Abstract
Background:
Women living in disadvantaged neighborhoods present with increased prevalence rates of triple negative breast cancer (TNBC). This study takes a spatio-temporal epidemiological approach to understand the impact of socio-environmental contextual factors on TNBC prevalence rates.
Methods:
We analyzed 935 TNBC cases from a major cancer center registry, between 2005 and 2017, to explore spatial and space-time clusters of TNBC prevalence rates at the census tract and neighborhood scales. Spatial regression analysis was performed to examine relationships between nine socio-environmental factors and TNBC prevalence rates at both ecological scales.
Results:
We observed spatial clustering of high TNBC prevalence rates along a north-south corridor of Miami-Dade County along Interstate 95, a region containing several majority non-Hispanic Black neighborhoods. Among the ecological measures, the percent of a region designated as a brownfield was associated with TNBC prevalence rates at the tract- (β = 4.27, SE = 1.08, P < 0.001) and neighborhood-level (β = 8.61, SE = 2.20, P < 0.001).
Conclusions:
Our spatio-temporal analysis identified robust patterns of hot spots of TNBC prevalence rates in a corridor of several disadvantaged neighborhoods in the northern half of the county. These patterns of TNBC align with the literature regarding at-risk groups and neighborhood-level effects on TNBC; however, remain to be validated in a population-based sample.
Impact:
Spatial epidemiological approaches can help public health officials and cancer care providers improve place-specific screening, patient care, and understanding of socio-environmental factors that may shape breast cancer subtype through gene-environment and epigenetic interactions.
Keywords: breast cancer, spatial analysis, neighborhood effects, health disparities, environmental determinants, structural determinants
INTRODUCTION
Triple negative breast cancer (TNBC) is an aggressive subtype that accounts for 10–15% of all breast cancer cases and disproportionate mortality (1,2). Previous epidemiological studies have shown that TNBC incidence and prevalence rates are higher among non-Hispanic Black and Hispanic Black women, thus making this subtype an early source of disparities in breast cancer survival outcomes (3,4). Recent studies have further identified that disadvantaged neighborhoods have higher rates of TNBC compared to ER+/HER2- (1, 5), even independent of Black race and West African genetic ancestry (1,5). These race and ancestry-independent disparities in TNBC may reflect the downstream effects of neighborhood disadvantage “getting under the skin” to impact tumor subtype development.
The ecosocial theory of disease distribution posits that health disparities may arise due to social, ecological, political and/or historical exposures within a neighborhood (6). Studies have hypothesized that health behaviors such as poor diet and limited physical activity may lead to the development of obesity and diabetes mellitus which have been associated with an increased risk of developing TNBC through the dysregulation of cell cycle regulation and cell proliferation signaling pathways (7–10). Synergistically, stress of living in a disadvantaged environment has been thought to shape tumor biology by altering gene expression and impacting inflammatory or immune response systems. Newman et al and Linnenbringer et al (11,12) also highlight that the allostatic load (“wear and tear on the body” or “weathering”) from chronic stress associated with social inequality may impact breast cancer subtype. More recently, a translational epidemiologic study by Goel et al identified that independent of West African ancestry, living in a disadvantaged neighborhood, was associated with higher odds of TNBC compared to less aggressive breast cancer subtypes, suggesting potential gene-environment and/or epigenetic alterations as a source of disparities in breast cancer subtype during tumor development. However, there remains a critical knowledge gap with respect to the potential social and environmental determinants associated with these disparities.
To better understand these contextual, or neighborhood-level, features associated with TNBC, geographic information systems (GIS) have been used to study breast cancer subtypes throughout the US at various spatial scales (13–17). Some studies have explored TNBC at the national level (18–20), while finer-scale TNBC studies have analyzed racial disparities using ecological regression (21) or in the context of all breast cancer subtypes (22). These studies have generally affirmed that neighborhoods of lower socioeconomic status and larger minority populations endure a greater TNBC health burden. Neighborhood-focused studies have analyzed breast cancer risk, breast cancer by subtype, as well as all cancer types, and have also found that neighborhood-level characteristics, especially neighborhood socioeconomic status, play an important role in racial and ethnic disparities and warrant further investigation (5,23–25). Few studies have conducted multi-scalar geospatial clustering analyses of TNBC prevalence rates, which is an important step toward understanding hot spots of TNBC, their underlying social and environmental determinants, and potential epigenetic drivers.
This study builds on the prior TNBC spatial epidemiology literature by assessing the spatial and spatiotemporal patterns of TNBC prevalence rates in Miami-Dade County, and by exploring ecological correlates of higher prevalence rates at different spatial scales. We hypothesize that TNBC prevalence rates will exhibit spatial and spatiotemporal clustering in Miami-Dade County related to social and environmental determinants at the neighborhood level. We discuss additional unmeasured social and environmental determinants that may be linked to TNBC and close with the implications for studying gene-environment interactions and the epigenetics of TNBC in other growing majority-minority cities like Miami.
MATERIALS AND METHODS
Study Site
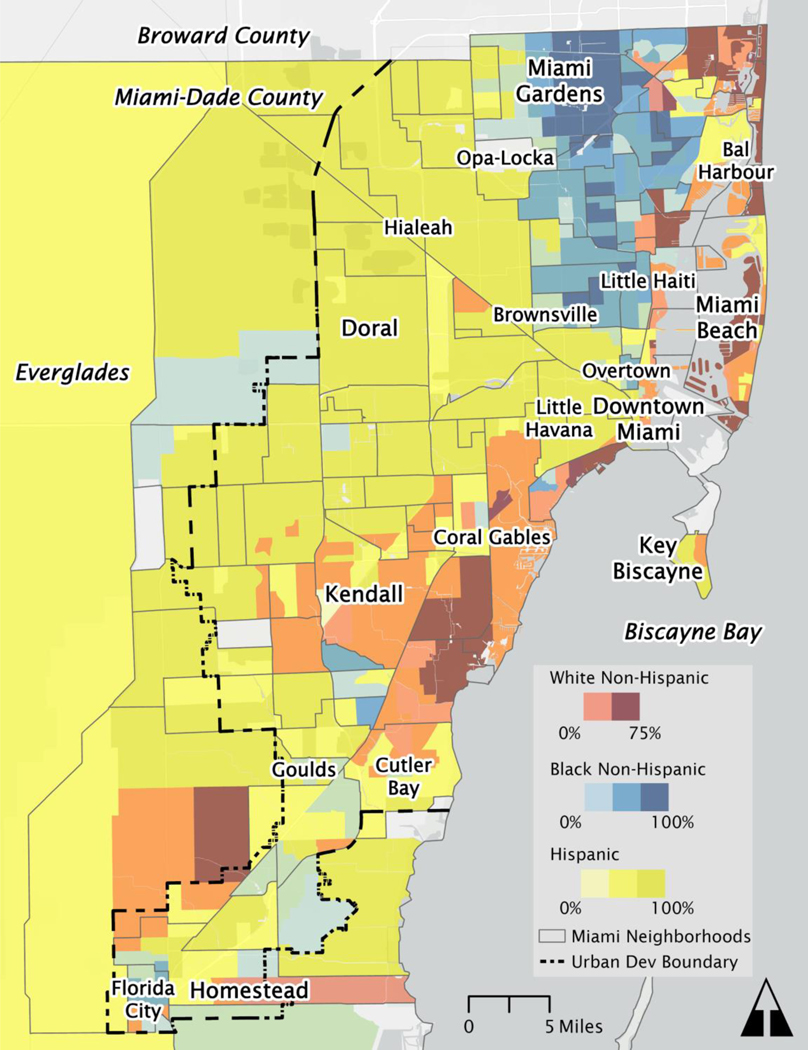
Miami-Dade County is the seventh-largest county in the US with over 2.7 million residents (26) and is known as the “Gateway of the Americas” due to its location, cultural connections to Latin America and the Caribbean, and diverse majority-Hispanic population. As presented in Figure 1, Miami-Dade County’s legacy of racial segregation has produced distinct geographies of social and economic inequality with wealthier non-Hispanic White residents concentrated along the beaches and coastal areas, and an inland patchwork of ethnic enclaves and marginalized communities (27). Miami-Dade County’s majority-minority makeup may well reflect the future composition of many US sun belt cities. Demographic studies have shown that as US cities diversify, they also tend to reinforce patterns of segregation (28,29), making Miami-Dade County an apt model for studying structural and social determinants of cancer.
Figure 1.
Race and ethnicity patterns in Miami-Dade County. Racial and ethnic groups (White Non-Hispanic, Black Non-Hispanic, and Hispanic) are visualized at the census tract level and contextualized with neighborhood boundaries.
Data
We used data from the institutional tumor registry of National Cancer Institute-designated Sylvester Comprehensive Cancer Center and Jackson Memorial Hospital (a partner safety-net hospital that is one of the largest public hospitals in the US, with similar standards of care) and identified cases of stage I-IV breast cancer diagnosed between 2005 and 2017. The study was approved by the Institutional Review Board at the University of Miami and Jackson Health System. The catchment area for Sylvester Comprehensive Cancer Center and Jackson Memorial Hospital is all of South Florida (about a third of Florida’s population), yet these hospitals tend to capture distinctly different populations by socioeconomic status, insurance coverage, and geography within Miami-Dade County. Specifically, the safety-net hospital ensures that all residents of Miami-Dade County can receive high-quality medical care regardless of their ability to pay. This study was therefore limited to Miami-Dade County to reduce bias secondary to catchment area.
We began by geocoding 6,415 breast cancer patient records using ArcGIS Pro 2.7 and Esri’s World Geocoding Service (Esri, Redlands, CA). We excluded 207 cases due to incomplete address information, and 1,498 cases for patients who resided outside of Miami-Dade County. Out of the remaining 4,440 cases, we analyzed 935 with a tumor subtype of TNBC and home address in Miami-Dade County at the time of hospital encounter. TNBC was determined based on pathology review showing the following tumor markers: estrogen receptor negative, progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2/neu) negative.
To protect patient privacy, these records were aggregated to two scales for analysis, US census tracts and Miami-Dade County neighborhoods. US Census tracts are commonly used in epidemiological studies because they approximate neighborhoods and are the smallest census unit at which demographic variables are considered statistically reliable (30). The Miami-Dade County neighborhood layer was created by aggregating US Census block groups into vernacular, socially recognized neighborhoods using local expert knowledge of Miami-Dade County and emphasizing natural landscape barriers (transportation corridors, land use changes, etc.), thus mimicking previous efforts to create appropriate neighborhoods for urban health research (31,32). Neighborhoods are more appropriate for communicating results to health officials and policy makers both for ease of interpretation and because they tend to match the scale of community-based interventions. We calculated the TNBC prevalence rate for the entire 15-year study period for each tract and neighborhood per 10,000 females 18 years and older using data from the US Census Bureau’s American Community Survey 2015–2019 5-year estimates.
We compiled a set of nine socio-environmental independent variables as potential proxies of structural determinants of TNBC. These variables included park acreage (the total area in acres of parkland in each region); number of correctional facilities; brownfields proportion (the percent area of each region designated by the Florida Department of Environmental Protection as a brownfield); education (proportion age 25+ with a bachelor’s degree); percent foreign-born (females > 18 years old born outside the US); a diversity index; number of hazardous waste facilities; the CDC/ATSDR 2018 Social Vulnerability Index (SVI), which is a composite of 16 US Census variables (33); and the US Census Bureau’s 2019 Community Resilience Estimate (CRE), which is a composite measure of ten demographic and economic risk factors related to resilience to local disasters (34). SVI and the diversity index were computed as the mean of each census tract and neighborhood. The CRE measure was the percent of the population facing 3 or more risk factors.
The rationale for choosing these variables is their downstream effects on comorbidities (e.g., obesity and diabetes mellitus) and stress (“weathering”) which have been hypothesized to be associated with TNBC (1,6,8–12). Studies have shown that limited green spaces and the presence of correctional facilities, both of which limit access to safe walking areas, were associated with increased obesity rates (7,35–38), and obesity has been associated with TNBC. Education, percent foreign-born, and the diversity index were included to control for contextual-level sociodemographic characteristics. The social vulnerability index and CRE were included as proxies for social stress from social inequality because studies have shown that the allostatic load (“wear and tear on the body” or “weathering”) from stress associated with social inequality may affect breast cancer subtype through epigenetic alterations (1,7). In addition, brownfields and hazardous waste facilities may lead to epigenetic alterations secondary to toxic chemicals or other factors associated with environmental injustices experienced by disinvested neighborhoods which are disproportionately located near brownfields (39–41).
The socio-environmental variables were gathered from multiple data sources, such as the Miami-Dade County Open Data Hub, Florida Geographic Data Library (FDGL), and US Census Bureau’s American Community Survey (ACS), and aggregated to the census tract and Miami-Dade County neighborhood scale.
Spatial Analysis
We began by computing the global Moran’s I statistic to test for the presence of statistically significant spatial patterning in prevalence rates of TNBC across Miami-Dade County. We then computed the local indicators of spatial association (LISA) statistic, a commonly used spatial clustering statistic which compared the TNBC prevalence rate in each geographic unit to those of neighboring units to assess the significance of spatial clusters of high and low prevalence rates, and potential spatial outliers (42).
We iteratively tested a variety of spatial weights matrices, which defined spatial neighbors in the spatial cluster analysis, at both the tract and neighborhood scales to ensure the robustness of our results, and we applied row standardization and a false discovery rate (FDR) correction to all models. Miami’s costal geography required that we characterize spatial relationships by distance rather than contiguity to avoid spatial “islands,” or units with no neighbors. We tested three different distance conceptualizations: inverse distance, inverse distance squared, and fixed distance, all using Euclidean distance. We explored these three conceptualizations using a minimum threshold distance (the minimum distance that ensures that every feature has at least one neighbor), and then again using alternative thresholds from 1–10 km. Ultimately, an inverse distance spatial weights matrix with a distance threshold of 5 km best characterized the spatial trends in prevalence rates of TNBC at the tract level, while inverse distance using the minimum threshold distance (21.4 km, reflecting the larger size of these units) was the best fit at the neighborhood level.
Spatiotemporal Analysis
In addition to exploring spatial trends, we also assessed spatiotemporal patterns of TNBC prevalence rates. This analysis was a two-step process. First, we defined a 3D space-time cube that used a case’s location coordinates as the x and y axes, and the date of diagnosis as the z axis. We created multiple space-time cubes at the tract and neighborhood scales, and with temporal bins of 3, 6, and 12 months that began January 1, 2005. We then used the Emerging Hot Spot Analysis (EHSA) tool to assess spatiotemporal clustering at the tract and neighborhood level. We computed multiple models using different conceptualizations of space and time relationships to once again ensure robustness across results, especially given the low numbers of cases distributed across each space-time bin when using shorter temporal parameters (i.e., months vs. years). The distance conceptualizations we ultimately selected were queen’s case contiguity for the tract level and fixed distance using default distance for neighborhoods. For time conceptualization, we tried multiple neighborhood time step variations which dictate the number of neighboring time intervals used to compare each space-time bin. Ultimately, we used a 12-month space-time cube with 3-time steps for the tract analysis, and a 6-month space-time cube with three time steps for the neighborhood analysis. For both spatial scales, we selected the individual time step as the comparison window.
Regression Modeling
To further our study of TNBC prevalence in Miami-Dade County, we performed a statistical analysis to compare TNBC prevalence rates to social and built environment factors based on previous calls in the literature (43). We began with the nine independent measures representing social and environmental risk factors in the environmental health literature: public park acreage, correctional facilities, brownfields, education, percent foreign-born, diversity index, hazardous waste facilities, SVI, and CRE. Each of these variables was summarized to both the tract and neighborhood level for comparison with the TNBC prevalence rate at each scale.
We began by using IBM SPSS Statistics v27 to compute bivariate Pearson’s correlations to explore associations between our independent variables that would suggest potential multicollinearity in a regression model. We used the results of this analysis to determine which variables to include in regression analysis and computed preliminary ordinary least squares (OLS) multivariable regression models to check for variance inflation factors > 3 that might suggest collinearity. Next, we computed OLS models at each scale (tract and neighborhood) with spatial model diagnostics using GeoDa v1.2 to assess the extent of spatial dependence in our data and potential need for fitting a spatial regression model.
We created spatial weights matrices for each scale based on the same principles used in the spatial and spatiotemporal analyses. We used an inverse distance spatial weights matrix with a 10 km distance band for the tract level analysis, and the neighborhood level analysis used an inverse distance spatial weights matrix with a 21 km distance band. If the OLS regression model demonstrated spatial dependence in the residuals, the Lagrange Multiplier test guided our decision of whether a spatial lag or spatial error model would be a better fit for our data.
Robustness Test for Spatial Representation
To further evaluate if our tumor-registry data is representative of the population-registry data in Florida we compared the distributions of breast cancer cases from our hospital system’s tumor registry to those from the population-based Florida Cancer Data System (FCDS) registry during the study period. To do this, we used the full sampling frame of 4,434 georeferenced breast cancer cases in MDC from which TNBC cases were originally derived for this study. These 4,434 cases included all subtypes, not just TNBC, from 2005–2017 with valid address data. Our comparison data was a FCDS file of all breast cancer cases during the same period. There were 27,729 breast cancer cases, of which 27,595 had a valid census tract and were geocoded as census tract centroids. To compare the two distributions, we first computed the proportion of each set of cases for 534 census tracts in MDC. Our null hypothesis was that the tract-level proportions of breast cancer cases from our study’s sampling frame would not be associated with the tract-level proportions of FCDS cases from the same period. The alternative hypothesis was that these distributions would be spatially related.
We also visualized the relationship between the two case distributions using the Local Bivariate Relationships tool in ArcGIS Pro, which uses an entropy statistic to quantify the amount of shared information between the two distributions over geographic space.
Data Availability
Data for this study were generated at Sylvester Comprehensive Cancer Center and Jackson Memorial Hospital. Derived data supporting the findings of this study are available from the corresponding author upon request.
RESULTS
Socio-environmental Determinants
The 935 TNBC cases with a home address in Miami-Dade County were aggregated to 518 census tracts and 96 neighborhoods. We computed descriptive statistics for each socio-environmental measure at both scales (Table 1). The mean proportion of the population age 25+ with a bachelor’s degree was 0.18 (SD = 0.09) for both tracts and neighborhoods. The mean SVI value was 0.64 (SD = 0.25) for tracts and 0.63 (SD = 0.21) for neighborhoods. The mean percentage of foreign-born women was 0.62 (SD = 0.19) for tracts and 0.51 (SD = 0.19) for neighborhoods. The mean park acreage was 26.56 (SD = 112.53) acres per tract and 143.53 (SD = 273.11) acres per neighborhood. The mean number of correctional facilities was 0.04 (SD = 0.31) in each tract and 0.22 (SD = 0.70) in each neighborhood. The mean proportion of area designated as a brownfield was 0.21 (SD = 0.37) for tracts and 0.21 (SD = 0.32) for neighborhoods. The mean diversity index was 48.44 (SD = 19.48) for tracts and 53.00 (SD = 16.36) for neighborhoods. The mean number of hazardous waste facilities was 0.12 (SD = 0.77) in each tract and 0.67 (SD = 2.08) in each neighborhood. The CRE’s proportion of population facing 3+ risk factors was 0.28 (SD = 0.11) for tracts and 0.26 (SD= 0.08) for neighborhoods. The TNBC prevalence rate was 0.08 (SD = 0.08) per 10,000 females 18+ for tracts and 0.08 (SD = 0.06) for neighborhoods.
Table 1.
Descriptive statistics of candidate socio-environmental measures at the census tract and neighborhood scales.
| Census Tract | Neighborhood | |||||
|---|---|---|---|---|---|---|
| Measure | n | Mean | SD | n | Mean | SD |
| Proportion 25+ with bachelor’s degree | 454 | 0.18 | 0.09 | 89 | 0.18 | 0.09 |
| Social Vulnerability Index | 509 | 0.64 | 0.25 | 93 | 0.63 | 0.21 |
| Percent foreign born (females > 18 years) | 509 | 0.62 | 0.19 | 95 | 0.51 | 0.19 |
| Park acreage | 518 | 26.56 | 112.53 | 96 | 143.53 | 273.11 |
| Correctional facilities (count) | 518 | 0.04 | 0.31 | 96 | 0.22 | 0.70 |
| Proportion brownfield area | 518 | 0.21 | 0.37 | 96 | 0.21 | 0.32 |
| Diversity index | 518 | 48.44 | 19.48 | 96 | 53.00 | 16.36 |
| Hazardous waste facilities (count) | 518 | 0.12 | 0.77 | 96 | 0.67 | 2.08 |
| CRE high risk (3+ risk factors) | 518 | 0.28 | 0.11 | 96 | 0.26 | 0.08 |
| TNBC cases per 10,000 females 18+ | 510 | 0.08 | 0.08 | 94 | 0.08 | 0.06 |
Spatial Analysis
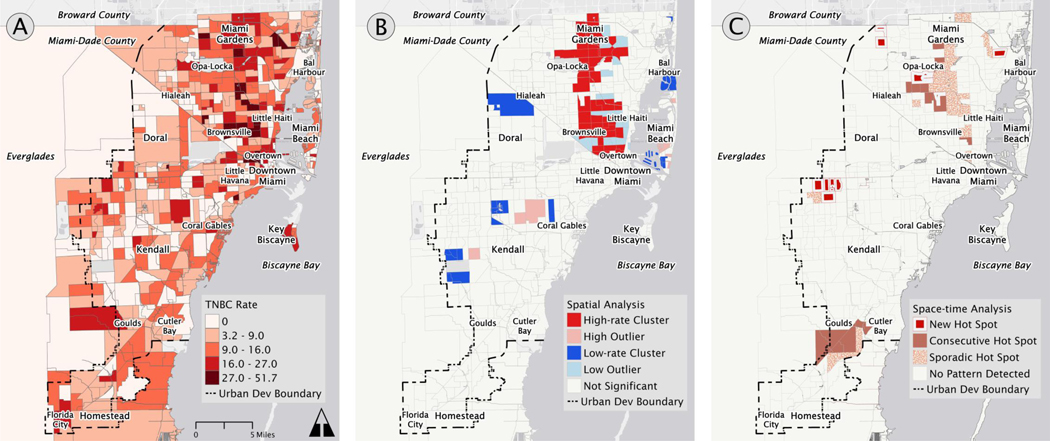
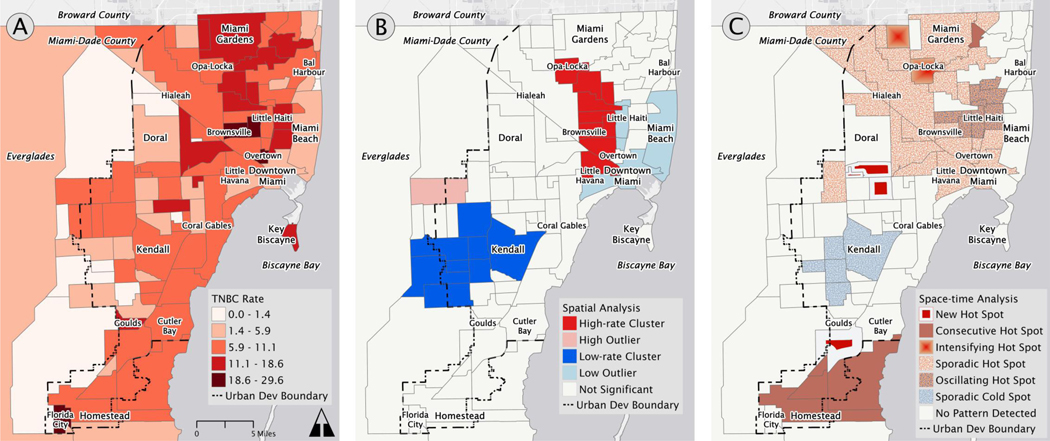
The overall spatial pattern of TNBC prevalence rates revealed more cases and higher prevalence rates in the northern half of Miami-Dade County, particularly north of Little Havana (Figures 2A and 3A). The northern half of the county is the primary service area of our hospital system, with many breast cancer patients having additional provider options in Coral Gables, Kendall, and other neighborhoods in the central and southern parts of Miami’s urban area. The LISA statistic detected statistically significant hot spots of TNBC prevalence rates throughout the northern half of the county, with occasional outliers of low prevalence rates (Figures 2B and 3B). These hot spot patterns persisted even when we constrained the models to be more conservative, consistently appearing in a vertical corridor that is constrained to the west by the industrial corridor between Hialeah and Brownsville, and to the east by Interstate 95. This region includes majority non-Hispanic Black neighborhoods such as Brownsville, Liberty City, Opa Locka, and Miami Gardens.
Figure 2.
Spatial analysis of triple negative breast cancer cases aggregated by census tract. The panels highlight: (A) the distribution of 935 triple negative breast cancer diagnosed between 2005 and 2017 in Miami-Dade County, (B) hot and cold spots identified by spatial cluster analysis, and (C) different forms of space-time clusters identified by spatiotemporal cluster analysis.
Figure 3.
Spatial analysis of triple negative breast cancer cases aggregated by neighborhood. The panels highlight: (A) the distribution of 935 triple negative breast cancer diagnosed between 2005 and 2017 in Miami-Dade County, (B) hot and cold spots identified by spatial cluster analysis, and (C) different forms of space-time clusters identified by spatiotemporal cluster analysis.
The analysis detected the presence of cold spots, i.e., clusters of low TNBC prevalence rates, in the southern half of Miami-Dade County, particularly at the neighborhood scale, with a few nearby high-rate outliers. The results in the southern half of county should not be over-interpreted given how patient volumes at this hospital system are less representative of neighborhood cancer prevalence in the southern half of the county. Cold spots were also identified at the tract scale in higher-income areas of Aventura, Bal Harbor, and Medley in the north, Miami Beach’s Venetian, Star, Palm, and Hibiscus Islands, and Kendall and Country Walk in the south.
Spatiotemporal Analysis
Our space-time analysis revealed three trends, as presented in Figure 2C and 3C: (1) hot spots in the south near Florida City, Goulds, Princeton; (2) hot spots in the northern corridor that correspond with the hot spots from the spatial analysis; and (3) emerging hot spots in the western central region of Miami-Dade County.
The hot spots in the northern corridor were primarily sporadic hot spots which means that they appeared on and off as hot spots over time and never constituted statistically significant cold spots. This suggests that while they are spatially significant, there is no consistent temporal pattern. Taken together, the results of the spatial and spatiotemporal analyses in Figures 2 and 3 demonstrate robust hot spots of TNBC in a corridor of low-income non-Hispanic Black neighborhoods in northeast Miami-Dade County stretching from Opa-Locka to Overtown, and spilling into adjacent low-income Hispanic neighborhoods.
Regression
Our bivariate correlation analysis led us to remove the education and SVI variables from our models due to a moderately strong association between them (r = −0.58, P < 0.001) as well as an association between education and CRE (r = −0.55, P < 0.001) at the census tract scale. We also removed the foreign-born variable due to a strong association with the diversity index (r = −.59, P < 0.001) at the census tract scale. Preliminary OLS models that included all candidate independent measures also revealed high VIFs for education and SVI, thus supporting their exclusion from multivariable modeling due to collinearity concerns. We used the remaining six measures in our regression models: total park area, count of correctional facilities, proportion brownfield area, count of hazardous waste facilities, the diversity index, and highest-risk CRE proportions.
The OLS models at both tract and neighborhood scales revealed non-normal residual plots, affirming the need for a spatially weighted model, and the Lagrange Multiplier tests confirmed that a spatial lag model with maximum likelihood estimation would be a better fit. Spatial lag models revealed that the only significant correlate of TNBC was brownfields at both the tract level (β = 4.27, Z = 3.97, P < 0.001) and neighborhood level (β = 8.61, Z = 3.92, P < 0.001). To ensure robustness of this result, we recoded the brownfield variable using three different operationalizations of brownfield presence and intensity to test the measure’s sensitivity. Each subsequent regression model using these alternative brownfield measures produced nearly the same results. The overall fit for these models was weak (AIC = 3616.18, adjusted R2 = 0.10 for tracts, and AIC = 581.34, adjusted R2 = 0.28 for neighborhoods), indicating that this combination of socio-environmental measures alone captured little of the variation in TNBC prevalence rates (Table 2).
Table 2.
Spatial lag regression model of the relationship between select socio-environmental measures and TNBC prevalence rates at the census tract and neighborhood scales.
| Census Tract (n = 514) | Neighborhood (n = 93) | |||
|---|---|---|---|---|
| Measure | β | SE | β | SE |
| Constant | 1.27 | 1.70 | 4.85 | 4.14 |
| Spatial lag of TNBC rate | 0.52* | 0.14 | 0.16 | 0.38 |
| Park acreage | 0.00 | 0.00 | 0.00 | 0.00 |
| Correctional facilities | −1.14 | 1.47 | 0.07 | 1.02 |
| Brownfield area | 4.27* | 1.08 | 8.61* | 2.20 |
| Diversity index | 0.01 | 0.02 | −0.01 | 0.03 |
| Hazardous waste facilities | −0.75 | 0.46 | −0.33 | 0.28 |
| CRE high risk (3+ risk factors) | 0.05 | 0.04 | 0.05 | 0.08 |
| Model diagnostics | AIC= 3616.18, R2 = 0.10 | AIC= 581.337, R2 = 0.28 | ||
P < .001
Robustness Tests
Finally, the Florida Cancer Data System (FCDS) and study hospital case distributions of breast cancer were correlated at the census tract level (r = 0.55, P = 0.001) (Figure S1). The local bivariate analysis demonstrated that 61% of census tracts in Miami-Dade County exhibited either a positive linear relationship (288 of 534, i.e., 54%) or a concave, i.e., non-linear positive relationship (37 of 534, 7%) (Figure S2). There was also general agreement in the distributions in areas that were identified as TNBC hot spots in the northern half of the county (Figure S2). The tracts with non-significant local relationships between the two distributions were concentrated in four regions of varying socioeconomic levels: low-income areas near Hialeah and Homestead, middle-class inland areas around Kendale Lakes and Tamiami, and a high-income coastal strip extending north from Miami Beach. These local patterns suggest local determinants of breast cancer origin, care, and decision-making—likely associated with a second cancer center’s location in the southern half of MDC—despite the global correlation of cases between the distributions. The county-wide pattern supports the likelihood that the observed spatiotemporal patterns were not geographical artefacts of the population served by the study hospitals, though these findings should still be validated in a population-based cohort.
DISCUSSION
This study aimed to improve our understanding of the socio-environmental contextual effects on TNBC prevalence rates in Miami-Dade County. Our spatial analysis identified robust patterns of hot spots of TNBC prevalence rates in a corridor of several disadvantaged neighborhoods in the northern half of the county. We also identified cold spots of low TNBC prevalence rates in the southern half of the county, reflecting greater distances from the hospital system, as well as in higher-income neighborhoods east of Interstate 95. The spatiotemporal analysis revealed consistent spatial trends, but only modest temporal trends. Our regression analysis found that among nine ecological measures commonly used as proxies of neighborhood advantage, higher percent area designated as brownfields was significantly associated with TNBC prevalence rates, which we interpret as reflecting relative degrees of environmental injustice experienced by communities. The regression models did not demonstrate strong model fit, indicating that this subset of ecological variables did not explain much variation in TNBC prevalence rates in the absence of individual-level factors.
The results of our spatial and spatiotemporal analysis were consistent with literature demonstrating that non-Hispanic Black women face higher TNBC prevalence rates than other groups (1–5, 29, 31). The hot spot corridor identified in our analysis is composed of majority-Black neighborhoods such as Brownsville, Liberty City, Opa-Locka, and Miami Gardens, communities which also feature extreme racial segregation (23,45). In contrast, cold spots of low TNBC prevalence rates were detected in majority-White neighborhoods such as Kendall, Country Walk, Miami Beach, and Aventura. Using iterative and multi-scalar spatial modeling, we demonstrated that racial disparities among TNBC patients existed at the census tract and neighborhood scales, as suggested by previous studies (24,25,43,46). The positive association between TNBC prevalence rates and brownfields may be novel, as we could not find other studies discussing brownfields in the context of breast cancer. We emphasize that this finding should not be interpreted as a causal link between brownfield-related environmental pollution and TNBC. Rather, brownfield presence likely serves as an indicator of broader contextual-level and environmental injustice, such as proximity to industrial land uses and discriminatory housing policies, that have shaped Miami’s socio-demographic and environmental landscapes.
This study serves as a first look at contextual effects of TNBC in Miami and a gateway to future studies of potential neighborhood-level epigenetic drivers. This work explicitly responds to calls for expanding cancer applications of geospatial analysis and neighborhood effects (43). We assessed nine social and environmental variables that are commonly used to study neighborhood effects on individual health, but these ecological models explained modest variation in TNBC prevalence rates. Future studies should expand upon this approach by broadening the set of contextual variables to capture neighborhood histories, such as a region’s redlining legacy, and socioeconomic trajectories, such as development or gentrification metrics over time. More importantly, these studies should also model individual-level effects such as demographic information, stage at diagnosis, tumor type, and residential history to understand how individual- and neighborhood-level effects interact to produce different cancer outcomes, and to assess impacts on screening, care, and treatment.
Although our study design and ecological measures do not permit causal inference between brownfields and TNBC, this relationship may warrant further study given previous studies that analyzed links between brownfield-related contaminants and human health. A Baltimore-based study conducted in 2002 analyzed the historical land use and hazardous substances detected at brownfields and found that nearby communities suffer excess mortality rates of multiple diseases, including cancer (47). Other studies have characterized the adverse human health impacts of contaminants found in brownfields in Canada (48), England (49), and China (50). Studies linking brownfields to human health are complicated by human mobility and the diversity of chemical exposures, but these hazards remain a compelling health threat for trapped-in-place populations enduring environmental injustice. Given these methodological challenges, the epigenetic effects of such environmental threats remain unclear.
The primary strength of our study is the multi-scalar use of tracts and neighborhoods in our analyses. The two scales of aggregation demonstrated robustness of results and reduced potential statistical bias from the modifiable aerial unit problem (51). Whereas census tracts are a common scale for health analysis, neighborhoods provide a scale that is easier for policy makers to understand and relate to. We also tested multiple sets of spatial and temporal parameters to ensure that our results were consistent across different space-time conceptualizations.
A limitation of this study is that it includes a catchment area based, case-only, two-institution population in South Florida, rather than a population-based sample, which may not be generalizable to other health systems caring for similar populations. Nevertheless, these two institutions are the only NCI designated cancer center and safety-net hospital in South Florida which capture much of the population and reflect diverse racial/ethnic and socioeconomic characteristics. In addition, the comparison of our study data to state cancer registry data suggested that our findings are probably minimally biased by the population distribution served by our hospitals; however, remain to be validated in a population-based cohort. This study population is also among the most diverse in the nation in terms of race/ethnicity, ancestry, and cultural identity with nearly half of South Florida residents born in Latin America or the Caribbean. The granularity of data analyzed in this study cannot be easily captured in national cancer databases. However, we were unable to capture BRCA 1 mutation data, which is a known to be associated with TNBC (7). The spatiotemporal cluster analysis was also limited by small numbers of cases in some areas and time intervals, which led to modest variation in hot and cold spots across models. Though the locations of patterns remained consistent across models, it is important to remember that the edges of hot and cold spots can be fluid given the underlying case distribution and quirks of boundary effects, particularly given Miami’s coastal geography. Given unknown TNBC caseloads treated at other cancer centers, the largest of which are located in the southern half of MDC, we caution against over-interpretation of TNBC cold spots in southern MDC.
This study demonstrated an approach to multi-scalar spatial and spatiotemporal analysis of cancer cases in Miami-Dade County. We identified consistent spatial patterns of TNBC prevalence rates that aligned with previous literature regarding at-risk groups and neighborhood-level effects on cancer outcomes. Cancer disparities will persist until clinical approaches fully account for social and structural determinants of health that produce yet-unknown epigenetic effects on cancer pathogenesis. As these pathways are still being understood, spatial modeling can help identify priority populations for screening and treatment and guide health communication efforts in neighborhoods where disparities in care are related to a legacy of social factors that include medical racism and physician mistrust. Such approaches are paramount in majority-minority cities where health equity means not only navigating disparities in income and education, but also housing, the built environment, and other neighborhood amenities.
Supplementary Material
ACKNOWLEDGEMENTS
Funding:
Dr. Goel is funded by National Institute of Health Grant #K12CA226330, the American Surgical Association Foundation Fellowship Research Award, and the American Society of Clinical Oncology Career Development Award, a CCSG Transdisciplinary Award, and a V Foundation Award.
Footnotes
Design: Retrospective cohort study utilizing local tumor registry data from 2005 to 2017.
Conflict of interest statement: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Goel N, Yadegarynia S, Kwon D, Kesmodel SB, Harbour JW, Kobetz E, et al. Translational Epidemiology: An Integrative Approach to Determine the Interplay Between Genetic Ancestry and Neighborhood Socioeconomic Status on Triple Negative Breast Cancer. Ann Surg. 2022;276:430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst. 2015;107:djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goel N, Yadegarynia S, Lubarsky M, Choi S, Kelly K, Balise R, et al. Racial and Ethnic Disparities in Breast Cancer Survival: Emergence of a Clinically Distinct Hispanic Black Population. Ann Surg. 2021;274:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinheiro PS, Callahan KE, Ragin C, Hage RW, Hylton T, Kobetz EN. Black heterogeneity in cancer mortality: US-Blacks, Haitians, and Jamaicans. Cancer Control. 2016;23:347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linnenbringer E, Geronimus AT, Davis KL, Bound J, Ellis L, Gomez SL. Associations between breast cancer subtype and neighborhood socioeconomic and racial composition among Black and White women. Breast Cancer Res Treat. 2020;180:437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger N. Methods for the scientific study of discrimination and health: An ecosocial approach. Am J Public Health. 2012;102:936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vargas-Hernández VM, Vargas-Aguilar V, Moreno-Eutimio MA, Acosta-Altamirano G, Tovar-Rodriguez J. Metabolic syndrome in breast cancer. Gland Surg. 2013;2:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis AA, Kaklamani VG. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer. 2012;2012:809291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010;121:479–83. [DOI] [PubMed] [Google Scholar]
- 11.Newman LA, Kaljee LM. Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA Surg. 2017;152:485–93. [DOI] [PubMed] [Google Scholar]
- 12.Linnenbringer E, Gehlert S, Geronimus AT. Black-White disparities in breast cancer subtype: The intersection of socially patterned stress and genetic expression. AIMS Public Health. 2017;4:526–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bambhroliya AB, Burau KD, Sexton K. Spatial analysis of county-jevel breast cancer mortality in Texas. J Environ Public Health. 2012;2012:e959343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buck KD. Modelling of geographic cancer risk factor disparities in US counties. Appl Geogr. 2016;75:28–35. [Google Scholar]
- 15.Hu L, Chun Y, Griffith DA. Uncovering a positive and negative spatial autocorrelation mixture pattern: a spatial analysis of breast cancer incidences in Broward County, Florida, 2000–2010. J Geogr Syst. 2020;22:291–308. [Google Scholar]
- 16.Salmeron B, Mamudu L, Liu X, Whiteside M, Williams F. Assessing health disparities in breast cancer incidence burden in Tennessee: geospatial analysis. BMC Womens Health. 2021;21:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott L, Mobley LR, Il’yasova D. Geospatial Analysis of Inflammatory Breast Cancer and Associated Community Characteristics in the United States. Int J Environ Res Public Health. 2017;14:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss JL, Tatalovich Z, Zhu L, Morgan C, Cronin KA. Triple-negative breast cancer incidence in the United States: ecological correlations with area-level sociodemographics, healthcare, and health behaviors. Breast Cancer Tokyo Jpn. 2021;28:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott LC, Mobley LR, Kuo T-M, Il’yasova D. Update on triple-negative breast cancer disparities for the United States: A population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer. 2019;125:3412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger N, Jahn JL, Waterman PD. Jim Crow and estrogen-receptor-negative breast cancer: US-born black and white non-Hispanic women, 1992–2012. Cancer Causes Control. 2017;28:49–59. [DOI] [PubMed] [Google Scholar]
- 21.Hossain F, Danos D, Prakash O, Gilliland A, Ferguson TF, Simonsen N, et al. Neighborhood Social Determinants of Triple Negative Breast Cancer. Front Public Health [Internet]. Frontiers; 2019. [cited 2021 Aug 4];0. Available from: https://www.frontiersin.org/articles/10.3389/fpubh.2019.00018/full?report=reader [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan A, Lichtensztajn DY, Oh D, Jain J, Tao L, Hiatt RA, et al. Breast Cancer in San Francisco: Disentangling Disparities at the Neighborhood Level. Cancer Epidemiol Biomark Amp Prev. 2019;cebp.0799.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel N, Westrick AC, Bailey ZD, Hernandez A, Balise RR, Goldfinger E, et al. Structural racism and breast cancer-specific survival: Impact of economic and racial residential segregation. Ann Surg [Internet]. 2022. [cited 2022 Mar 8]; Available from: https://journals.lww.com/annalsofsurgery/Abstract/9000/Structural_Racism_and_Breast_Cancer_Specific.93090.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conroy SM, Shariff-Marco S, Koo J, Yang J, Keegan THM, Sangaramoorthy M, et al. Racial/Ethnic differences in the impact of neighborhood social and built environment on breast cancer risk: The Neighborhoods and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2017;26:541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol Off J Am Soc Clin Oncol. 2017/10/16 ed. 2018;36:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Census Bureau. Annual estimates of the resident population: April 1, 2010 to July 1, 2019 [Internet]. Population Division; 2020. Available from: https://www.census.gov/data/tables/time-series/demo/popest/2010s-counties-total.html [Google Scholar]
- 27.Kohn-Wood LP, Samson F, Braddock J. Race, Social Identity, and Generative Spaces: Miami as a Microcosm of Categorical Complexity in a 21st-Century Global City. Am Behav Sci. 2015;59:386–405. [Google Scholar]
- 28.Ellis M, Wright R, Holloway S, Fiorio L. Remaking white residential segregation: metropolitan diversity and neighborhood change in the United States. Urban Geogr. 2018;39:519–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright R, Ellis M, Holloway SR, Wong S. Patterns of racial diversity and segregation in the United States: 1990–2010. Prof Geogr. 2014;66:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spielman SE, Folch D, Nagle N. Patterns and causes of uncertainty in the American Community Survey. Appl Geogr. 2014;46:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engstrom R, Ofiesh C, Rain D, Jewell H, Weeks JR. Defining Neighborhood Boundaries for Urban Health Research: A Case Study of Accra, Ghana. In: Weeks JR, Hill AG, Stoler J, editors. Spat Inequalities Health Poverty Place Accra Ghana [Internet]. Dordrecht: Springer Netherlands; 2013. [cited 2022 Apr 7]. page 27–38. Available from: 10.1007/978-94-007-6732-4_2 [DOI] [Google Scholar]
- 32.Weiss L, Ompad D, Galea S, Vlahov D. Defining Neighborhood Boundaries for Urban Health Research. Am J Prev Med. 2007;32:S154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC/ATSDR. CDC/ATSDR Social Vulnerability Index [Internet]. CDC; 2022. [cited 2022 Nov 1]. Available from: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html [Google Scholar]
- 34.US Census Bureau. About Community Resilience Estimates [Internet]. US Census Bureau; 2021. [cited 2021 Nov 1]. Available from: https://www.census.gov/programs-surveys/community-resilience-estimates/about.html [Google Scholar]
- 35.Iyer HS, James P, Valeri L, Hart JE, Pernar CH, Mucci LA, et al. The association between neighborhood greenness and incidence of lethal prostate cancer: A prospective cohort study. Environ Epidemiol. 2020;4:e091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding D, Sallis JF, Kerr J, Lee S, Rosenberg DE. Neighborhood environment and physical activity among youth a review. Am J Prev Med. 2011;41:442–55. [DOI] [PubMed] [Google Scholar]
- 37.Feng J, Glass TA, Curriero FC, Stewart WF, Schwartz BS. The built environment and obesity: a systematic review of the epidemiologic evidence. Health Place. 2010;16:175–90. [DOI] [PubMed] [Google Scholar]
- 38.Frank LD, Schmid TL, Sallis JF, Chapman J, Saelens BE. Linking objectively measured physical activity with objectively measured urban form: findings from SMARTRAQ. Am J Prev Med. 2005;28:117–25. [DOI] [PubMed] [Google Scholar]
- 39.Lodge EK, Engel LS, Ferrando-Martínez S, Wildman D, Uddin M, Galea S, et al. The association between residential proximity to brownfield sites and high-traffic areas and measures of immunity. J Expo Sci Environ Epidemiol. 2020;30:824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi H, Ossip DJ, Mayo NL, Lopez DA, Block RC, Post WS, et al. Role of DNA methylation on the association between physical activity and cardiovascular diseases: results from the longitudinal multi-ethnic study of atherosclerosis (MESA) cohort. BMC Genomics. 2021;22:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chi GC, Liu Y, MacDonald JW, Barr RG, Donohue KM, Hensley MD, et al. Long-term outdoor air pollution and DNA methylation in circulating monocytes: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Glob Access Sci Source. 2016;15:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anselin L. Local Indicators of Spatial Association—LISA. Geogr Anal. 1995;27:93–115. [Google Scholar]
- 43.Gomez SL, Shariff-Marco S, DeRouen M, Keegan THM, Yen IH, Mujahid M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer. 2015;121:2314–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaels EK, Canchola AJ, Beyer KMM, Zhou Y, Shariff-Marco S, Gomez SL. Home mortgage discrimination and incidence of triple-negative and Luminal A breast cancer among non-Hispanic Black and non-Hispanic White females in California, 2006–2015. Cancer Causes Control; [Internet]. 2022. [cited 2022 Mar 8]; Available from: 10.1007/s10552-022-01557-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohl RA. Whitening Miami: Race, Housing, and Government Policy in Twentieth-Century Dade County. Fla Hist Q. Florida Historical Society; 2001;79:319–45. [Google Scholar]
- 46.Echeverría SE, Borrell LN, Brown D, Rhoads G. A Local Area Analysis of Racial, Ethnic, and Neighborhood Disparities in Breast Cancer Staging. Cancer Epidemiol Biomarkers Prev. 2009;18:3024–9. [DOI] [PubMed] [Google Scholar]
- 47.Litt JS, Burke TA. Uncovering the historic environmental hazards of urban brownfields. J Urban Health. 2002;79:464–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siciliano SD, Laird BD, Lemieux CL. Polycyclic aromatic hydrocarbons are enriched but bioaccessibility reduced in brownfield soils adhered to human hands. Chemosphere. 2010;80:1101–8. [DOI] [PubMed] [Google Scholar]
- 49.Bambra C, Robertson S, Kasim A, Smith J, Cairns-Nagi JM, Copeland A, et al. Healthy Land? An examination of the area-level association between brownfield land and morbidity and mortality in England. Environ Plan Econ Space. 2014;46:433–54. [Google Scholar]
- 50.Chen X, Liu M, Ma J, Liu X, Liu D, Chen Y, et al. Health risk assessment of soil heavy metals in housing units built on brownfields in a city in China. J Soils Sediments. 2017;17:1741–50. [Google Scholar]
- 51.Fotheringham AS, Wong DWS. The Modifiable Areal Unit Problem in multivariate statistical analysis. Environ Plan Econ Space. 1991;23:1025–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study were generated at Sylvester Comprehensive Cancer Center and Jackson Memorial Hospital. Derived data supporting the findings of this study are available from the corresponding author upon request.