Abstract
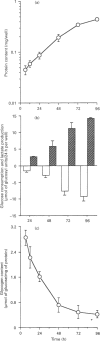
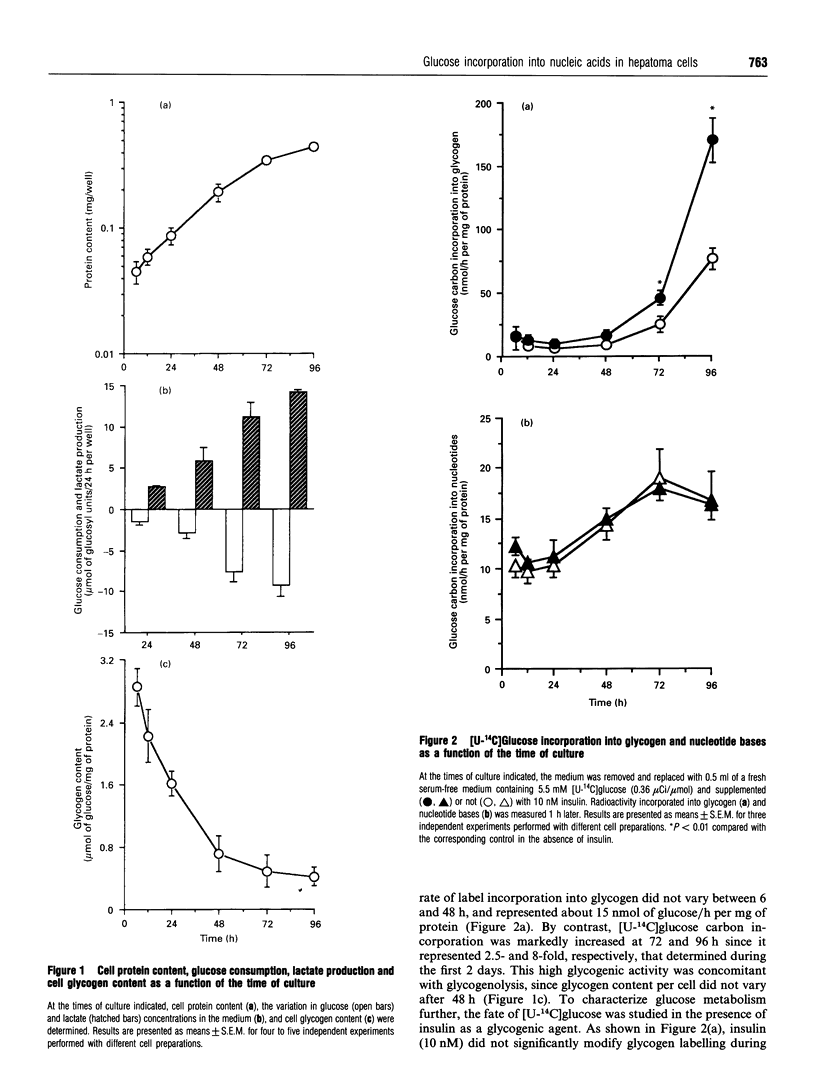
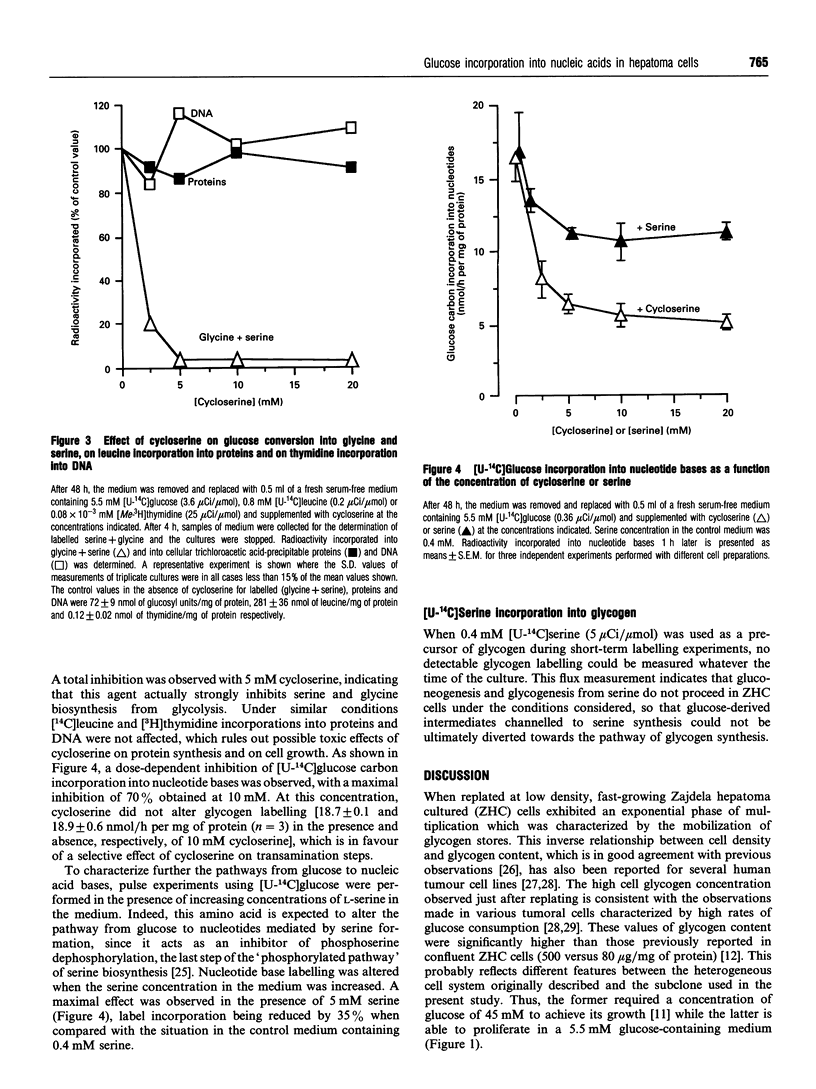
The coupling of glycolysis to serine and glycine metabolism was studied in fast-growing Zajdela hepatoma cultured cells. During the exponential phase of growth, occurring between 12 and 72 h, cells exhibited a decreased glycogen content together with a high glycolytic activity. Glycogen labelling, evaluated by 1 h-pulse experiments with [U-14C]glucose (5.5 mM), was minimal during the first 48 h and increased 2.5-fold at 72 h and 8-fold at 96 h, at which times it was also stimulated 2-fold by 10 nM insulin. [U-14C]Glucose carbons were incorporated into nucleic acid bases, with maximal incorporation at 72 h, the rate of nucleotide base labelling exceeding that of glycogen during the first 2 days of culture. Incubation of the cells with [U-14C]glucose resulted in the release into the medium of 14C-labelled glycine, the first intermediate formed on the route from serine to DNA. The rate of release per cell decreased as a function of cell growth, concomitantly with an increased rate of glucose carbon incorporation into nucleotide bases. The latter implied the intermediary formation of amino acids since the transaminase inhibitor cycloserine (10 mM), which totally inhibited [14C]glycine release, decreased by 65% nucleotide labelling from [U-14C]glucose. A dose-dependent inhibition by serine of the rate of [U-14C]glucose carbon incorporation into nucleotide bases was observed, which was maximal at 5 mM serine. These metabolic flux measurements indicate that glucose can be used as a precursor of nucleic acid synthesis. These results strongly suggest that this process is to a large extent mediated by a serine/glycine-biosynthesis-mediated pathway, and reinforce the hypothesis that glycolysis contributes to enhancing the provision of precursors required for cell proliferation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORKENHAGEN L. F., KENNEDY E. P. The enzymatic exchange of L-serine with O-phospho-L-serine catalyzed by a specific phosphatase. J Biol Chem. 1959 Apr;234(4):849–853. [PubMed] [Google Scholar]
- Bismut H., Plas C. Pathways of glycogen synthesis from glucose during the glycogenic response to insulin in cultured foetal hepatocytes. Biochem J. 1989 Nov 1;263(3):889–895. doi: 10.1042/bj2630889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismut H., Plas C. Role of serine biosynthesis and its utilization in the alternative pathway from glucose to glycogen during the response to insulin in cultured foetal-rat hepatocytes. Biochem J. 1991 Jun 15;276(Pt 3):577–582. doi: 10.1042/bj2760577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismut H., Poggi-Bach J., Plas C. Consumption and production of amino acids by insulin-responsive cultured fetal rat hepatocytes: the particular case of serine. Biol Neonate. 1992;62(1):37–46. doi: 10.1159/000243851. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caron M., Cherqui G., Wicek D., Melin B., Reynet C., Capeau J., Picard J. Evidence for the involvement of vicinal sulfhydryl groups in the insulin stimulation of intracellular glucose metabolism in Zajdela hepatoma cells. Biosci Rep. 1990 Feb;10(1):23–29. doi: 10.1007/BF01116847. [DOI] [PubMed] [Google Scholar]
- Chantret I., Rodolosse A., Barbat A., Dussaulx E., Brot-Laroche E., Zweibaum A., Rousset M. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994 Jan;107(Pt 1):213–225. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- Davis J. L., Fallon H. J., Morris H. P. Two enzymes of serine metabolism in rat liver and hepatomas. Cancer Res. 1970 Dec;30(12):2917–2920. [PubMed] [Google Scholar]
- DeSante D. C., Little L., Peavy D. E., Vinicor F. Insulin-responsive cultured foetal-rat hepatocytes. Their preparation and characterization. Biochem J. 1984 Oct 1;223(1):39–46. doi: 10.1042/bj2230039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell D. A., Snell K. Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem J. 1988 Nov 15;256(1):97–101. doi: 10.1042/bj2560097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON B. E., WALSH D. A., SALLACH H. J. CHANGES IN THE ACTIVITIES OF D-GLYCERATE AND D-3-PHOSPHOGLYCERATE DEHYDROGENASES IN THE DEVELOPING RAT LIVER. Biochim Biophys Acta. 1964 May 4;85:202–205. doi: 10.1016/0926-6569(64)90241-x. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Greengard O. Phosphoserine phosphatase: development formation and hormonal regulation in rat tissues. Arch Biochem Biophys. 1969 Oct;134(1):228–232. doi: 10.1016/0003-9861(69)90270-7. [DOI] [PubMed] [Google Scholar]
- Kitagawa T. Protein synthesis in transformed 3T3 cells permeabilized by exogenous ATP. J Cell Physiol. 1980 Jan;102(1):37–43. doi: 10.1002/jcp.1041020106. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Herzfeld A., Hudson J. Phosphoserine phosphatase distribution in normal and neoplastic rat tissues. Arch Biochem Biophys. 1969 Jul;132(2):397–403. doi: 10.1016/0003-9861(69)90381-6. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Van Dam K. The metabolic significance of anion transport in mitochondria. Biochim Biophys Acta. 1974 Dec 30;346(3-4):213–244. doi: 10.1016/0304-4173(74)90001-9. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C., BYRNE W. L. Metabolism of phosphoserine. III. Mechanism of O-phosphoserine phosphatase. J Biol Chem. 1960 Jul;235:2019–2024. [PubMed] [Google Scholar]
- PIZER L. I. ENZYMOLOGY AND REGULATION OF SERINE BIOSYNTHESIS IN CULTURED HUMAN CELLS. J Biol Chem. 1964 Dec;239:4219–4226. [PubMed] [Google Scholar]
- Parniak M., Kalant N. Incorporation of glucose into glycogen in primary cultures of rat hepatocytes. Can J Biochem Cell Biol. 1985 May;63(5):333–340. doi: 10.1139/o85-049. [DOI] [PubMed] [Google Scholar]
- Plas C., Chapeville F., Jacquot R. Development of glycogen storage ability under cortisol control in primary cultures of rat fetal hepatocytes. Dev Biol. 1973 May;32(1):82–91. doi: 10.1016/0012-1606(73)90221-2. [DOI] [PubMed] [Google Scholar]
- Plas C., Menuelle P., Moncany M. L., Fulchignoni-Lataud M. C. Time dependence of the glycogenic effect of insulin in cultured fetal hepatocytes. Diabetes. 1979 Aug;28(8):705–712. doi: 10.2337/diab.28.8.705. [DOI] [PubMed] [Google Scholar]
- Rousset M., Paris H., Chevalier G., Terrain B., Murat J. C., Zweibaum A. Growth-related enzymatic control of glycogen metabolism in cultured human tumor cells. Cancer Res. 1984 Jan;44(1):154–160. [PubMed] [Google Scholar]
- Rowe P. B., Sauer D., Fahey D., Craig G., McCairns E. One-carbon metabolism in lectin-activated human lymphocytes. Arch Biochem Biophys. 1985 Jan;236(1):277–288. doi: 10.1016/0003-9861(85)90627-7. [DOI] [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- Salhanick A. I., Chang C. L., Amatruda J. M. Hormone and substrate regulation of glycogen accumulation in primary cultures of rat hepatocytes. Biochem J. 1989 Aug 1;261(3):985–992. doi: 10.1042/bj2610985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze P. E., Solheim A. E., Seglen P. O. Amino acid and energy requirements for rat hepatocytes in primary culture. In Vitro. 1982 Jan;18(1):43–54. doi: 10.1007/BF02796384. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal and neoplastic rat tissues. Biochim Biophys Acta. 1985 Dec 13;843(3):276–281. doi: 10.1016/0304-4165(85)90149-7. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv Enzyme Regul. 1984;22:325–400. doi: 10.1016/0065-2571(84)90021-9. [DOI] [PubMed] [Google Scholar]
- Snell K. Liver enzymes of serine metabolism during neonatal development of the rat. Biochem J. 1980 Aug 15;190(2):451–455. doi: 10.1042/bj1900451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Natsumeda Y., Weber G. The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem J. 1987 Jul 15;245(2):609–612. doi: 10.1042/bj2450609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Weber G. Enzymic imbalance in serine metabolism in rat hepatomas. Biochem J. 1986 Jan 15;233(2):617–620. doi: 10.1042/bj2330617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedel-Flaig C., Capeau J., Picard J., Beck J. P. Action of insulin on glycogen metabolism in cultured hepatoma cells. Mol Cell Endocrinol. 1981 Dec;24(3):283–291. doi: 10.1016/0303-7207(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Staedel C., Beck J. P. Resurgence of glycogen synthesis and storage capacity in cultured hepatoma cells. Cell Differ. 1978 Apr;7(1-2):61–71. doi: 10.1016/0045-6039(78)90007-6. [DOI] [PubMed] [Google Scholar]
- Staedel C., Castagna M., Beck J. P. Modifications of the activities of key enzymes and intracellular levels of cyclic nucleotides, in correlation with the glyogen deposition in a cultured hepatoma cell line. Cell Differ. 1979 Feb;8(1):29–38. doi: 10.1016/0045-6039(79)90015-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Kishi K., Ichihara A. Biochemical studies on liver functions in primary cultured hepatocytes of adult rats. II. Regulation of protein and amino acid metabolism. J Biochem. 1979 Oct;86(4):863–870. doi: 10.1093/oxfordjournals.jbchem.a132618. [DOI] [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Williams J. F., Arora K. K., Longenecker J. P. The pentose pathway: a random harvest. Impediments which oppose acceptance of the classical (F-type) pentose cycle for liver, some neoplasms and photosynthetic tissue. The case for the L-type pentose pathway. Int J Biochem. 1987;19(9):749–817. doi: 10.1016/0020-711x(87)90239-4. [DOI] [PubMed] [Google Scholar]
- Zweibaum A., Pinto M., Chevalier G., Dussaulx E., Triadou N., Lacroix B., Haffen K., Brun J. L., Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985 Jan;122(1):21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]