deoxynucleoside triphosphate
- ELISA
Enzyme-Linked Immunosorbent Assay
- EPM
Elevated Plus Maze
- FST
Forced Swim Test
- IDO1
Indoleamine 2,3-dioxygenase 1
- IL-10
Interleukin-10
- IL-1β
Interleukin-1 beta
- IL-6
Interleukin-6
- LPS
Lipopolysaccharide
- LSD
Least significant difference
- MAPK
Mitogen-activated protein kinase
- M-MLV
Moloney murine leukemia virus
- MOR
Mu opioid receptor
- NF-κB
Nuclear factor kappa B
- NLRP3
NOD-like receptor family pyrin domain-containing protein 3
- RNA
Ribonucleic acid
- rRNA
Ribosomal ribonucleic acid
- RT-qPCR
Reverse transcription-quantitative polymerase chain reaction
- SEM
Standard error of mean
- STAT
Signal transducer and activator of transcription
- TLR
Toll-like receptor
- TLR4
Toll-like receptor 4
Authors’ contributions
K.H. designed and performed the experiments, performed data analyses, prepared figures, and wrote the manuscript. D.B. assisted with experiments and assays and preparation of the methods. S.D. was instrumental in assay performance, data interpretation, and manuscript writing and editing. R.D. was involved in experimental design, data interpretation, and writing the manuscript.
Funding
This study was supported in part by Oklahoma Center for Advancement of Science & Technology Health Research Program-HR 18–033 (RLD) and the Oklahoma State University Center for Health Sciences, Office of the Vice President for Research (RLD).
Availability of data and materials
Data can be made available upon reasonable request.
Declarations
Ethics approval and consent to participate
OSU-CHS Institutional Animal Care and Use Committee approved all experimental processes and animal manipulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Henein MY, et al. The role of inflammation in Cardiovascular Disease. Int J Mol Sci. 2022;23:12906. 10.3390/ijms232112906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman D, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32. 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straub RH, Schradin C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol Med Public Health. 2016;2016:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. 10.2174/187221309787158371 [DOI] [PubMed] [Google Scholar]
- 5.Afify SM, et al. Cancer-inducing niche: the force of chronic inflammation. Br J Cancer. 2022;127:193–201. 10.1038/s41416-022-01775-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23:50. 10.1186/s12199-018-0740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page MJ, Kell DB, Pretorius E. The role of Lipopolysaccharide-Induced cell signalling in chronic inflammation. Chronic Stress. 2022;6:1–18. 10.1177/24705470221076390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer ME, Teixeira AL. Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci. 2019;1437:57–67. 10.1111/nyas.13712 [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Pereira JS, Rea K, Nolan YM, O’Leary OF, Dinan TG, Cryan JF. Depression’s Unholy Trinity: dysregulated stress, immunity, and the Microbiome. Annu Rev Psychol. 2020;71:49–78. 10.1146/annurev-psych-122216-011613 [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues FTS, et al. Major depression model induced by repeated and intermittent lipopolysaccharide administration: long-lasting behavioral, neuroimmune and neuroprogressive alterations. J Psychiatr Res. 2018;107:57–67. 10.1016/j.jpsychires.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 11.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172:1075–91. 10.1176/appi.ajp.2015.15020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gałecki P, Talarowska M. Inflammatory theory of depression. Psychiatr Pol. 2018;52:437–47. 10.12740/PP/76863 [DOI] [PubMed] [Google Scholar]
- 13.Lyman M, et al. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12. 10.1016/j.neures.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 14.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(Suppl 2):136–53. 10.1111/jnc.13607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis RL, Stevens CW, Thomas Curtis J. The opioid antagonist, β-funaltrexamine, inhibits lipopolysaccharide-induced neuroinflammation and reduces sickness behavior in mice. Physiol Behav. 2017;173:52–60. 10.1016/j.physbeh.2017.01.037 [DOI] [PubMed] [Google Scholar]
- 16.Myers S, et al. Anti-inflammatory actions of β-funaltrexamine in a mouse model of lipopolysaccharide-induced inflammation. Inflammopharmacology. 2023;31:349–58. 10.1007/s10787-022-01113-9 [DOI] [PubMed] [Google Scholar]
- 17.Myers S, et al. Anti-inflammatory effects of β-FNA are sex-dependent in a pre-clinical model of LPS-induced inflammation. J Inflamm. 2023;20(1):4. 10.1186/s12950-023-00328-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis RL, et al. β-Funaltrexamine inhibits chemokine (CXCL10) expression in normal human astrocytes. Neurochem Int. 2013;62:478–85. 10.1016/j.neuint.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis RL, et al. The opioid antagonist, β-funaltrexamine, inhibits NF-κB signaling and chemokine expression in human astrocytes and in mice. Eur J Pharmacol. 2015;5:762:193–201. 10.1016/j.ejphar.2015.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis RL, McCracken K, Buck DJ. β-funaltrexamine differentially modulates chemokine and cytokine expression in normal human astrocytes and C20 human microglial cells. Neuroimmunol Neuroinflamm. 2020;7:300–10. [Google Scholar]
- 21.Walker AK, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38:1609–16. 10.1038/npp.2013.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skrzypczak-Wiercioch A, Salat K. Lipopolysaccharide-induced model of neuroinflammation: mechanisms of action, research application and future directions for its use. Molecules. 2022;27:5481. 10.3390/molecules27175481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Q, et al. B355252 supresses LPS-induced neuroinflammation in the mouse brain. Brain Sci. 2024;14:467. 10.3390/brainsci14050467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connor JC, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–22. 10.1038/sj.mp.4002148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dantzer R. Cytokine, sickness behavior, and depression. Neurol Clin. 2006;24:441–60. 10.1016/j.ncl.2006.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley KW, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–118. 10.1016/S0889-1591(02)00077-6 [DOI] [PubMed] [Google Scholar]
- 27.Nandi A, et al. DBscorer: an Open-Source Software for Automated Accurate Analysis of Rodent Behavior in forced swim test and tail suspension test. eNeuro. 2021;8:0305–21. 10.1523/ENEURO.0305-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toni LS, et al. Optimization of phenol-chloroform RNA extraction. MethodsX. 2018;5:599–608. 10.1016/j.mex.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Y, et al. Brain-spleen axis in health and diseases: a review and future perspective. Brain Res Bull. 2022;182:130–40. 10.1016/j.brainresbull.2022.02.008 [DOI] [PubMed] [Google Scholar]
- 30.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313–35. 10.1146/annurev-immunol-020711-075015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci. 2004;61:2322–31. 10.1007/s00018-004-4102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dantzer R. Neuroimmune interactions: from the brain to the Immune System and Vice Versa. Physiol Rev. 2018;98:477–504. 10.1152/physrev.00039.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medrano MC, et al. Characterization of functional µ opioid receptor turnover in rat locus coeruleus: an electrophysiological and immunocytochemical study. Br J Pharmacol. 2017;174:2758–72. 10.1111/bph.13901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuitavi J, et al. Crosstalk between Mu-Opioid receptors and neuroinflammation: consequences for drug addiction and pain. Neurosci Biobehav Rev. 2023;145:105011. 10.1016/j.neubiorev.2022.105011 [DOI] [PubMed] [Google Scholar]
- 35.Adams JU, Paronis CA, Holtzman SG. Assessment of relative intrinsic activity of mu-opioid analgesics in vivo by using beta-funaltrexamine. J Pharmacol Exp Ther. 1990;255:1027–32. [PubMed] [Google Scholar]
- 36.Mjanger E, Yaksh TL. Characteristics of dose-dependent antagonism by beta-funaltrexamine of the antinociceptive effects of intrathecal mu agonists. J Pharmacol Exp Ther. 1991;258:544–50. [PubMed] [Google Scholar]
- 37.Tam SW, Liu-Chen LY. Reversible and irreversible binding of beta-funaltrexamine to mu, delta and kappa opioid receptors in guinea pig brain membranes. J Pharmacol Exp Ther. 1986;239:351–7. [PubMed] [Google Scholar]
- 38.Ward SJ, Portoghese PS, Takemori AE. Pharmacological characterization in vivo of the novel opiate, beta-funaltrexamine. J Pharmacol Exp Ther. 1982;220:494–8. [PubMed] [Google Scholar]
- 39.Stevens CW, et al. Pharmacological characterization of LPS and opioid interactions at the toll-like receptor 4. Br J Pharmacol. 2013;168:1421–9. 10.1111/bph.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis RL, et al. The opioid antagonist, beta-funaltrexamine, inhibits chemokine expression in human astroglial cells. J Neuroimmunol. 2007;186:141–9. 10.1016/j.jneuroim.2007.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin R, et al. Lipopolysaccharide-induced depression-like model in mice: meta-analysis and systematic evaluation. Front Immunol. 2023;14:1181973. 10.3389/fimmu.2023.1181973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murray CL, et al. Endogenous IL-1 in cognitive function and anxiety: a study in IL-1RI-/- mice. PLoS One. 2013;8(10):e78385. 10.1371/journal.pone.0078385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong M-L, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol Psychiatry. 2016;21:797–805. 10.1038/mp.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi S, et al. Interleukin-1β causes anxiety by interacting with the endocannabinoid system. J Neurosci. 2012;32:13896–905. 10.1523/JNEUROSCI.1515-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther. 2014;20:119–24. 10.1111/cns.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurung P, et al. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and Caspase-8 activation. Sci Rep. 2015;5:14488. 10.1038/srep14488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong Q, et al. Comparison of the TLR4/NFκB and NLRP3 signalling pathways in major organs of the mouse after intravenous injection of lipopolysaccharide. Pharm Biol. 2019;57:555–63. 10.1080/13880209.2019.1653326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salazar A, et al. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav. 2012;62:202–9. 10.1016/j.yhbeh.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C-C, et al. β-Funaltrexamine displayed anti-inflammatory and neuroprotective effects in cells and rat model of stroke. Int J Mol Sci. 2020;21:3866. 10.3390/ijms21113866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dresselhaus EC, Meffert MK. Cellular specificity of NF-κB function in the nervous system. Front Immunol. 2019;10:1043. 10.3389/fimmu.2019.01043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattson MP, Meffert MK. Roles for NF-κB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–60. 10.1038/sj.cdd.4401837 [DOI] [PubMed] [Google Scholar]
- 52.Kaltschmidt C, et al. Constitutive NF-κB activity in neurons. Mol Cell Biol. 1994;14:3981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fridmacher V, et al. Forebrain-specific neuronal inhibition of nuclear factor-kappab activity leads to loss of neuroprotection. J Neurosci. 2003;23:9403–8. 10.1523/JNEUROSCI.23-28-09403.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhakar AL, et al. Constitutive nuclear factor-κB activity is required for central neuron survival. J Neurosci. 2002;22:8466–75. 10.1523/JNEUROSCI.22-19-08466.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaltschmidt B, Kaltschmidt C. NF-κB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1:a001271. 10.1101/cshperspect.a001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J-G, Ruckle MB, Prather PL. Constitutively active µ-opioid receptors inhibit adenylyl cyclase activity in intact cells and activate G-proteins differently than the agonist [d-Ala2, N-MePhe4, Gly-ol5] enkephalin. J Biol Chem. 2001;276:37779–86. 10.1074/jbc.M106104200 [DOI] [PubMed] [Google Scholar]
- 57.Lengler J, Jug F, Steger A. Reliable neuronal systems: the importance of heterogeneity. PLoS One. 2013;8:e80694. 10.1371/journal.pone.0080694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spurgat MS, Tang SJ. Single-Cell RNA-Sequencing: Astrocyte and Microglial Heterogeneity in Health and Disease. Cells. 2022;11:2021. 10.3390/cells11132021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dadwal S, Heneka MT. Microglia heterogeneity in health and disease. FEBS Open Bio. 2024;14:217–29. 10.1002/2211-5463.13735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan Y-L, Yuan Y, Tian L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry. 2020;25:351–67. 10.1038/s41380-019-0609-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilhelm I, et al. Heterogeneity of the blood-brain barrier. Tissue Barriers. 2016;4:e1143544. 10.1080/21688370.2016.1143544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–9. 10.1016/j.neulet.2013.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be made available upon reasonable request.