Abstract
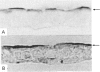
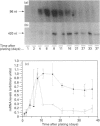
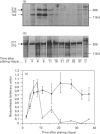
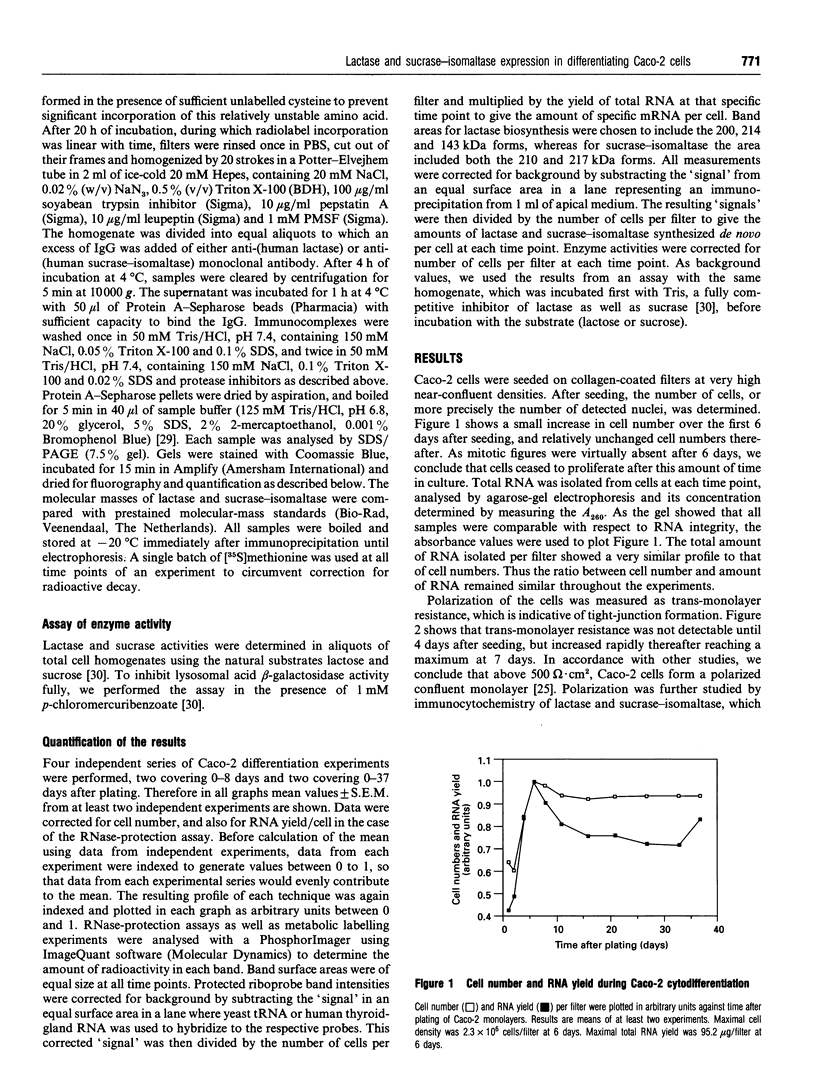
The Caco-2 cell line is derived from a human colon adenocarcinoma and differentiates in vitro into small-intestinal enterocyte-like cells, expressing the hydrolases lactase and sucrase-isomaltase. We cultured Caco-2 cells on permeable supports from 0 to 37 days after plating to study endogenous lactase and sucrase-isomaltase gene expression in relation to cell differentiation. Profiles of lactase and sucrase-isomaltase mRNA, protein and enzyme activity were analysed on a per-cell basis, using immunocytochemistry, RNase protection assays, metabolic polypeptide labelling and enzyme activity assays. Tight-junction formation was complete 6 days after plating. Immunocytochemistry of Caco-2 cross-sections showed lactase and sucrase-isomaltase predominantly in the microvillar membrane of polarized cells. mRNA, protein and enzyme activity of lactase appeared consecutively, reaching maximum levels 8-11 days after plating. Whereas lactase mRNA and protein biosynthesis showed a sharp decline after peak levels, lactase activity remained high until 37 days after plating. In contrast, mRNA and protein biosynthesis and activity of sucrase-isomaltase peaked successively 11-21 days after plating, and exhibited comparable levels throughout the entire experiment. The following conclusions were reached. (1) In Caco-2 cells, biosynthesis of lactase and sucrase-isomaltase is regulated by the amount of their mRNAs, indicating transcriptional control. (2) Sucrase-isomaltase activity is most probably transcriptionally controlled at all time points. (3) In contrast, lactase activity is initially regulated by its level of biosynthesis. After its peak at 8 days, the slow decline in activity compared with its biosynthesis indicates high stability. (4) Different mRNA profiles for lactase and sucrase-isomaltase indicate different mechanisms of transcriptional regulation of these genes.
Full text
PDF

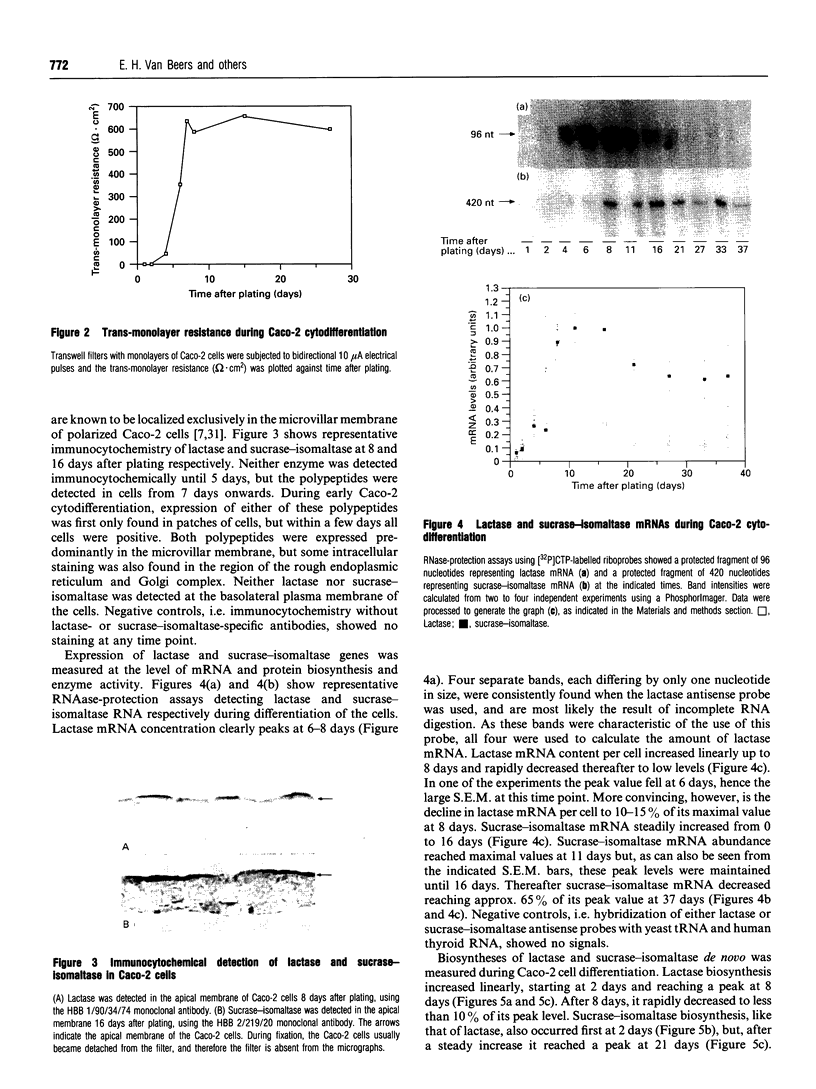
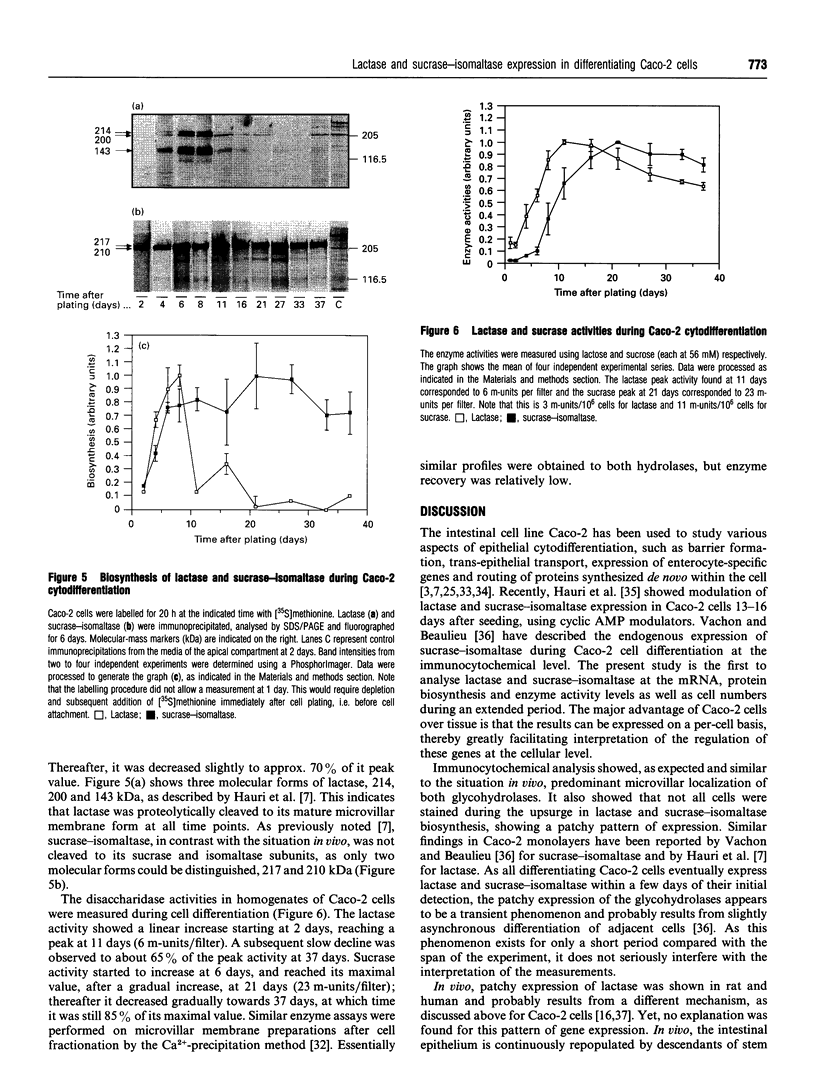


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockhausen I., Romero P. A., Herscovics A. Glycosyltransferase changes upon differentiation of CaCo-2 human colonic adenocarcinoma cells. Cancer Res. 1991 Jun 15;51(12):3136–3142. [PubMed] [Google Scholar]
- Büller H. A., Kothe M. J., Goldman D. A., Grubman S. A., Sasak W. V., Matsudaira P. T., Montgomery R. K., Grand R. J. Coordinate expression of lactase-phlorizin hydrolase mRNA and enzyme levels in rat intestine during development. J Biol Chem. 1990 Apr 25;265(12):6978–6983. [PubMed] [Google Scholar]
- Büller H. A., Rings E. H., Pajkrt D., Montgomery R. K., Grand R. J. Glycosylation of lactase-phlorizin hydrolase in rat small intestine during development. Gastroenterology. 1990 Mar;98(3):667–675. doi: 10.1016/0016-5085(90)90287-b. [DOI] [PubMed] [Google Scholar]
- CUATRECASAS P., LOCKWOOD D. H., CALDWELL J. R. LACTASE DEFICIENCY IN THE ADULT. A COMMON OCCURRENCE. Lancet. 1965 Jan 2;1(7375):14–18. doi: 10.1016/s0140-6736(65)90922-0. [DOI] [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. Studies of small intestine during development. I. Distribution and activity of beta-galactosidase. Biochim Biophys Acta. 1962 Aug 13;62:353–362. doi: 10.1016/0006-3002(62)90097-5. [DOI] [PubMed] [Google Scholar]
- Danielsen E. M., Skovbjerg H., Norén O., Sjöström H. Biosynthesis of intestinal microvillar proteins. Intracellular processing of lactase-phlorizin hydrolase. Biochem Biophys Res Commun. 1984 Jul 18;122(1):82–90. doi: 10.1016/0006-291x(84)90442-x. [DOI] [PubMed] [Google Scholar]
- Escher J. C., de Koning N. D., van Engen C. G., Arora S., Büller H. A., Montgomery R. K., Grand R. J. Molecular basis of lactase levels in adult humans. J Clin Invest. 1992 Feb;89(2):480–483. doi: 10.1172/JCI115609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo O., Naim H. Y., Lacey S. W. The polymorphic expression of lactase in adults is regulated at the messenger RNA level. Gastroenterology. 1994 May;106(5):1233–1241. doi: 10.1016/0016-5085(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Fogh J., Fogh J. M., Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977 Jul;59(1):221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Duluc I., Raul F. Lactase expression is controlled differently in the jejunum and ileum during development in rats. Gastroenterology. 1991 Feb;100(2):388–394. doi: 10.1016/0016-5085(91)90207-2. [DOI] [PubMed] [Google Scholar]
- Gilat T., Russo S., Gelman-Malachi E., Aldor T. A. Lactase in man: a nonadaptable enzyme. Gastroenterology. 1972 Jun;62(6):1125–1127. [PubMed] [Google Scholar]
- Gilbert T., Le Bivic A., Quaroni A., Rodriguez-Boulan E. Microtubular organization and its involvement in the biogenetic pathways of plasma membrane proteins in Caco-2 intestinal epithelial cells. J Cell Biol. 1991 Apr;113(2):275–288. doi: 10.1083/jcb.113.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Sander B., Naim H. Induction of lactase biosynthesis in the human intestinal epithelial cell line Caco-2. Eur J Biochem. 1994 Jan 15;219(1-2):539–546. doi: 10.1111/j.1432-1033.1994.tb19969.x. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Wacker H., Rickli E. E., Bigler-Meier B., Quaroni A., Semenza G. Biosynthesis of sucrase-isomaltase. Purification and NH2-terminal amino acid sequence of the rat sucrase-isomaltase precursor (pro-sucrase-isomaltase) from fetal intestinal transplants. J Biol Chem. 1982 Apr 25;257(8):4522–4528. [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Klumperman J., Fransen J. A., Boekestijn T. C., Oude Elferink R. P., Matter K., Hauri H. P., Tager J. M., Ginsel L. A. Biosynthesis and transport of lysosomal alpha-glucosidase in the human colon carcinoma cell line Caco-2: secretion from the apical surface. J Cell Sci. 1991 Oct;100(Pt 2):339–347. doi: 10.1242/jcs.100.2.339. [DOI] [PubMed] [Google Scholar]
- Koldovský O., Asp N. G., Dahlqvist A. A method for the separate assay of "neutral" and "acid" beta-galactosidase in homogenates of rat small-intestinal mucosa. Anal Biochem. 1969 Mar;27(3):409–418. doi: 10.1016/0003-2697(69)90054-2. [DOI] [PubMed] [Google Scholar]
- Krasinski S. D., Estrada G., Yeh K. Y., Yeh M., Traber P. G., Rings E. H., Büller H. A., Verhave M., Montgomery R. K., Grand R. J. Transcriptional regulation of intestinal hydrolase biosynthesis during postnatal development in rats. Am J Physiol. 1994 Oct;267(4 Pt 1):G584–G594. doi: 10.1152/ajpgi.1994.267.4.G584. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Quaroni A., Nichols B., Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990 Oct;111(4):1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri L., Raia V., Potter J., Swallow D., Ho M. W., Fiocca R., Finzi G., Cornaggia M., Capella C., Quaroni A. Mosaic pattern of lactase expression by villous enterocytes in human adult-type hypolactasia. Gastroenterology. 1991 Feb;100(2):359–369. doi: 10.1016/0016-5085(91)90203-w. [DOI] [PubMed] [Google Scholar]
- Maiuri L., Rossi M., Raia V., D'Auria S., Swallow D., Quaroni A., Auricchio S. Patchy expression of lactase protein in adult rabbit and rat intestine. Gastroenterology. 1992 Dec;103(6):1739–1746. doi: 10.1016/0016-5085(92)91429-8. [DOI] [PubMed] [Google Scholar]
- Montgomery R. K., Büller H. A., Rings E. H., Grand R. J. Lactose intolerance and the genetic regulation of intestinal lactase-phlorizin hydrolase. FASEB J. 1991 Oct;5(13):2824–2832. doi: 10.1096/fasebj.5.13.1916106. [DOI] [PubMed] [Google Scholar]
- Naim H. Y., Lacey S. W., Sambrook J. F., Gething M. J. Expression of a full-length cDNA coding for human intestinal lactase-phlorizin hydrolase reveals an uncleaved, enzymatically active, and transport-competent protein. J Biol Chem. 1991 Jul 5;266(19):12313–12320. [PubMed] [Google Scholar]
- Naim H. Y. Processing of human pro-lactase-phlorizin hydrolase at reduced temperatures: cleavage is preceded by complex glycosylation. Biochem J. 1992 Jul 1;285(Pt 1):13–16. doi: 10.1042/bj2850013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim H. Y., Sterchi E. E., Lentze M. J. Biosynthesis and maturation of lactase-phlorizin hydrolase in the human small intestinal epithelial cells. Biochem J. 1987 Jan 15;241(2):427–434. doi: 10.1042/bj2410427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim H. Y., Sterchi E. E., Lentze M. J. Biosynthesis of the human sucrase-isomaltase complex. Differential O-glycosylation of the sucrase subunit correlates with its position within the enzyme complex. J Biol Chem. 1988 May 25;263(15):7242–7253. [PubMed] [Google Scholar]
- Peterson M. D., Bement W. M., Mooseker M. S. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci. 1993 Jun;105(Pt 2):461–472. doi: 10.1242/jcs.105.2.461. [DOI] [PubMed] [Google Scholar]
- Peterson M. D., Mooseker M. S. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci. 1993 Jun;105(Pt 2):445–460. doi: 10.1242/jcs.105.2.445. [DOI] [PubMed] [Google Scholar]
- Quezada-Calvillo R., Markowitz A. J., Traber P. G., Underdown B. J. Murine intestinal disaccharidases: identification of structural variants of sucrase-isomaltase complex. Am J Physiol. 1993 Dec;265(6 Pt 1):G1141–G1149. doi: 10.1152/ajpgi.1993.265.6.G1141. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Traber M. G. A specific sorting signal is not required for the polarized secretion of newly synthesized proteins from cultured intestinal epithelial cells. J Cell Biol. 1988 Aug;107(2):471–479. doi: 10.1083/jcb.107.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rings E. H., Krasinski S. D., van Beers E. H., Moorman A. F., Dekker J., Montgomery R. K., Grand R. J., Büller H. A. Restriction of lactase gene expression along the proximal-to-distal axis of rat small intestine occurs during postnatal development. Gastroenterology. 1994 May;106(5):1223–1232. doi: 10.1016/0016-5085(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Rings E. H., de Boer P. A., Moorman A. F., van Beers E. H., Dekker J., Montgomery R. K., Grand R. J., Büller H. A. Lactase gene expression during early development of rat small intestine. Gastroenterology. 1992 Oct;103(4):1154–1161. doi: 10.1016/0016-5085(92)91498-s. [DOI] [PubMed] [Google Scholar]
- Rossi M., Maiuri L., Russomanno C., Auricchio S. In vitro biosynthesis of lactase in preweaning and adult rabbit. FEBS Lett. 1992 Nov 30;313(3):260–264. doi: 10.1016/0014-5793(92)81205-z. [DOI] [PubMed] [Google Scholar]
- Sjöström H., Norén O., Christiansen L., Wacker H., Semenza G. A fully active, two-active-site, single-chain sucrase.isomaltase from pig small intestine. Implications for the biosynthesis of a mammalian integral stalked membrane protein. J Biol Chem. 1980 Dec 10;255(23):11332–11338. [PubMed] [Google Scholar]
- Traber P. G., Wu G. D., Wang W. Novel DNA-binding proteins regulate intestine-specific transcription of the sucrase-isomaltase gene. Mol Cell Biol. 1992 Aug;12(8):3614–3627. doi: 10.1128/mcb.12.8.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber P. G., Yu L., Wu G. D., Judge T. A. Sucrase-isomaltase gene expression along crypt-villus axis of human small intestine is regulated at level of mRNA abundance. Am J Physiol. 1992 Jan;262(1 Pt 1):G123–G130. doi: 10.1152/ajpgi.1992.262.1.G123. [DOI] [PubMed] [Google Scholar]
- Troelsen J. T., Olsen J., Mitchelmore C., Hansen G. H., Sjöström H., Norén O. Two intestinal specific nuclear factors binding to the lactase-phlorizin hydrolase and sucrase-isomaltase promoters are functionally related oligomeric molecules. FEBS Lett. 1994 Apr 11;342(3):297–301. doi: 10.1016/0014-5793(94)80520-2. [DOI] [PubMed] [Google Scholar]
- Troelsen J. T., Olsen J., Norén O., Sjöström H. A novel intestinal trans-factor (NF-LPH1) interacts with the lactase-phlorizin hydrolase promoter and co-varies with the enzymatic activity. J Biol Chem. 1992 Oct 5;267(28):20407–20411. [PubMed] [Google Scholar]
- Vachon P. H., Beaulieu J. F. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology. 1992 Aug;103(2):414–423. doi: 10.1016/0016-5085(92)90829-n. [DOI] [PubMed] [Google Scholar]
- Witte J., Lloyd M., Lorenzsonn V., Korsmo H., Olsen W. The biosynthetic basis of adult lactase deficiency. J Clin Invest. 1990 Oct;86(4):1338–1342. doi: 10.1172/JCI114843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweibaum A., Triadou N., Kedinger M., Augeron C., Robine-Léon S., Pinto M., Rousset M., Haffen K. Sucrase-isomaltase: a marker of foetal and malignant epithelial cells of the human colon. Int J Cancer. 1983 Oct 15;32(4):407–412. doi: 10.1002/ijc.2910320403. [DOI] [PubMed] [Google Scholar]