Abstract
Background
The use of antioxidant-rich foods to treat female infertility has received significant attention in recent years. The aim of this study was to investigate the potential correlation between the composite dietary antioxidant index (CDAI) and female infertility.
Methods
The participants in the cross-sectional data were women between the ages of 20 and 45 who had complete CDAI-related data and infertility information, which were taken from the National Health and Nutrition Examination Survey (NHANES) conducted between 2013 and 2020. The independent association between CDAI and infertility was investigated using multivariate logistic regression analysis. Trends between the two variables were examined using smoothed curve fitting, and subgroup analysis and interaction tests were conducted.
Results
The prevalence of infertility was 12.57% of the 3,259 participants included in the study; individuals in higher CDAI quartiles tended to have a lower percentage of infertility. The risk of infertility was 44% lower among individuals in the highest quartile of the CDAI compared to those in the lowest quartile (OR = 0.56, 95%CI: 0.36–0.85, P = 0.0072), and the test for trend was also significant (P for trend = 0.0235). Smoothed curve fitting showed a negative non-linear relationship between CDAI and infertility. Subgroup analysis and interaction tests showed that there was an interaction of BMI in the relationship between CDAI and infertility risk (P for interaction = 0.0497) and that education, PIR, marital status, smoking status, hypertension, diabetes, age at menarche, ever having been treated for pelvic infection, ever having used female hormones, and ever been pregnant had no significant dependence on this negative association (all P for interaction > 0.05).
Conclusion
There was a negative non-linear correlation between CDAI and infertility among reproductive-aged women in the US. The risk of infertility may be reduced by increasing the intake of antioxidant-rich foods.
Keywords: CDAI, Infertility, NHANES, Oxidative stress
Introduction
Infertility is defined as the inability to conceive after 12 months or more of regular, unprotected sexual behavior or due to reduced fertility, either alone or with a partner [1]. The prevalence of infertility has been increasing annually in recent years, affecting 15% of couples of reproductive age globally [2, 3]. In addition to its definition as the inability to conceive, infertility has been demonstrated to have a significant impact on women’s psychological well-being, quality of life, and long-term health [4, 5]. Consequently, it has become a significant public health issue.
An individual’s dietary antioxidant capacity is measured by the composite dietary antioxidant index (CDAI), which is calculated based on the dietary intake of minerals and vitamins that possess antioxidant qualities, such as zinc, magnesium, selenium, and vitamins A, C, and E [6]. The current literature indicates that diseases related to oxidative stress (OS), such as heart failure, hyperlipidemia, and chronic obstructive pulmonary disease, are all associated with CDAI [7–9].
OS is characterized by an imbalance between the body’s reactive oxygen species (ROS) and antioxidants. This imbalance represents a significant contributing factor to the pathogenesis of reproductive disorders, including endometriosis, premature ovarian failure, and infertility [10, 11]. Research has been shown that excessive ROS production can lead to damage and senescence of oocyte, failure of implantation, and placental damage, ultimately leading to infertility [12, 13]. Therefore, antioxidants are now commonly included in infertility treatment to mitigate the damage of OS and enhance blood circulation of endometrium [14]. In recent years, there has been growing interest in the relationship between dietary intake of antioxidants and female fertility. Studies have reported that naturally occurring antioxidant compounds found in dietary sources may be useful in treating OS-mediated infertility across both natural and assisted reproductive settings [15]. It has also been observed that supplementing selenium and vitamin E helps improve OS status in women with ovulatory disorders [16, 17]. A greater intake of vitamin C and vitamin E has been shown to reduce the time taken for infertile couples to conceive, according to a study examining the relationship between women’s dietary antioxidant consumption and the time to pregnancy in couples undergoing treatment for infertility-related diseases [18]. Previous research has also indicated a link between a decreased rate of infertility and consumption of various antioxidant substances [19]. However, less attention has been given to evaluating the effect of CDAI, an indicator of dietary antioxidant capacity, on infertility risk.
Therefore, the purpose of this study is to investigate the association between CDAI and infertility using information from the National Health and Nutrition Examination Survey (NHANES), which may provide new approaches to infertility treatments and dietary recommendations.
Materials and methods
Data sources
The NHANES database (www.cdc.gov/nchs/nhanes.com) provided the data for this study. It is designed to assess the general health and nutritional status of the US population by collecting data on demographics, socioeconomic status, dietary habits, and health-related data. NHANES was approved by the Ethics Review Board of the National Center for Health Statistics, and informed permission was obtained from each survey respondent before their participation in the study.
Study population
This cross-sectional analysis used data from 2013 to 2020 for a total of 35,706 study participants, excluding 17,616 males, 13,430 females younger than 20 or older than 45 years of age, and 1,401 who did not respond to the “history of infertility” question or had missing data related to the CDAI, and finally. The final analysis sample consisted 3,259 participants. Details of the screening process and the size of the participants are shown in Fig. 1.
Fig. 1.
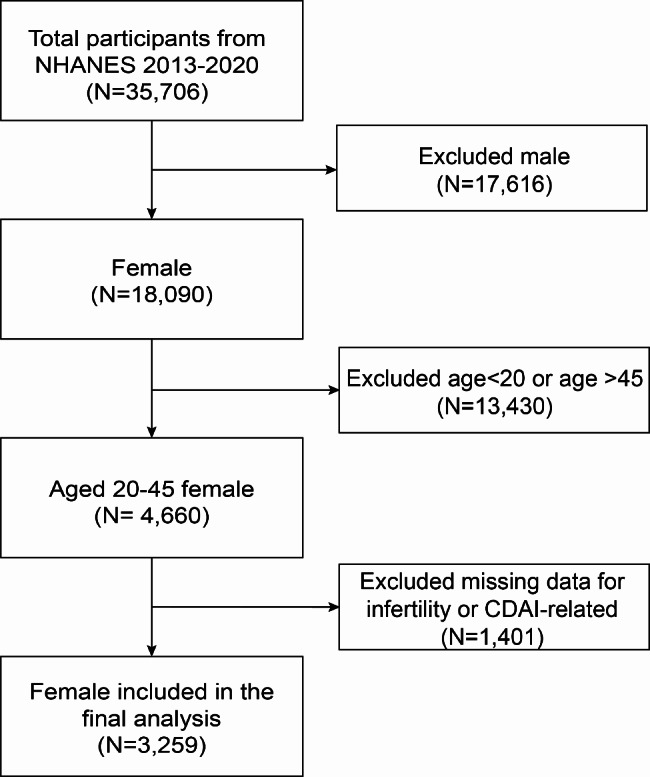
Flowchart of the sample selection from NHANES 2013–2020
Assessment of CDAI
The food and nutrient intake of each participant was collected during the dietary interview section of the NHANES database. Each participant was required to complete a non-continuous 24-hour dietary recall interview for two days. The first dietary interview was conducted in person at the Mobile Examination Center, and the second was conducted by telephone 3 to 10 days later, with the amounts per-intake calculated from dietary recall data on both days. We determined the CDAI using the Wright et al. suggested measure, which is consists of six dietary antioxidants, including magnesium, selenium, zinc, and vitamins A, C, and E [6].
The following formula was calculated by subtracting the global mean from the average daily intake of the six antioxidants and dividing the result by the global standard deviation:
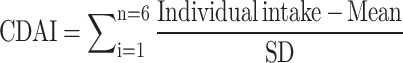 |
Assessment of infertility
Infertility is measured by self-reported Reproductive Health Questionnaires for each woman: “Have you ever tried to conceive a child for at least one year without getting pregnant?” Women who answered “yes” were considered infertile.
Covariates
Covariates in this study included age, race (Mexican American/other Hispanic/non-Hispanic white/non-Hispanic black/other races), education level (Less than 9th grade/9th–11th grade/High school or GED/Some college or AA degree/College graduate or above), ratio of family income to poverty(PIR) (< 1.3/1.3–3.49/≥3.5), energy(kcal), body mass index (BMI) (< 25/25–29.9/≥30), marital status (married or living with a partner/widowed or divorced or separated/never married), smoking status (according to the standard of at least 100 cigarettes per year, it is divided into current > 100 cigarettes, former > 100 cigarettes and have quit smoking, and never < 100 cigarettes), hypertension (yes/no), Diabetes (yes/no), age when first menstrual period occurred (Age ≤ 10/10 < Age ≤ 15/Age > 15), ever treated for a pelvic infection/pelvic inflammatory disease(PID) (yes/no), ever use female hormones (yes/no), ever been pregnant (yes/no).
Statistical analysis
Infertility was categorized as a dichotomous variable with or without infertility. CDAI as a continuous variable is presented as quartiles. Continuous covariates are expressed as mean ± standard deviation, and categorical variables are expressed as percentages. We used weighted chi-squared tests for categorical variables and weighted linear regression models for continuous variables to assess differences between individuals categorized by CDAI quartiles. The independent association between CDAI and infertility was examined in three separate models using multivariate logistic regression models. Model 1 did not adjust for covariates. Model 2 was adjusted for age and race. Model 3 was adjusted for age, race, education level, PIR, energy, BMI, marital status, smoking status, hypertension, diabetes, age when first menstrual period occurred, ever treated for a pelvic infection/PID, ever use female hormones, and ever been pregnant. Subgroup analyses were performed to assess the stability of the association between CDAI and infertility, with stratification factors including education level, PIR, BMI, marital status, smoking status, hypertension, diabetes, age when first menstrual period occurred, ever treated for a pelvic infection/PID, ever use female hormones, and ever been pregnant. An interaction term was included to test for heterogeneity of associations between subgroups. Missing values were input by median for continuous variables or mode for categorical variables of existing cases of those variables. All statistical analyses were performed using EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA) and R software, and P < 0.05 was considered statistically significant.
Results
Baseline characteristics of study participants
Table 1 shows the baseline characteristics of all participants. A total of 3,259 participants were enrolled in this study, with a mean age of 32.32 ± 7.60 years. The overall prevalence of infertility was 12.57%, and the prevalence of infertility in quartiles 1–4 was 14.52%, 12.57%, 14.00%, and 9.48%respectively. Compared with the lowest quartile of the CDAI, participants in the highest quartile of the CDAI had lower BMI, smoking rates, age of menarche, PID prevalence, and ever been pregnant, and higher levels of education, family income, calorie intake, and marriage rate when compared to those in the lowest quartile. (all P < 0.05). There was no statistically significant difference in age, hypertension and diabetes between the different quartiles (P > 0.05).
Table 1.
Baseline characteristics of the study population according to CDAI quartiles
| CDAI | Overall | Q1 | Q2 | Q3 | Q4 | P-value |
|---|---|---|---|---|---|---|
| N = 815 | N = 814 | N = 815 | N = 815 | |||
| Age, mean ± SD (years) | 32.32 ± 7.60 | 32.44 ± 8.07 | 31.92 ± 7.60 | 32.10 ± 7.54 | 32.79 ± 7.21 | 0.0908 |
| Race(%) | 0.0062 | |||||
| Mexican American | 12.53 | 11.31 | 11.31 | 11.72 | 15.53 | |
| Other Hispanic | 7.83 | 10.10 | 6.40 | 7.39 | 7.64 | |
| Non-Hispanic White | 55.63 | 52.94 | 55.77 | 58.35 | 55.11 | |
| Non-Hispanic Black | 13.60 | 15.41 | 15.97 | 11.82 | 11.58 | |
| Other Race | 10.42 | 10.24 | 10.55 | 10.72 | 10.14 | |
| Education Level (%) | < 0.0001 | |||||
| Less than 9th grade | 2.93 | 3.93 | 2.88 | 2.58 | 2.46 | |
| 9th–11th grade | 7.40 | 9.73 | 7.84 | 6.09 | 6.31 | |
| High school or GED | 19.41 | 28.64 | 18.72 | 17.34 | 14.20 | |
| Some college or AA degree | 35.22 | 38.19 | 41.65 | 29.23 | 32.59 | |
| College graduate or above | 35.03 | 19.52 | 28.91 | 44.76 | 44.45 | |
| PIR (%) | < 0.0001 | |||||
| < 1.3 | 25.95 | 32.93 | 29.21 | 22.85 | 19.95 | |
| 1.3–3.49 | 40.24 | 39.57 | 38.85 | 40.07 | 42.29 | |
| ≥ 3.5 | 33.81 | 27.50 | 31.94 | 37.08 | 37.76 | |
| Energy, mean ± SD (kcal) | 1847.07 ± 636.89 | 1230.15 ± 388.86 | 1731.77 ± 400.07 | 1950.07 ± 430.73 | 2383.99 ± 660.28 | < 0.0001 |
| BMI (%) | 0.0316 | |||||
| < 25 | 35.09 | 35.19 | 32.20 | 35.25 | 37.54 | |
| 25–29.9 | 24.85 | 22.30 | 25.42 | 27.87 | 23.49 | |
| ≥ 30 | 40.07 | 42.51 | 42.38 | 36.88 | 38.97 | |
| Marital status (%) | < 0.0001 | |||||
| Married/Living with partner | 59.78 | 52.04 | 61.25 | 60.37 | 64.47 | |
| Widowed/Divorced/Separated | 9.69 | 14.75 | 9.13 | 7.99 | 7.54 | |
| Never married | 30.53 | 33.21 | 29.62 | 31.64 | 27.99 | |
| Smoking status (%) | < 0.0001 | |||||
| Current | 17.35 | 28.54 | 18.94 | 12.59 | 10.96 | |
| Former | 13.60 | 9.76 | 13.70 | 13.87 | 16.54 | |
| Never | 69.05 | 61.70 | 67.35 | 73.53 | 72.51 | |
| Hypertension (%) | 0.1183 | |||||
| Yes | 12.35 | 13.82 | 11.52 | 10.56 | 13.66 | |
| No | 87.65 | 86.18 | 88.48 | 89.44 | 86.34 | |
| Diabetes (%) | 0.0894 | |||||
| Yes | 3.80 | 3.96 | 4.75 | 2.46 | 4.13 | |
| No | 96.20 | 96.04 | 95.25 | 97.54 | 95.87 | |
| Age when first menstrual period occurred (%) | 0.0010 | |||||
| Age ≤ 10 | 9.44 | 12.88 | 9.60 | 6.44 | 9.31 | |
| 10 < Age ≤ 15 | 84.48 | 79.94 | 84.32 | 87.84 | 85.17 | |
| Age > 15 | 6.09 | 7.18 | 6.08 | 5.72 | 5.52 | |
| Ever treated for a pelvic infection/PID (%) | 0.0001 | |||||
| Yes | 4.56 | 7.61 | 3.46 | 3.73 | 3.79 | |
| No | 95.44 | 92.39 | 96.54 | 96.27 | 96.21 | |
| Ever use female hormones (%) | < 0.0001 | |||||
| Yes | 4.36 | 5.28 | 5.02 | 1.20 | 6.09 | |
| No | 95.64 | 94.72 | 94.98 | 98.80 | 93.91 | |
| Ever been pregnant (%) | 0.0491 | |||||
| Yes | 69.17 | 72.12 | 70.88 | 66.48 | 67.73 | |
| No | 30.83 | 27.88 | 29.12 | 33.52 | 32.27 | |
| Tried for a year to become pregnant (%) | 0.0089 | |||||
| Yes | 12.57 | 14.52 | 12.57 | 14.00 | 9.48 | |
| No | 87.43 | 85.48 | 87.43 | 86.00 | 90.52 |
Association of CDAI with infertility
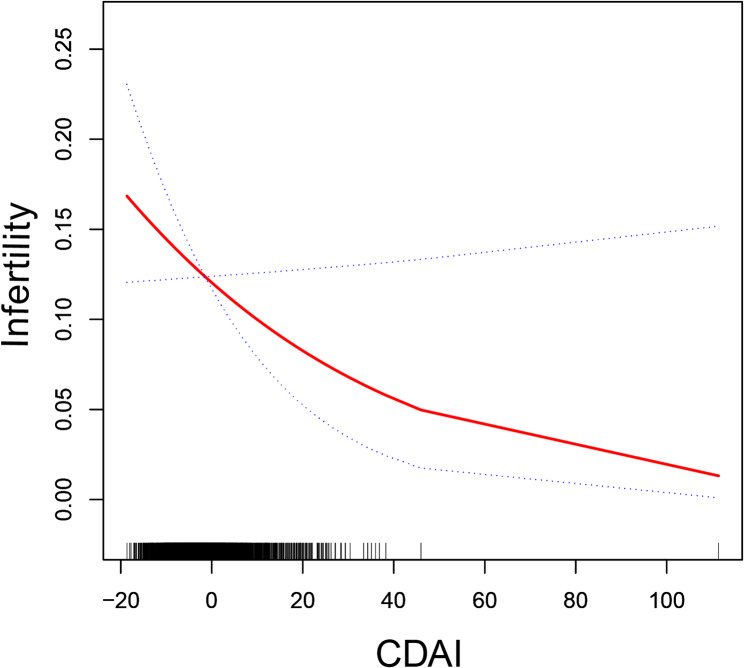
Table 2 shows the association between CDAI and infertility. In comparison to the lowest quartile of CDAI, participants with the highest quartile of CDAI exhibited a 44% reduction in the likelihood of developing infertility, which was statistically significant (OR = 0.56, 95% CI: 0.36–0.85; P = 0.0072), and the test for trend was also statistically significant (P for trend = 0.0235). The relationship between CDAI and infertility was further investigated with smoothed curve fitting, which showed negative nonlinear associations (Fig. 2).
Table 2.
Association of CDAI with infertility
| CDAI | OR(95%CI), P-value | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Q1 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.70 (0.52, 0.94) 0.0184 | 0.71 (0.53, 0.96) 0.0256 | 0.65 (0.47, 0.90) 0.0086 |
| Q3 | 0.83 (0.62, 1.10) 0.1941 | 0.84 (0.63, 1.12) 0.2467 | 0.76 (0.53, 1.07) 0.1122 |
| Q4 | 0.75 (0.56, 1.00) 0.0478 | 0.74 (0.55, 0.99) 0.0442 | 0.56 (0.36, 0.85) 0.0072 |
| P for trend | 0.1167 | 0.1073 | 0.0235 |
Model 1 adjusted for none
Model 2 adjusted for age and race
Model 3 adjusted for age, race, education level, PIR, energy, BMI, marital status, smoking status, hypertension, diabetes, age when first menstrual period occurred, ever treated for a pelvic infection/PID, ever use female hormones, ever been pregnant
Fig. 2.
Smooth curve fitting for CDAI and infertility
Subgroup analysis
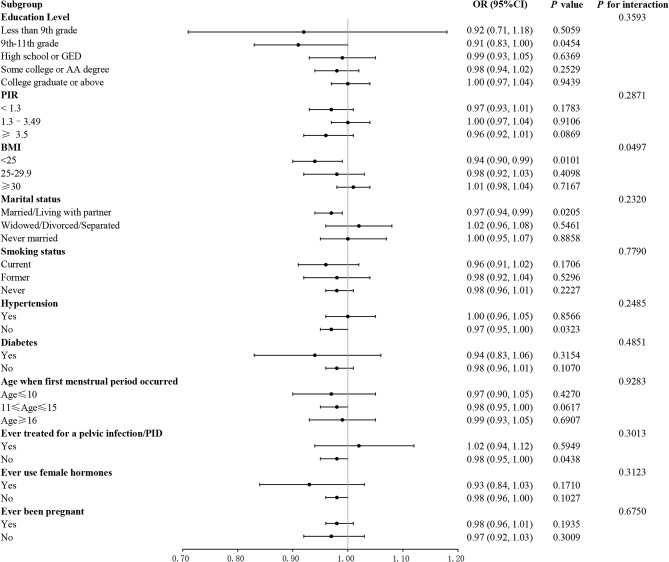
To further explore the factors associated with the association between CDAI and infertility risk, we performed subgroup analysis. All covariables in each subgroup analysis model were adjusted, with the exception of the stratified variables themselves. The results indicated a substantial inverse relationship between CDAI and infertility among individuals with grades 9–11 in education (OR = 0.91, 95% CI: 0.83–1.00), BMI < 25 kg/m2 (OR = 0.94, 95% CI: 0.90–0.99), who were married or living with a partner (OR = 0.97, 95% CI: 0.94–0.99), had no history of hypertension (OR = 0.97, 95% CI: 0.95–1.00) and had not been infected with pelvic inflammatory disease (OR = 0.98, 95% CI: 0.95–1.00) (all P < 0.05). The interaction test demonstrated that there was an interaction between BMI in the relationship between CDAI and infertility risk (P for interaction = 0.0497). The negative association between CDAI and infertility risk was more pronounced in those with a BMI < 25 kg/m2. Furthermore, no significant association was observed between the negative association and other factors, including education level, PIR, marital status, smoking status, hypertension, diabetes, age of menarche, previous treatment for pelvic infection, previous use of female hormones, and previous pregnancy (all P for interaction > 0.05) (Fig. 3).
Fig. 3.
Subgroup analysis of the association between CDAI and infertility
Discussion
In the cross-sectional study of 3,259 participants, we observed an inverse association between CDAI and infertility. Subgroup analyses showed that the association between CDAI and infertility risk was influenced by BMI. Our study suggest that increased intake of antioxidant-rich foods may help reduce the risk of infertility.
Effects of antioxidants on infertility
Research on the connection between diet and male fertility have been extensively studied in the past, while there have been few studies on the relationship between CDAI and female infertility. Nowadays, using dietary antioxidants to intervene female infertility has gradually gained attention. Previous research has indicated that antioxidants may be beneficial for women experiencing infertility. In a randomized controlled clinical experiment conducted by Safiyeh et al. on 70 infertility individuals with occult ovarian dysfunction, it was found that supplementation of selenium and vitamin E may decrease ROS overexpression and boost ovarian reserve [20]. In their study, Jurczewska et al. concluded that antioxidant vitamins (A, C, and E) and minerals have a positive effect on ovulation and fertility in women [21]. In a cross-sectional study involving 1,713 women, Ji et al. found that a high intake of vitamins A, C, magnesium, and other nutrients was associated with a lower incidence of female infertility [19]. In cross-sectional research, Adeniyi et al. observed serum zinc and selenium concentrations in Nigerian women with unexplained infertility were significantly lower than in fertile women [22]. In a case-control study, Kabodmehri et al. identified that the dietary antioxidant index was inversely related to the risk of infertility among Iranian women [23]. Consistent with previous research reports, this study shows that CDAI is inversely associated with the risk of infertility, suggesting that increased dietary antioxidant intake has a positive effect on infertility.
Effect of OS on infertility
OS is an important pathological process that leads to infertility. It has been demonstrated that OS can accelerate oocyte senescence by increasing the level of inflammatory factors, proteins, and lipid peroxidation, and disrupting vascular homeostasis, thereby reducing ovarian reserve function, potentially leading to infertility and other serious consequences [24]. Furthermore, excessive ROS may also exert an influence on the normal separation of chromosomes during oocyte meiosis, impair oocyte viability, and reduce the possibility of fertilization [12]. In addition, OS can also induce endometrial inflammation to make embryo implantation fail and hinder embryo development by altering gene expression [25]. OS is associated with various reproductive diseases, and excessive ROS in endometriosis may cause adhesions, impair uterine peristalsis, cause developmental inconsistencies between the endometrium and the embryo, and ultimately lead to infertility [26]. In patients with polycystic ovary syndrome, increased OS markers in oocyte follicular fluid lead to abnormal follicular growth and maturation, poor oocyte or embryo quality, and thus infertility [27]. Therefore, reducing the detrimental effects of OS on the female reproductive system is of great significance to prevent and improve the reduction of female fertility.
Dietary antioxidants can improve OS to increase pregnancy rates
Antioxidant vitamins and minerals can restore or maintain the oxidation-antioxidant balance in the blood and tissues and are essential for normal ovulation, maintaining pregnancy and reducing adverse pregnancy outcomes [21, 28]. According to research by Amini et al., vitamin C and E supplements can effectively reduce the expression level of OS markers in women with endometriosis [29]. Shi YQ et al. found that natural antioxidants such as vitamins C and E could delay the disease process of premature ovarian aging by reducing ROS levels in the body [24]. Ozkaya et al. observed that multivitamin and mineral supplementation can reduce OS levels in the serum and follicular fluid of women undergoing in vitro fertilization [30]. Numerous studies have confirmed that antioxidant supplementation can effectively prevent or assist in the treatment of infertility-related diseases and improve the pregnancy rate [31, 32]. The benefits of consuming natural antioxidants from food include their high safety and minimal side effects, garnering increasing interest in its application for treating infertility [33]. The CDAI is a well-established marker for evaluating the intake of dietary antioxidants, which mirrors an individual’s antioxidant status. Therefore, using the CDAI to assess a patient’s antioxidant capacity might be a more effective approach to assess the relationship between intake of antioxidant-rich food intake and infertility. In Model 3, the risk of infertility in the Q4 group was lower than that in the Q1 group, suggesting that higher CDAI had a positive effect on infertility. Subgroup analysis showed that this negative association was significant in people with grades 9–11, a BMI < 25 kg/m2, who were married or living with a partner, had no history of hypertension, and had not been infected with pelvic inflammatory disease. The results showed that for these participants, higher CDAI was associated with a lower risk of infertility. Interaction tests showed that the negative association between CDAI and infertility risk was more significant in those with a BMI < 25 kg/m2. However, education, PIR, marital status, smoking status, hypertension, diabetes, age at menarche, previous treatment for pelvic infection, previous use of female hormones, and ever having been pregnant were not dependent on the negative association between CDAI and infertility, suggesting that these negative associations were similar across populations. The results of this study complement and confirm the positive effect of higher CDAI on reducing the risk of infertility in the general population.
This study used NHANES data to make the study more reliable and representative. In addition, based on the information we could find, we included the largest sample size of previous research looking at the relationship between CDAI and the risk of infertility. Furthermore, we adjusted covariates related to exposure and outcome to ensure that our results are applicable to a broad population. However, this study has certain limitations. First, the cross-sectional research design of the NHANES made it impossible for us to establish a causal relationship between CDAI and the risk of infertility. Second, the NHANES database lacks information on the causes of infertility, and some infertile populations may be caused by factors unrelated to dietary intake, such as uterine malformations and tubal obstruction. It was also not possible to explore in depth the relationship between CDAI and infertility-related gynecological disorders, such as polycystic ovary syndrome and endometriosis. Third, the evaluation of an individual’s dietary intake relies on the mean of two 24-hour dietary recall interviews, and it could be subject to bias.
Conclusion
Our study shows that there is a negative non-linear correlation between CDAI and infertility among reproductive-aged women in the US, suggesting that increasing dietary intake of antioxidants may play a key role in reducing the risk of infertility. Further prospective studies are needed to verify our findings.
Acknowledgements
Special thanks to NHANES for providing public data. Thanks to all the staff and participants who participated in NHANES 2013-2020.
Abbreviations
- BMI
Body mass index
- CDAI
Composite dietary antioxidant index
- NHANES
National Health and Nutrition Examination Survey
- OS
Oxidative stress
- PID
Pelvic inflammatory disease
- PIR
Ratio of family income to poverty
- ROS
Reactive oxygen species
Author contributions
YS and ZWT designed the study. ZBD and JXC collected the data. ZWT and ZJY analyzed the data. YS edited the original manuscript. XHL reviewed and edited the manuscript. All the authors read and approved the final manuscript.
Funding
The study was funded by the Youth Science Foundation of the National Natural Science Foundation of China (81403428), the Scientific Research Project of the Administration of Traditional Chinese Medicine of Hebei Province (2024019), and the Natural Science Foundation of Hebei Province (H2024423019).
Data availability
The data used for this study are available at the NHANES online website (https://www.cdc.gov/nchs/nhanes/index.htm).
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the guidelines set forth in the Declaration of Helsinki and was approved by the Research Ethics Review Board of the National Center for Health Statistics. All NHANES participants provided written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Shen and Zhanwang Tan contributed equally to this work.
References
- 1.Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm. Org. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2020;113(3):533–5. 10.1016/j.fertnstert.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 2.Li HY, Liu YM, Dong L, Zhang RH, Zhou WD, Wu HT, Li YF, Wang YX, Wei WB. Global, regional, and national prevalence, disability adjusted life years, and time trends for refraction disorders, 1990–2019: findings from the global burden of disease study. BMC Public Health. 2019;21(1):1619. 10.1186/s12889-021-11648-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerrits T, Van Rooij F, Esho T, Ndegwa W, Goossens J, Bilajbegovic A, Jansen A, Kioko B, Koppen L, Kemunto Migiro S, Mwenda S, Bos H. Infertility in the global south: raising awareness and generating insights for policy and practice. Facts Views Vis ObGyn. 2017;9(1):39–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Barnhart KT, Introduction. Fertility as a window to health. Fertil Steril. 2018;110(5):781–2. 10.1016/j.fertnstert.2018.08.031 [DOI] [PubMed] [Google Scholar]
- 5.Teklemicheal AG, Kassa EM, Weldetensaye EK. Prevalence and correlates of infertility related psychological stress in women with infertility: a cross-sectional hospital based survey. BMC Psychol. 2022;10(1):91. 10.1186/s40359-022-00804-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, Virtamo J, Albanes D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004;160(1):68–76. 10.1093/aje/kwh173 [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Liu J, Sun J, Cui Y, Wu P, Wei F, Gao X, Ma T, Zhang X, Kuang X, Fan J. Composite dietary antioxidant index and the risk of heart failure: a cross-sectional study from NHANES. Clin Cardiol. 2023;46(12):1538–43. 10.1002/clc.24144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, Li T, Li J, Zheng D, Yang J, Zhuang X. Linear association of compound dietary antioxidant index with hyperlipidemia: a cross-sectional study. Front Nutr. 2024;11:1365580. 10.3389/fnut.2024.1365580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Li J, Chen T, Zhao X, Chen Q, Xiao L, Peng Z, Zhang H. Association between dietary antioxidant levels and chronic obstructive pulmonary disease: a mediation analysis of inflammatory factors. Front Immunol. 2024;14:1310399. 10.3389/fimmu.2023.1310399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reproductive biology and endocrinology: RB&E. 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, Li F. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. 2021;236(12):7966–83. 10.1002/jcp.30468 [DOI] [PubMed] [Google Scholar]
- 12.Aitken RJ. Impact of oxidative stress on male and female germ cells: implications for fertility. Reprod (Cambridge England). 2020;159(4):R189–201. 10.1530/REP-19-0452 [DOI] [PubMed] [Google Scholar]
- 13.Adeoye O, Olawumi J, Opeyemi A, Christiania O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist Reprod. 2018;22(1):61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devi N, Boya C, Chhabra M, Bansal D. N-acetyl-cysteine as adjuvant therapy in female infertility: a systematic review and meta-analysis. J Basic Clin Physiol Pharmacol. 2020;32(5):899–910. 10.1515/jbcpp-2020-0107 [DOI] [PubMed] [Google Scholar]
- 15.Bhardwaj JK, Panchal H, Saraf P. Ameliorating effects of natural antioxidant compounds on female infertility: a review. Reproductive sciences (Thousand Oaks, Calif.). 2021;28(5):1227–56. [DOI] [PubMed]
- 16.Jamilian M, Mansury S, Bahmani F, Heidar Z, Amirani E, Asemi Z. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res. 2018;11(1):80. 10.1186/s13048-018-0457-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Guo Q, Pei YH, Ren QL, Chi L, Hu RK, Tan Y. Effect of a short-term vitamin E supplementation on oxidative stress in infertile PCOS women under ovulation induction: a retrospective cohort study. BMC Womens Health. 2020;20(1):69. 10.1186/s12905-020-00930-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruder EH, Hartman TJ, Reindollar RH, Goldman MB. Female dietary antioxidant intake and time to pregnancy among couples treated for unexplained infertility. Fertil Steril. 2014;101(3):759–66. 10.1016/j.fertnstert.2013.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji X, Ye Y, Wang L, Liu S, Dong X. Association between nutrient intake and female infertility: a study based on NHANES database. J Obstet Gynaecology: J Inst Obstet Gynecol. 2023;43(2):2285025. 10.1080/01443615.2023.2285025 [DOI] [PubMed] [Google Scholar]
- 20.Safiyeh FD, Mojgan M, Parviz S, Sakineh MA, Behnaz SO. The effect of selenium and vitamin E supplementation on anti-mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: a randomized controlled clinical trial. Complement Ther Med. 2021;56:102533. 10.1016/j.ctim.2020.102533 [DOI] [PubMed] [Google Scholar]
- 21.Jurczewska J, Szostak-Węgierek D. The influence of diet on ovulation disorders in women-a narrative review. Nutrients. 2022;14(8):1556. 10.3390/nu14081556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adeniyi AA, Ogunbode OO, Adeyanju AS, Oladokun A. Serum copper, zinc and selenium levels in women with unexplained infertility in Ibadan Nigeria: a cross-sectional analytical study. Niger Postgrd Med J. 2023;30(4):269–74. 10.4103/npmj.npmj_144_23 [DOI] [PubMed] [Google Scholar]
- 23.Kabodmehri R, Javaheri FSH, Alami F, Mahmoudi Z, Amjadi A, Saeedirad Z, Omidi S, Sadeghi S, Hoseini MSM, Mohamadiyan Z, et al. Female infertility and dietary antioxidant index (DAI); a case-control study. BMC Womens Health. 2023;23(1):608. 10.1186/s12905-023-02747-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi YQ, Zhu XT, Zhang SN, Ma YF, Han YH, Jiang Y, Zhang YH. Premature ovarian insufficiency: a review on the role of oxidative stress and the application of antioxidants. Front Endocrinol. 2023;14:1172481. 10.3389/fendo.2023.1172481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaltsas A, Zikopoulos A, Moustakli E, Zachariou A, Tsirka G, Tsiampali C, Palapela N, Sofikitis N, Dimitriadis F. The silent threat to women’s fertility: uncovering the devastating effects of oxidative stress. Antioxid (Basel Switzerland). 2023;12(8):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13(1):126–34. 10.1016/S1472-6483(10)62026-3 [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui S, Mateen S, Ahmad R, Moin S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J Assist Reprod Genet. 2022;39(11):2439–73. 10.1007/s10815-022-02625-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathak P, Kapil U. Role of trace elements zinc, copper and magnesium during pregnancy and its outcome. Indian J Pediatr. 2004;71(11):1003–5. 10.1007/BF02828116 [DOI] [PubMed] [Google Scholar]
- 29.Amini L, Chekini R, Nateghi MR, Haghani H, Jamialahmadi T, Sathyapalan T, Sahebkar A. The effect of combined vitamin C and vitamin E supplementation on oxidative stress markers in women with endometriosis: a randomized, triple-blind placebo-controlled clinical trial. Pain Res Manag. 2021:5529741. [DOI] [PMC free article] [PubMed]
- 30.Ozkaya MO, Nazıroğlu M. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil Steril. 2010;94(6):2465–6. 10.1016/j.fertnstert.2010.01.066 [DOI] [PubMed] [Google Scholar]
- 31.Gharaei R, Mahdavinezhad F, Samadian E, Asadi J, Ashrafnezhad Z, Kashani L, Amidi F. Antioxidant supplementations ameliorate PCOS complications: a review of RCTs and insights into the underlying mechanisms. J Assist Reprod Genet. 2021;38(11):2817–31. 10.1007/s10815-021-02342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Florou P, Anagnostis P, Theocharis P, Chourdakis M, Goulis DG. Does coenzyme Q10 supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J Assist Reprod Genet. 2020;37(10):2377–87. 10.1007/s10815-020-01906-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vašková J, Klepcová Z, Špaková I, Urdzík P, Štofilová J, Bertková I, Kľoc M, Rabajdová M. The importance of natural antioxidants in female reproduction. Antioxid (Basel Switzerland). 2023;12(4):907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study are available at the NHANES online website (https://www.cdc.gov/nchs/nhanes/index.htm).