Abstract
Background
Globally, gastroenteritis (GE) significantly impacts children’s health and contributes to societal, economic, and health burdens. Previous studies reporting risk factors of GE in children in high-income settings mainly rely on outbreak investigations, which inherently capture only a fractional representation of the overall spectrum of GE occurrences. In addition, there is paucity of comprehensive information pertaining to modifiable risk factors of GE. This scoping review aims to synthesize existing evidence concerning modifiable and behavioural risk factors associated with GE among children in high-income countries.
Methods
PubMed, Embase, CINAHL, and Scopus were the databases from which articles were retrieved. A descriptive synthesis of the evidence was performed, following the Arksey and O’Malley scoping studies framework and enhanced by the Preferred Reporting Items for Systematic Reviews and Meta-analysis extension for Scoping Reviews checklist (PRISMA-ScR).
Results
The systematic search identified 13,395 journal articles, which were subsequently screened, and duplicates removed, resulting in 19 articles for inclusion in the review. The majority of these studies (63.2%) employed a case-control design and were predominantly conducted in community settings (68.4%). Factors such as parental literacy, contact with individuals exhibiting gastrointestinal symptoms, and nappy-wearing were identified as significantly associated with childhood GE within domestic environments. Childcare-related variables, including enrolment size, mixing of personnel between child groups, the presence of central cleaning stations, and the implementation of hygiene and disease prevention policies, showed significant association with GE. In addition, the presence of sand pits, paddling pools, and animals in childcare centers correlated with increased incidences of GE among attending children.
Conclusions
The scoping review reveals a complex and varied research landscape on factors influencing gastroenteritis (GE) for children in high-income countries. The findings suggest that while some variables are closely linked to specific pathogens, others may not be, highlighting variability across GE aetiology. The significant association between various household level and childcare-related factors and childhood GE points to a valuable direction for future research and public health intervention.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13690-024-01375-5.
Keywords: Modifiable, Behaviour, Factor, Gastroenteritis, Enteric pathogen, High-income, Scoping review
| Text box 1. Contributions to the literature |
|---|
| • It has been a challenge to have a single definition of gastroenteritis because there are many causes, symptoms, and terms used to describe it. |
| • This review clarifies the use of “gastroenteritis” as a unifying term in high-income countries, distinguishing it from related terms like diarrhea or gastric flu, infectious intestinal diseases thereby standardizing terminology in the literature. |
| • It also introduces the specific term “modifiable risk factors” in the context of gastroenteritis (GE) among children, providing a clear direction for future research and intervention strategies. |
Background
Infectious gastroenteritis (GE) is an illness caused by infection with pathogenic organisms such as viruses, bacteria, and parasites [1], that are transmitted via the faecal-oral route either directly through person-to-person contact or through contaminated food or water [2, 3]. Foodborne [4, 5] as well as zoonotic [6–8] transmission pathways also play significant roles. Most cases GE do not require medical attention [9]. However, some higher risk populations such as young children and malnourished or immunocompromised people could develop persistent diarrhoea [10].
GE accounts for approximately 89.5 million disability-adjusted life years (DALYs) and is ranked fifth among the major causes of DALYs worldwide [11]. It ranks as the 8th most common cause of death across all age groups, accounting for 1.65 million fatalities, and is the 5th leading cause of mortality in children under the age of five, resulting in 446,000 deaths. The majority of these burdens are in low- and middle-income countries (LMICs) [12, 13]. In high-income countries, GE is a common cause of illness, with incidence varying between 0.274 and 1.4 episodes per person per year (pppy). GE commonly leads to hospital admissions, particularly among young children [14]. However, the burden of GE is underreported as research shows individuals often don’t seek medical attention for mild-moderate GE symptoms [15, 16], medical practitioners don’t often refer patients for diagnostic testing [17, 18] and not all pathogens are subject to mandatory reporting through public health surveillance systems [19].
The incidence of GE varies among high-income countries because of variation in the population related to health literacy levels, dietary habits, health behaviour, and the structure of healthcare services [20–22]. Despite consistent underreporting of GE [23–26], it is estimated that there are still 179 million illnesses annually in USA [19], 16.6 million in Australia [27], 21 million in France [28], 10. 06 million in Belgium [29], 42.2 million in Germany [14], and 9.4 million in England [4]. In children, GE is a common reason for emergency department visits and hospitalizations [30]. In developed countries, children under 5 years old experience 1.0 to 2.5 episodes of diarrhea per child per year. Of these, 0.1 to 0.4 episodes result in attendance at a general-practice clinic, and 0.001 to 0.003 episodes lead to hospitalization [16, 31, 32]. The majority of the economic burden in high-income countries is associated with morbidity and financial impacts of illness (direct and indirect costs) [11, 33], imposing G significant monetary costs on communities, and controlling childhood GE would lead to considerable health-care cost savings worldwide [34].
Current knowledge of the epidemiology primarily relies on cross-sectional studies aimed at estimating the prevalence of GE and case-control studies utilized to examine outbreaks [35–39]. Determinants and associated sources are usually inferred from data collected during outbreaks [40], despite outbreaks accounting for less than 10% of all GE occurrences [41]. While accurate estimates of GE incidence and prevalence are crucial for the development of effective public health policies [11], identifying behavioural and modifiable risk factors enables the identification of barriers and facilitators to maximizing public health compliance and ultimately mitigating transmission and burden [42]. Better information on modifiable and behavioural risk factors would also facilitate the design of models to evaluate of the effectiveness of behavioural interventions [43].
The epidemiology of gastroenteritis varies between high-income countries (HICs) and low and middle-income countries (LMICs). In HICs, the burden of disease may be more closely linked to specific behavioural factors such as food handling practices, hygiene behaviours, and daycare attendance. In contrast, in LMICs, the disease burden may be more heavily influenced by broader structural issues such as access to clean water and basic sanitation [44–46]. Despite higher standards of living, better access to healthcare, advanced sanitation and hygiene infrastructure, and generally higher levels of public health awareness, gastroenteritis remains a huge societal burden in high-income countries [27, 45–47]. However, there is a paucity of comprehensive information on modifiable and behavioural drivers of gastroenteritis in HICs. Since high-income countries often have the resources to implement comprehensive public health campaigns and policy interventions, understanding these drivers in the context of HICs can inform more effective and tailored interventions that are appropriate for these settings.
The aim of this study was to carry out a comprehensive scoping review of modifiable and behavioural factors in domestic and childcare settings associated with gastroenteritis among children in high-income countries.
Methods
This review adhered to the five iterative stages of the Arksey and O’Malley scoping studies framework [48], namely setting the research questions, sourcing studies, selecting studies, recording data, and summarizing. It was further enhanced by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews (PRISMA-ScR) [49] checklist. Due to the current policy that prohibits the registration of scoping reviews on International Prospective Register Of Systematic Reviews (PROSPERO) [50], this review was not registered. Furthermore, the quality of studies was not evaluated.
Research question
The Population, Concept, and Context (PCC) framework was used to develop the research question. The population was identified as children aged 0–14 years in high-income countries, and the study settings encompass community, childcare, healthcare, and school. The research question involves factors associated with GE that are modifiable, defined as behaviours or set up that can be changed or manipulated in some way. Those modifiable factors could be at the individual (e.g. awareness, handwashing) or community (childcare facility design, disease control policy etc.) levels. There term gastroenteritis is used due to the inconsistent terminology found in the literature concerning gastrointestinal conditions such as gastroenteritis, diarrhoea, intestinal infectious disease, etc. The scoping review aimed to address the fundamental research question: “What does the existing body of literature reveal about the modifiable and behavioural factors linked to gastroenteritis in children from high-income countries? and to what extent do the factors differ with aetiology?”
Information source and search strategy
Search terms used included “risk factors”, “epidemiology”, “transmission”, “enteric, pathogen”, “gastroenteritis”, “diarrhoea”, “diarrhea”, “cryptosporidiosis”, “campylobacter”, “giardia”, “child”, “infant”, “preschool”, “developed country”. A search strategy was developed using the search terms, comprising a combination of MeSH terms and title/abstract searches. Boolean operators “AND” and “OR” were employed to refine the search strategy, ensuring relevance and comprehensiveness. Search strategies were performed using PubMed, EMBASE, EBSCO for Medline, CINAHL, and Scopus.
Search results from each database was imported into EndNote (EndNote 21.0.1 for Windows, Clarivate Plc) and subsequently transferred to Covidence to eliminate duplicates, screen studies, and extract data. An initial search was performed in July 2023 and updated in November 2023 to capture any new relevant studies. No new article was found. The full search strategy is provided in a supplementary file (Supplementary material 1).
Study selection
Screening of title and abstract of each paper were conducted within Covidence. Studies were included if they presented information on modifiable or behavioural factors associated with sporadic (non-outbreak) GE. They included household factors (e.g., parental literacy, hygiene practices), characteristics of childcare facilities (e.g., facility design, hygiene protocols, policies regarding exclusion of ill children), and factors such as exposure to and interactions with animals, and recreational activities. Inclusion criteria for the studies encompassed those published in English, irrespective of the publication date.
Studies solely focused on epidemiological investigation and factors not categorized as behavioural or modifiable were excluded from the review. In addition, studies investigating risk factors solely within community-wide or population-based outbreaks of gastroenteritis were excluded, as such outbreaks represent only a subset of GE occurrences.
Data charting process
A customized data charting form, adapted from the Joanna Briggs Institute (JBI) scoping review data extraction tool, was used for the extraction of data from the selected studies. This form encompassed the following principal elements:
Author and publication year.
Country of origin.
Study design.
Study setting.
Study population.
Pathogen/Gastrointestinal condition reported.
Outcome (Modifiable risk factors).
Significance.
Collation, summarization, and report of results
This constitutes the conclusive phase of the Arksey and O’Malley’s methodological framework, wherein articles underwent compilation and synthesis based on predetermined categories. Extracted data were systematically summarized into distinct categories, including household characteristics, childcare attributes, recreational activities, and interactions with animals. Furthermore, factors demonstrating significant associations with GE were elucidated, accompanied by their respective measures of association for clarity and interpretability.
Results
Study characteristics
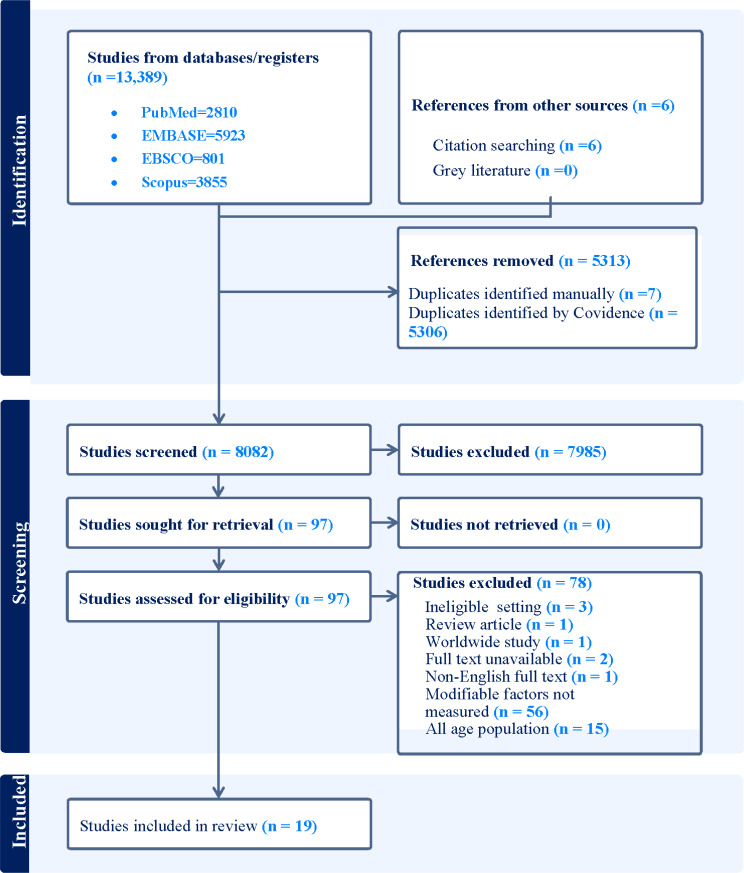
We initially retrieved 13,395 studies from various databases: PubMed (2,810), EMBASE (5,923), EBSCO for Medline and CINAHL (801), Scopus (3,855), and through citation searching (6). Once duplications were removed, 8,082 studies were screened by title and abstract. Of these, 7,985 were deemed irrelevant and excluded, leaving 97 studies for full-text review. After this review, 78 studies were further excluded due to various reasons, as illustrated in Fig. 1. Finally, 19 studies were selected for inclusion in the review (Fig. 1).
Fig. 1.
PRISMA-ScR flow chart for study selection process for a review of a review of modifiable and behavioural factors associated with childhood gastroenteritis in high-income countries, 1990–2018. Initially, 13,395 studies were found across four databases and citation searches. After removing duplicates (5313), 7985 more were excluded based on irrelevant titles and abstracts. 97 articles underwent full text review, with 78 being excluded. Reasons for exclusion included lack of consideration for modifiable and behavioural factors (56 articles), absence of subgroup analysis for all-age group participants (15 articles), and other factors such as ineligible study settings, lack of full-text availability, non-English texts, and review articles
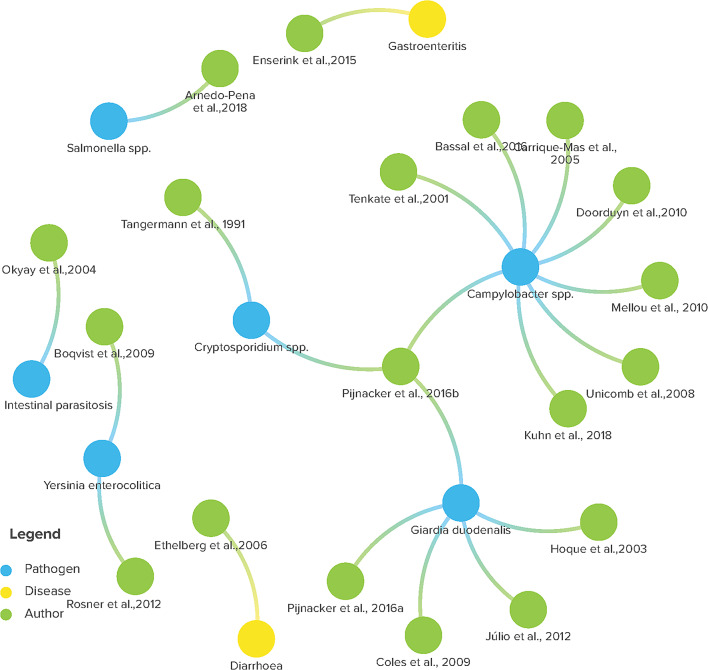
The majority of the included studies were case control design (63.2%), based in community settings (68.4%) and were published in last 15 years (60%). The majority of included studies featured children as research participants, although a handful included individuals of all ages with subgroup analysis of risk factors of GE (Table 1). Figure 2 below presents the specific citations, including the authors and the year of publication, as well as the reported enteropathogens or gastrointestinal conditions. Campylobacter spp. and G. duodenalis rank as the pathogens most commonly reported, with Cryptosporidium species being the next most frequent.
Table 1.
Summary of studies on modifiable and behavioural factors associated with childhood gastroenteritis in high-income countries, 1990–2018
| Characteristics | Frequency (n = 19) | ||
|---|---|---|---|
| % | n | ||
| Publication year | 1990–2000 | 5.3 | 1 |
| 2001–2010 | 52.6 | 10 | |
| 2011-present | 42.1 | 8 | |
| Country | The Netherlands | 21.1 | 4 |
| Australia | 10.5 | 2 | |
| Denmark | 10.5 | 2 | |
| Israel | 10.5 | 2 | |
| Sweden | 10.5 | 2 | |
| Germany | 5.3 | 1 | |
| Greek | 5.3 | 1 | |
| New Zealand | 5.3 | 1 | |
| Portugal | 5.3 | 1 | |
| Spain | 5.3 | 1 | |
| Turkey | 5.3 | 1 | |
| United States of America | 5.3 | 1 | |
| Study design | Case-control | 63.2 | 12 |
| Cross-sectional | 21.1 | 4 | |
| Cohort | 10.5 | 2 | |
| Case-series | 5.3 | 1 | |
| Population/sample | Children | 84.2 | 16 |
| All age group | 15.8 | 3 | |
| Setting | Community | 68.4 | 13 |
| Childcare facilities | 21.1 | 4 | |
| Healthcare facilities | 5.3 | 1 | |
| School | 5.3 | 1 | |
| Enteropathogen/ condition studied | Campylobacter | 42.1 | 8 |
| Giardia | 26.3 | 5 | |
| Cryptosporidium | 10.5 | 2 | |
| Yersinia | 10.5 | 2 | |
| Salmonella | 5.3 | 1 | |
| Gastroenteritis | 5.3 | 1 | |
| Diarrhoea | 5.3 | 1 | |
Fig. 2.
Diagram illustrating relationships between various microorganisms/gastrointestinal conditions indicated by interconnected coloured nodes labelled with organism names and corresponding authors and year for a review of modifiable and behavioural factors associated with childhood gastroenteritis in high-income countries, 1990–2018 (Image is created using Kumu, data visualization and exploration platform)
Household factors
Parental literacy
Five included studies reported testing for parental literacy (48, 49, 50, 51, 52), and low parental literacy status showed significant association with increased rates of GE in two studies: childhood diarrhea [51], and giardiasis [52] (Table 2). The odds of giardiasis were about 4.5 times higher in children of uneducated mothers compared to those with educated mothers. Similarly, children of uneducated fathers were 12 times more likely to have symptomatic giardia infection compared to children whose fathers were educated [52]. According to Ethelberg et al. the odds of diarrhea were 1.39 times higher in children of parents with ten years or less of basic schooling compared to those with more than 10 years of basic schooling [51].
Table 2.
Socio-demographic, hygiene, and toileting behaviours associated with gastroenteritis among children in high-income countries, 1990–2018
| Risk factors | Pathogen/GI condition | Study citation | Significance |
|---|---|---|---|
| Parental factors | |||
| Mother’s literacy | G. duodenalis | [52] | OR = 4.49; 95% CI: 1.20-16.84 |
| Father’s literacy | G. duodenalis | [52] | OR = 12.26;95% CI: 4.08–36.82 |
| Parent literacy | G. duodenalis | [63] | OR = 1.94; 95% CI: 0.89–4.20, p = 0.93 |
| Diarrhoea | [51] | OR=; 95% CI: 1.39 (1.11–1.81) | |
| Mother’s literacy | Intestinal parasite | [62] | X2 = 4.44, p = 0.035 |
| Contact with ill person | |||
| Contact with a person in household having gastroenteritis | Giardia | [56] | OR = 11.3, 95% CI: 2.6–35.7; P < 0.001 |
| Campylobacter | [57] | OR = 9.0, 95% CI: 1.10–72.5 | |
| Contact with symptomatic persons in childcare centres | Gastroenteritis | [55] | OR = 2.19, 95% CI:1.64–2.92 |
| Diarrhoea | [51] | OR = 2.19, 95% CI:1.64–2.92 | |
| Contact with persons with gastroenteritis symptoms “in other places” | Campylobacter | [54] | OR = 1.5, 95% CI: 1.10–1.90 |
| Hygiene and toileting behaviour | |||
| Lack of toilet training and nappy wearing | Campylobacter | [58] | aMOR = 7.36, 95% CI: 1.66–32.70, p < 0.01 |
| Nappy wearing | Giardia duodenalis | [61] | OR = 3.0, P < 0.05 |
| Cryptosporidium | [61] | aOR = 1.4, 95% CI: 0.4-7.0 | |
| Lack of toilet training | Cryptosporidium | [61] | aOR = 2.9, 95% CI:0.90–9.20 |
| Giardia duodenalis | [56] | OR = 3.8, 95% CI: 1.80–7.80, p < 0.001 | |
| Washing anal area by hand after defecation | Intestinal parasite | [62] | X2 = 5.50, p = 0.019 |
** Univariate analysis only; GI condition = Gastrointestinal condition
Contact with symptomatic person
The association between contact with a person with gastroenteritis and the risk of gastroenteritis was investigated in six studies [51, 53–57]. Five of studies found a significant association [51, 54–57], while the remaining study did not reveal any association [53]. Among the five studies that found a significant association, two of them revealed a higher risk of GE among children who had contact with persons with gastroenteritis in childcare settings [51, 55], while the other two showed that household member contact increased the risk of acquiring GE [56, 57]. According to Ethelberg et al. the odds of viral diarrhea in children who had contact with symptomatic individuals were respectively 5.8 and 4.1 times higher when contact occurred “in daycare” and “other places” compared to contacts “at home” [51]. The remaining study showed that children who had contact with a symptomatic person outside the household were at higher odds of GE [54] (Table 2).
Hygiene, sanitation, and toileting
Five included studies investigated hygiene-related factors associated with GE. The variables studied include toileting and diaper use, kitchen hygiene, handwashing after pet contact, and handwashing before eating [54, 57–60]. The studies reported inconclusive findings regarding hygiene, specifically kitchen hygiene and handwashing. However, significant associations between GE and diaper use, and the protective role of toilet training were observed in two studies [58, 61]. According to Bassal et al. the odds of Campylobacter infection were 7.4 times higher in diapered children compared to non-diapered ones [54]. Similarly, Tangermann et al. reported 2.9 higher odds of cryptosporidiosis in non-toilet trained daycare children compared to the trained ones [61] Handwashing after pet contact did not reduce the risk of GE in two studies [59, 60]. Similarly, handwashing before eating was not found to be associated with reduced incidence of GE according to Tenkate et al. [60]. Two studies reported contradictory findings regarding kitchen hygiene. While Doorduyn et al. reported significantly higher odds of Campylobacter infection in the absence of knife cleaning after use with raw meat and other foods [54], poor cutting board practices were unexpectedly associated with a decreased incidence of campylobacteriosis in the second study [57]. According to Tenkate et al. there was no significant association between washing utensils between use with raw and cooked food and Campylobacter infection [60] (Table 2).
In three included studies nappies wearing showed significant association with GE incidence. The odds of developing giardiasis [56], cryptosporidiosis [61], and campylobacteriosis [58] were, respectively, 3, 1.4, and 2.14 times higher among diapered children than toilet-trained children. In contrast, children who were toilet-trained had a reduced risk of giardiasis in comparison to those who were not toilet-trained or only partially toilet-trained (OR = 3.8, 1.8–7.8; P < 0.001) [56]. Another study showed a higher likelihood of intestinal parasitosis in children who did not use toilet paper or who used water to clean their anal area after defecation [62] (Table 2).
Childcare facility characteristics
Enrolment size
Two studies investigated the relationship between childcare enrolment size (capacity) and the risk of gastroenteritis. According to Enserink et al. the median capacity of childcare centers showed a statistically significant association with higher incidence rate for all-cause gastroenteritis and norovirus infection, indicating that a 1% increase in overcrowding rates increased the risk of gastroenteritis by 0.7% (aIRR1·007,95%CI1·003–1·011). Similarly, Pijnacker et al. reported 3 times higher odds of norovirus infection in childcare centers with higher capacity compared to those with lower capacity [63] (Table 3).
Table 3.
Childcare centre characteristics associated with gastroenteritis among children in high-income countries, 1990–2018
| Risk factors | Pathogen/GI condition | Study citation | Significance |
|---|---|---|---|
| Childcare capacity | All-cause gastroenteritis | [55] | aIRRs = 1.001, 95% CI: 1.001–1.003 |
| Norovirus | [55] | aIRRs = 1.001, 95% CI: 1.001–1.002 | |
| [64] | aOR = 2·40, 95% CI: 1·61–3·56 | ||
| Personnel mixing between child groups | Rotavirus | [55] | aIRRs = 1·80, 95% CI: 1·00–3·60 |
| GAB | [65] | aOR = 4.70, 95% CI: 2.80–8.10 | |
| Staff multitasking | C. hominis | [55] | aIRRs = 2·70, 95% CI: 1·00–7·70 |
| Sandpits | Norovirus | [55] | aIRRs = 1·90, 95% CI:1·30–2·80 |
| Paddling pools | GUA | [65] | aOR = 2.40, 95% CI: 1.10–5.10 |
| Norovirus | [55] | aIRRs = 1·50, 95% CI:1·30–1·90 | |
| [64] | OR = 4·30, 95% CI: 2·27–8·15 | ||
| G. lamblia | [64] | OR = 2·03, 95% CI:1·16–3·55 | |
| Central diaper changing area | GAB | aOR = 3.60, 95% CI 1.70–7.60 | |
| Central laundry room | GAB | [65] | aOR = 2.30, 95% CI:1.10–4.90 |
| Using chlorine tablets to clean vomit | G. lamblia | [55] | aIRRs = 0.10, 95% CI: 0.04–0.30 |
| G. lamblia | [64] | aOR = 0.29, 95% CI: 0.11–0.76 | |
| Using paper towels and detergent for vomit cleanup | All-cause gastroenteritis | [55] | aIRRs = 0.6, 95% CI: 0.40–0.70 |
| Daily cleaning of toys | Astrovirus | [55] | aIRRs = 0.5, 95% CI: 0.20–0.90 |
| Cryptosporidium | [64] | aOR = 0.27, 95% CI: 0.08–0.92 | |
| Cleaning kids’ potties in a normal sink | Rotavirus | [55] | aIRRs = 1·90, 95% CI: 1·20–3·00 |
| Daily cleaning of bed linens | Norovirus | [55] | aIRRs = 0.70, 95% CI: 0.50–0.90 |
| Cleaning kids’ potties at a disposal area | Norovirus | [64] | aOR = 0·37, 95% CI: 0·20–0·69 |
| Disease control policies during a suspected outbreak | |||
| Extra cleaning of toys | G. lamblia | [55] | aIRRs = 0.40, 95% CI: 0.20–0.70 |
| [64] | aOR = 0.25, 95% CI: 0.11–0.60 | ||
| Norovirus | [64] | aOR = 0.54, 95% CI: 0.31–0.94 | |
| Excluding personnel with gastroenteritis | G. lamblia | [55] | aIRRs = 0.50, 95% CI: 0.30–0.80 |
| All-cause gastroenteritis | [55] | aIRRs = 0.50, 95% CI: 0.40–0.70 | |
| Excluding children with gastrointestinal symptoms | Cryptosporidium | [64] | aOR = 0.09, 95% CI: 0.02–0.37 |
| Not mixing of staff members in child groups | Norovirus | [55] | aIRRs = 0.60, 95% CI: 0.50–0.90 |
| Separate toilet for ill children | Rotavirus | [55] | aIRRs = 0.40, 95% CI: 0.20–0.80 |
GAB, Giardia Assemblage B, GAA, Giardia Assemblage A, GUA, Giardia Unknown Assemblage
Mixing of personnel among child groups
Enserink et al. found a significant association between personnel mixing and staff multitasking with rotavirus infection, and Cryptosporidium hominis (C. hominis) infection, respectively, [55], while Pijnacker et al. noted a significantly increased occurrence of GAB infection [64]. Enserink et al. reported 1.8 times higher adjusted incidence rate rations (aIRRs) of rotavirus infection in children attending childcare centres where there is mixing of staff among child groups. Similarly, the aIRRs of C. hominis infection increased by 2.7 times higher when children attended childcare centers where staff handle multiple daily duties [55]. Conversely, the other study found no significant association for personnel mixing and GE [63] (Table 3).
Hygiene and disease control policies
Enserink et al. found that certain childcare hygiene practices like using chlorine tablets for cleaning and daily toy cleaning were associated with lower rates of gastroenteritis. The aIRRs in G. lamblia in children decreased by 10% when childcare centres use chlorine tablets for cleaning. Daily cleaning of toys on the other hand decreased the aIRRs in astrovirus in children by 50%. The aIRRs of rotavirus infection decreased by 40% when ill children were provided with separate toilets during a suspected outbreak. When mixing of staff members between child groups was avoided during a suspected outbreak, the aIRRs of norovirus decreases by 60%. However, cleaning vomit with paper towels alone increased the aIRRs of norovirus by 30% [55] (Table 3).
Pijnacker et al. supported this, showing that cleaning child potties in designated areas and using chlorine-based products for vomit reduced norovirus occurrences. They also found that extra toy cleaning during outbreaks and excluding symptomatic children decreased infections (Table 3). However, no significant links were found between gastroenteritis and hand or food hygiene or routine kitchen cleaning [63].
Facility design
Sandpits in childcare centres were found to significantly increase the incidence of G. lamblia, norovirus, rotavirus, astrovirus infections [55, 63, 64]. In fact a significant association was demonstrated only when sandpits co-exist with cats in one of the studies [64] (Table 3).
Paddling pools were also identified to be significantly associated with norovirus and G. lamblia infections [55, 63] (Table 3).
Central cleaning stations, like diaper-changing areas and laundry rooms, were linked to GAB infection [64]. A central diaper-changing area was also linked to higher rates of all-cause gastroenteritis [55]. However, central waste disposal stations and cleaning child potties in a dedicated sink were associated with lower rates of norovirus infection [63] (Table 3).
Childcare ownership of animals was associated with was associated with increased incidence of all-cause gastroenteritis [55]. In the other studies, the significance couldn’t be demonstrated when presence of sandpits was controlled [63, 64].
Miscellaneous exposure
Interaction with animals
Owning dogs [59, 65], farm animal ownership [54], visiting farms [54], and exposure to animal faeces [65] were linked to higher rates of Campylobacter infection. Symptomatic giardiasis was associated with exposure to chicken or livestock at home [66]. Children in contact with dogs or cats had increased odds of Y. enterocolitica infection [67] (Table 4).
Table 4.
Recreational and animal contact behaviours associated with gastroenteritis among children in high-income countries, 1990–2018
| Risk factors | Pathogen/GI condition | Study citation | Significance |
|---|---|---|---|
| Interaction with domestic animals | |||
| Contact with dogs or cats | Y. enterocolitica | [67] | aMOR = 3.10 95% CI:1.20–8.30 |
| Having a dog | Campylobacter | [59] | aOR = 3.80, 95% CI: 1.50–9.70, p < 0.001 |
| Campylobacter | [66] | OR = 3.80, 95% CI: 1.10–13.10 | |
| Exposure to chicken or livestock | G. duodenalis | [63] | aOR = 4.36, 95% CI: 1.62–11.70, p = 0.004 |
| Ownership of farm animals | Campylobacter | [54] | OR = 2.40, 95% CI: 1.40–4.40 |
| Visiting farm animals | Campylobacter | [54] | OR = 2.80, 95% CI:1.20–6.40 |
| Contact with animal faeces | Campylobacter | [66] | OR = 62.40, 95% CI: 8.20–472.60 |
| Recreational behaviours | |||
| Playing in soil | S. typhimurium | [53] | aOR = 3.00, 95% CI:1.10–8.17, p = 0.032 |
| Playing in garden | Campylobacter | [68] | OR = 1.83, 95% CI:1.05–3.19, p = 0.033 |
| Playing in a sand box | Y. enterocolitica | [69] | aOR = 1.70, 95% CI:1.30–2.40; p = 0.001 |
| Bathing in paddling pools | Campylobacter | [66] | OR = 13.6,95% CI: 1.90–97.00 |
| Swimming in rivers | G. lamblia | [56] | OR = 4.40, 95% CI:1.20–15.70 |
| Eating at swimming pools | Cryptosporidium | [56] | OR 6.46, 95% CI: 2.60-16.05; p < 0.01 |
| Using the restrooms at pools | Cryptosporidium | [56] | OR 3.40; 95% CI: 1.42–8.17; p < 0.01 |
Recreational behaviours
Playing in the garden [68], soil [53], and sandbox was linked to higher rates of C. jejuni, Salmonella typhimurium (S. typhimurium), and Y. enterocolitica infections, respectively [69] (Table 4).
Bathing in paddling pools [65], swimming in rivers [56], and using public swimming pools, including eating and restroom use [56] were associated with Campylobacter, G. lamblia and Cryptosporidium infections, respectively (Table 4).
Discussion
This scoping review aimed to chart the landscape of research on modifiable factors linked to childhood gastroenteritis in high-income countries, as well as the degree to which these factors vary across the aetiologic agents of gastroenteritis. The study pinpointed several modifiable factors within home settings and childcare centers. Maternal literacy and the facility design of childcare centers have shown a consistent significant association with the occurrence of gastroenteritis. Nonetheless, it is important to highlight that the importance of these factors does not remain uniform across the different aetiologies. The review identified that parental literacy, in particular maternal literacy is associated with an increased risk of GE is likely to occur through direct and indirect effects [70]. Low levels of maternal literacy have been associated with poorer health and nutrition outcomes in children [62, 71]. This is consistent with previous studies that have demonstrated an inverse correlation of parental literacy and childhood infections including those that are responsible for gastroenteritis [71–73]. The higher odds of G. lamblia infection among children of mothers with lower literacy could be because maternal literacy can impact child health through differences in the utilization of health services, and acquisition of positive childcare behaviours [74], adoption of personal health practices, and understanding the consequences of unhealthy lifestyles [75]. Furthermore, literacy level also influences disease knowledge, transmission awareness, and attitudes toward disease control [76]. Higher knowledge leads to better adherence to protective behaviours, indicating a positive link between literacy, awareness, and compliance with preventive measures [77, 78]. Considering the significant impact of enhanced maternal literacy on reducing childhood gastrointestinal infections (GE [79], health promotion initiatives that target maternal literacy improvement would be advantageous for disease control programs.
Our review reports that Giardia lamblia/duodenalis (G. lamblia/duodenalis) spreads person-to-person, as seen in cases where contact with another infected person is a significant factor [56]. This is backed by observations of higher numbers of GE cases where there’s no common infection source [80]. Additionally, infection is more common in younger children with poor personal hygiene and toilet training [80]. The low infectious dose of as few as 10 cysts supports the idea that transmission can occur through contaminated fingers or fomites [81]. Similarly, the association between Campylobacter infections and contact with someone who has gastroenteritis in our review suggests that person-to-person transmission might be more common than previously thought [54, 57]. Studies comparing the rate of ant-C. jejuni and C. pylori in institutionalized patients versus healthy controls have found significantly higher antibody rates in patients [82]. Additionally, endoscopy workers, who were more likely to be in close contact with infected patients also show an increased incidence of C. pylori infection compared to general practitioners [83], supporting the idea of person-to-person transmission of Campylobacter infections. Therefore, preventive measures against Campylobacter infections should consider the person-to-person transmission pathway, which is often overlooked.
Our review shows nappy use in young children poses risks for campylobacteriosis [58], cryptosporidiosis [61], and giardiasis [56]. Children wear nappies in their first 2–3 years of life, [84] which is the age most episodes of gastroenteritis peak. Moreover, staff combining nappy changing and food preparation duties might spread infections to other children directly or through food contact [85]. Improper disposal of nappies could also contribute to the persistence of diarrheal illnesses in the home environment [86]. Conversely, it was shown that using specialized diapering equipment together with improved carer knowledge, skills, and awareness significantly reduced diarrheal illness in children [84]. These hygiene practices are also concern in childcare settings. This review also shows that Giardia assemblage B (GAB) infections were associated with dedicated nappy changing in a childcare centre. This could highlight the role of centralizing diaper changing areas in cross infection of children having their diapers changed, as these centralized areas could serve as a ‘hub’ for pathogens [55, 87]. Moreover, the anthroponotic transmission tendency of GAB infections compared to Giardia assemblage A (GAA) infections [64] could result in person-to-person spread in contaminated environments. Similarly, the significant association between having central laundry room in a childcare centre and GAB infection [64] and diarrhoea [55] could be related to the hub hypothesis. Additionally, childcare personnel may contract Giardia by handling or washing infected clothes, and in turn infect susceptible children [64]. Therefore, it is advisable to take extra precautions regarding the hygiene standards of these designated areas when a childcare center opts to centralize nappy-changing operations.
Several characteristics of childcare centers have been linked to different enteropathogen infections. Specifically, a larger number of children in a childcare setting was significantly associated with increased prevalence of norovirus, underscoring the virus’s significant potential for person-to-person transmission [55, 63]. The extent to which a child’s likelihood of encountering infectious agents within the childcare environment is determined predominantly by the facility’s size and the level of crowding therein [88]. Increased capacity could result in increased child/staff ratio and mixing of staff between child groups. This could potentially decrease the ability of staff to monitor hygiene closely, consequently raising the risk of children being exposed to prevalent enteropathogens [89] through the chances of contact between staff and toilet untrained children, leading to cross contamination. Despite existing guidelines for caregiver/child ratio, there are inconsistencies of compliance. For example, in Scandinavia, staff that may not directly care for children (e.g., preschool managers, cleaners, and others) are included in the calculation of the average caregiver/child ratio. This in turn affects the number of staff that are directly involved in childcare [90].
Temporary exclusion ill children from a childcare centre reduces GE, but implementing this policy depends on factors like beliefs about its effectiveness, staff availability, parental work pressure, and concerns about exposure [91]. Not all childcare centres have such policies [92, 93], and even if they do, they may lack clear procedures to implement them (e.g. in-house ill-child standard operational procedures (SOPs) [94]. Moreover, directors sometimes rely on personal judgment to exclude ill children, leading to inconsistencies and confusion [91, 94]. Working parents may also struggle to comply due to employment concerns, as their absence to care for their ill child may jeopardize their employment [55, 95]. Strict exclusion policies might inadvertently lead parents to enrol their sick children in childcare centers that have less stringent disease control protocols [96] .
The significant association of sandpits in the incidence and clustering of enteropathogens [55, 63] could be due to the contamination of sandpits with animal faeces (including free-ranging cats and other animals), or children during vomiting or faecal accidents [55, 97]. The idea that sandpits could be contaminated by animals can be strengthened by the fact that strong statistical association was observed for gastroenteritis when sandpits co-exist with animals in the childcare centre [63, 64]. For some of the enteropathogen infections, this association was lost when animal ownership was controlled [55]. Similarly, presence in the childcare centres of paddling pools was also found to posing significant risk of acquiring GE in children, mainly G. lamblia [63] and norovirus [55, 63]. Evidence has shown that recreational water acts as a vehicle for anthroponotic transmission of several waterborne enteropathogens [98]. Additionally, many of the enteropathogens survive longer in wet environment that accommodates viability of the extra-corporeal forms [56, 65, 69].
Animal contact represents another factor associated with an increased incidence of Campylobacter [54, 59, 65], Giardia [66], and Yersinia enterocolitica (Y. enterocolitica) [67] infections in children. Giardia has been identified in the faeces of livestock [99], and epidemiological evidence has indicated a correlation between animal exposure and giardiasis [100, 101]. The correlation between participation in activities involving soil and gardens and the occurrence of GE, as demonstrated in studies by Arnedo-Pena et al. and Mellou et al. may be ascribed to the potential contamination of soil and gardens with animal faeces [53, 68]. The substantial increase in the risk of enteropathogen infection associated with contact with animals’ faeces has already been substantiated [65]. Consequently, children engaging in such contact with animals should be under adult supervision. Emphasizing the importance of adhering to proper hygiene measures during and after contact with animals and their faeces, especially for children is imperative [65].
Limitations
The scoping review only included studies published in English, which means important literature in other languages might have been left out. Also, the focus was on gathering existing evidence rather than rigorously assessing the quality of the studies included. This lack of formal assessment increases the risk of bias in the included studies, potentially impacting how the results are interpreted.
Conclusion
The scoping review highlights the diverse landscape of research on modifiable factors associated with gastroenteritis, shedding light on the complex interplay between various pathogens, gastrointestinal conditions, and measured variables. While heterogeneity exists across studies, key insights emerge regarding the significant impact of factors within domestic environments and childcare settings.
Parental literacy in domestic settings and facility design in childcare settings stand out as particularly influential factors in the rate of GE. However, the review underscores the nuanced nature of these associations, with certain variables exhibiting significant correlations with specific pathogens while not with others. Despite variations in findings, the review identifies a range of potential modifiable factors contributing to gastroenteritis in both domestic and childcare settings. From literacy levels and hygiene practices to facility design and policy implementation, there exists a broad spectrum of variables that can be targeted for intervention.
Moreover, the consistency of findings regarding the association between parental literacy, particularly maternal literacy, and gastroenteritis suggests a promising avenue for future research. Understanding community awareness, perceptions, and preventive practices related to gastroenteritis can further elucidate the intricate relationship between literacy and public health outcomes, informing more targeted interventions. Moving forward, engaging with stakeholders to gather their perspectives on the issue of gastroenteritis will be crucial in developing effective policies and interventions. By incorporating diverse viewpoints and aligning strategies with community needs, public health efforts can be more responsive and impactful in mitigating the burden of GE and improving overall community health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- DALYs
Disability-adjusted life years
- GAA
Giardia assemblage A
- GAB
Giardia assemblage B
- GE
Gastroenteritis
- IP
Intestinal parasitosis
- JBI
Joanna Briggs Institute
- PCC
Population, Concept, and Context
- PRISMA-ScR
Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension for Scoping Reviews
- PROSPERO
Prospective Register Of Systematic Reviews
Author contributions
MA and AR performed qualitative synthesis of the findings and wrote core text of the manuscript. SR, YA and YL were involved in editing and reviewing the manuscript. All authors reviewed the manuscript and gave consent for publication.
Funding
The authors received no specific funding for this work.
Data availability
The data supporting the study’s conclusions can be found within the supplementary files, while all other data is presented in the results section.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petri WA Jr., Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118(4):1277–90. 10.1172/JCI34005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher SM, McLaws ML, Ellis JT. Prevalence of gastrointestinal pathogens in developed and developing countries: systematic review and meta-analysis. J Public Health Res. 2013;2(1):42–53. 10.4081/jphr.2013.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert GL. Improving foodborne disease surveillance in NSW. N S W public health bulletin. 2008;19(1):1. [DOI] [PubMed]
- 4.Flint JA, Van Duynhoven YT, Angulo FJ, DeLong SM, Braun P, Kirk M, et al. Estimating the Burden of Acute Gastroenteritis, Foodborne Disease, and Pathogens commonly transmitted by Food: An International Review. Clin Infect Dis. 2005;41(5):698–704. 10.1086/432064 [DOI] [PubMed] [Google Scholar]
- 5.Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5(5):607–25. 10.3201/eid0505.990502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter PR, Thompson RCA. The zoonotic transmission of Giardia and Cryptosporidium. Int J Parasitol. 2005;35(11):1181–90. 10.1016/j.ijpara.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 7.Monis PT, Thompson RCA. Cryptosporidium and Giardia-zoonoses: fact or fiction? Infect Genet Evol. 2003;3(4):233–44. 10.1016/j.meegid.2003.08.003 [DOI] [PubMed] [Google Scholar]
- 8.Rohde A, Hammerl JA, Al Dahouk S. Detection of foodborne bacterial zoonoses by fluorescence in situ hybridization. Food Control. 2016;69:297–305. 10.1016/j.foodcont.2016.05.008 [DOI] [Google Scholar]
- 9.Lamps LW. Infective disorders of the gastrointestinal tract. Histopathology. 2007;50(1):55–63. 10.1111/j.1365-2559.2006.02544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heymann DL. Control of Communicable diseases Manual. J Environ Health. 2023;85(10):35. [Google Scholar]
- 11.Roy SL, Scallan E, Beach MJ. The rate of acute gastrointestinal illness in developed countries. J Water Health. 2006;4(2):31–70. 10.2166/wh.2006.017 [DOI] [PubMed] [Google Scholar]
- 12.Hay SI, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. 2017. [DOI] [PMC free article] [PubMed]
- 13.Troeger C, Rao PC, Cao S, Zimsen SRM, Albertson SB, Stanaway JD, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–28. 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karsten C, Baumgarte S, Friedrich A, Von Eiff C, Becker K, Wosniok W, et al. Incidence and risk factors for community-acquired acute gastroenteritis in north-west Germany in 2004. Eur J Clin Microbiol Infect Dis. 2009;28:935–43. 10.1007/s10096-009-0729-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallowell BD, Burke RM, Salas SB, Groom H, Donald JL, Mattison CP, et al. Correlates of healthcare-seeking behavior for acute gastroenteritis—United States, October 1, 2016–September 30, 2017. PLoS ONE. 2023;18(10):e0293739. 10.1371/journal.pone.0293739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scallan E, Majowicz SE, Hall G, Banerjee A, Bowman CL, Daly L, et al. Prevalence of diarrhoea in the community in Australia, Canada, Ireland, and the United States. Int J Epidemiol. 2005;34(2):454–60. 10.1093/ije/dyh413 [DOI] [PubMed] [Google Scholar]
- 17.MacDonald PD, Torok MR, Salyers M, Wolf L, Nelson AL. Practices around acute diarrheal illness diagnosis, counseling, and reporting: Laboratory and health-care practitioners in North Carolina, 2004. Foodborne Pathog Dis. 2007;4(3):359–65. 10.1089/fpd.2007.0016 [DOI] [PubMed] [Google Scholar]
- 18.van den Brandhof WE, Bartelds AI, Koopmans MP, van Duynhoven YT. General practitioner practices in requesting laboratory tests for patients with gastroenteritis in the Netherlands, 2001–2002. BMC Fam Pract. 2006;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt MA, Groom HC, Rawlings AM, Mattison CP, Salas SB, Burke RM, et al. Incidence, etiology, and Healthcare Utilization for Acute Gastroenteritis in the community, United States. Emerg Infect Dis. 2022;28(11):2234–42. 10.3201/eid2811.220247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Wit M, Koopmans M, Kortbeek LM, van Leeuwen NJ, Bartelds A, Van Duynhoven Y. Gastroenteritis in sentinel general practices, the Netherlands. Emerg Infect Dis. 2001;7(1):82. 10.3201/eid0701.010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Wit M, Koopmans M, Kortbeek L, Wannet W, Vinje J, Van Leusden F, et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am J Epidemiol. 2001;154(7):666–74. 10.1093/aje/154.7.666 [DOI] [PubMed] [Google Scholar]
- 22.Ecollan M, Guerrisi C, Souty C, Rossignol L, Turbelin C, Hanslik T, et al. Determinants and risk factors of gastroenteritis in the general population, a web-based cohort between 2014 and 2017 in France. BMC Public Health. 2020;20(1):1146. 10.1186/s12889-020-09212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herikstad H, Yang S, Van Gilder TJ, Vugia D, Hadler J, Blake P, et al. A population-based estimate of the burden of diarrhoeal illness in the United States: FoodNet, 1996–7. Epidemiol Infect. 2002;129(1):9–17. 10.1017/S0950268801006628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirk M, Ford L, Glass K, Hall G. Foodborne illness, Australia, Circa 2000 and Circa 2010. Emerg Infect Dis. 2014;20(11):1857–64. 10.3201/eid2011.131315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MÜLler L, Korsgaard H, Ethelberg S. Burden of acute gastrointestinal illness in Denmark 2009: a population-based telephone survey. Epidemiol Infect. 2012;140(2):290–8. 10.1017/S0950268811000471 [DOI] [PubMed] [Google Scholar]
- 26.Tam CC, Rodrigues LC, Viviani L, Dodds JP, Evans MR, Hunter PR, et al. Longitudinal study of infectious intestinal disease in the UK (IID2 study): incidence in the community and presenting to general practice. Gut. 2012;61(1):69–77. 10.1136/gut.2011.238386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiona Barker S, Zomer E, O’Toole J, Sinclair M, Gibney K, Liew D, Leder K. Cost of gastroenteritis in Australia: a healthcare perspective. PLoS ONE. 2018;13(4):e0195759–e. 10.1371/journal.pone.0195759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Cauteren D, De Valk H, Vaux S, Le Strat Y, Vaillant V. Burden of acute gastroenteritis and healthcare-seeking behaviour in France: a population-based study. Epidemiol Infect. 2012;140(4):697–705. 10.1017/S0950268811000999 [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos T, Klamer S, Jacquinet S, Catry B, Litzroth A, Mortgat L, et al. The health and economic impact of acute gastroenteritis in Belgium, 2010–2014. Epidemiol Infect. 2019;147:e146. 10.1017/S095026881900044X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black RE. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine. 1993;11(2):100–6. 10.1016/0264-410X(93)90002-F [DOI] [PubMed] [Google Scholar]
- 31.Glass RI, Lew JF, Gangarosa RE, LeBaron CW, Ho M-S. Estimates of morbidity and mortality rates for diarrheal diseases in American children. J Pediatr. 1991;118(4):S27–33. 10.1016/S0022-3476(05)81422-2 [DOI] [PubMed] [Google Scholar]
- 32.Ryan M, Ramsay M, Brown D, Gay N, Farrington C, Wall PG. Hospital admissions attributable to rotavirus infection in England and Wales. J Infect Dis. 1996;174(Supplement1):S12–8. 10.1093/infdis/174.Supplement_1.S12 [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman CM, Bresee JS, Parashar UD, Riggs TL, Holman RC, Glass RI. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr Infect Dis J. 2001;20(1):14–9. 10.1097/00006454-200101000-00004 [DOI] [PubMed] [Google Scholar]
- 34.Barker SF, Zomer E, O’Toole J, Sinclair M, Gibney K, Liew D, Leder K. Cost of gastroenteritis in Australia: a healthcare perspective. PLoS ONE. 2018;13(4):e0195759. 10.1371/journal.pone.0195759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes GL, Uren E, Stevens KB, Bishop RF. Etiology of Acute Gastroenteritis in Hospitalized Children in Melbourne, Australia, from April 1980 to March 1993. J Clin Microbiol. 1998;36(1):133–8. 10.1128/JCM.36.1.133-138.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budu-Amoako E, Greenwood SJ, Dixon BR, Sweet L, Ang L, Barkema HW, McClure JT. Molecular Epidemiology of Cryptosporidium and Giardia in humans on Prince Edward Island, Canada: evidence of zoonotic transmission from cattle: Molecular Epidemiology of Cryptosporidium and Giardia. Zoonoses Public Health. 2012;59(6):424–33. 10.1111/j.1863-2378.2012.01474.x [DOI] [PubMed] [Google Scholar]
- 37.Clavel A, Olivares JL, Fleta J, Castillo J, Varea M, Ramos FJ, et al. Seasonality of cryptosporidiosis in children. Eur J Clin Microbiol Infect Dis. 1996;15(1):77–9. 10.1007/BF01586190 [DOI] [PubMed] [Google Scholar]
- 38.Fitzenberger J, Uphoff H, Gawrich S, Hauri AM. Urban-rural differences of age- and species-specific campylobacteriosis incidence, Hesse, Germany, July 2005 - June 2006. Euro Surveill. 2010;15(42):1–7. 10.2807/ese.15.42.19693-en [DOI] [PubMed] [Google Scholar]
- 39.Lal A, Fearnley E, Kirk M. The risk of reported cryptosporidiosis in children aged < 5 years in Australia is highest in very remote regions. Int J Environ Res Public Health. 2015;12(9):11815–28. 10.3390/ijerph120911815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chalmers RM, Giles M. Zoonotic cryptosporidiosis in the UK - challenges for control. J Appl Microbiol. 2010;109(5):1487–97. 10.1111/j.1365-2672.2010.04764.x [DOI] [PubMed] [Google Scholar]
- 41.Ryan MJ, Wall PG, Adak GK, Evans HS, Cowden JM. Outbreaks of infectious intestinal disease in residential institutions in England and Wales 1992–1994. J Infect. 1997;34(1):49–54. 10.1016/S0163-4453(97)80009-6 [DOI] [PubMed] [Google Scholar]
- 42.Weston D, Ip A, Amlôt R. Examining the application of behaviour change theories in the context of infectious disease outbreaks and emergency response: a review of reviews. BMC Public Health. 2020;20(1):1–1483. 10.1186/s12889-020-09519-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keeling MJ, Danon L. Mathematical modelling of infectious diseases. Br Med Bull. 2009;92(1):33–42. 10.1093/bmb/ldp038 [DOI] [PubMed] [Google Scholar]
- 44.Hellard ME, Sinclair M, Harris AH, Kirk M, Fairley CK. Cost of community gastroenteritis. J Gastroenterol Hepatol. 2003;18(3):322–8. 10.1046/j.1440-1746.2003.02959.x [DOI] [PubMed] [Google Scholar]
- 45.Majowicz S, McNab W, Sockett P, Henson S, Doré K, Edge V, et al. Burden and cost of gastroenteritis in a Canadian community. J Food Prot. 2006;69(3):651–60. 10.4315/0362-028X-69.3.651 [DOI] [PubMed] [Google Scholar]
- 46.Van Den Brandhof W, De Wit G, De Wit M, Van Duynhoven Y. Costs of gastroenteritis in the Netherlands. Epidemiol Infect. 2004;132(2):211–21. 10.1017/S0950268803001559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henson S, Majowicz S, Masakure O, Sockett P, MacDougall L, Edge V, et al. Estimation of the costs of acute gastrointestinal illness in British Columbia. Can Int J food Microbiol. 2008;127(1–2):43–52. 10.1016/j.ijfoodmicro.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 48.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 49.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 50.Plaatjie MT, Onyiche TE, Ramatla T, Bezuidenhout JJ, Legoabe L, Nyembe NI, Thekisoe O. A scoping review on efficacy and safety of medicinal plants used for the treatment of diarrhea in sub-saharan Africa. Trop Med Health. 2024;52(1):6. 10.1186/s41182-023-00569-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ethelberg S, Olesen B, Neimann J, Schiellerup P, Helms M, Jensen C et al. Risk factors for diarrhea among children in an industrialized country. Epidemiology. 2006:24–30. [DOI] [PubMed]
- 52.Júlio C, Vilares A, Oleastro M, Ferreira I, Gomes S, Monteiro L, et al. Prevalence and risk factors for Giardia duodenalis infection among children: a case study in Portugal. Parasit Vectors. 2012;5:22. 10.1186/1756-3305-5-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnedo-Pena A, Vivas-Fornas I, Meseguer-Ferrer N, Tirado-Balaguer MD, Yagüe-Muñoz A, Herrera-León S, et al. Comparison of sporadic cases of Salmonella Typhimurium with other Salmonella serotypes in Castellon (Spain): case-case study. Enferm Infecc Microbiol Clin. 2018;36(8):478–83. 10.1016/j.eimc.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 54.Doorduyn Y, Van Den Brandhof WE, Van Duynhoven YT, Breukink BJ, Wagenaar JA, Van Pelt W. Risk factors for indigenous Campylobacter jejuni and Campylobacter coli infections in the Netherlands: a case-control study. Epidemiol Infect. 2010;138(10):1391–404. 10.1017/S095026881000052X [DOI] [PubMed] [Google Scholar]
- 55.Enserink R, Mughini-Gras L, Duizer E, Kortbeek T, Van Pelt W. Risk factors for gastroenteritis in child day care. Epidemiol Infect. 2015;143(13):2707–20. 10.1017/S0950268814003367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoque ME, Hope VT, Scragg R, Kjellström T. Children at risk of giardiasis in Auckland: a case-control analysis. Epidemiol Infect. 2003;131(1):655–62. 10.1017/S0950268803008598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Unicomb LE, Dalton CB, Gilbert GL, Becker NG, Patel MS. Age-specific risk factors for sporadic Campylobacter infection in regional Australia. Foodborne Pathog Dis. 2008;5(1):79–85. 10.1089/fpd.2007.0047 [DOI] [PubMed] [Google Scholar]
- 58.Bassal R, Ovadia A, Bromberg M, Stein M, Shainberg B, Loewenthal S, et al. Risk factors for sporadic infection with Campylobacter Spp. Among children in Israel: a case-control study. Pediatr Infect Dis J. 2016;35(3):249–52. 10.1097/INF.0000000000000989 [DOI] [PubMed] [Google Scholar]
- 59.Carrique-Mas J, Andersson Y, Hjertqvist M, Svensson Å, Torner A, Giesecke J. Risk factors for domestic sporadic campylobacteriosis among young children in Sweden. Scand J Infect Dis. 2005;37(2):101–10. 10.1080/00365540510027165 [DOI] [PubMed] [Google Scholar]
- 60.Tenkate TD, Stafford RJ. Risk factors for campylobacter infection in infants and young children: a matched case-control study. Epidemiol Infect. 2001;127(3):399–404. 10.1017/S0950268801006306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tangermann RH, Gordon S, Wiesner P, Kreckman L. An outbreak of cryptosporidiosis in a day-care center in Georgia. Am J Epidemiol. 1991;133(5):471–6. 10.1093/oxfordjournals.aje.a115914 [DOI] [PubMed] [Google Scholar]
- 62.Okyay P, Ertug S, Gultekin B, Onen O, Beser E. Intestinal parasites prevalence and related factors in school children, a western city sample–Turkey. BMC Public Health. 2004;4:64. 10.1186/1471-2458-4-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pijnacker R, Mughini-Gras L, Vennema H, Enserink R, Van Den Wijngaard CC, Kortbeek T, W VANP. Characteristics of child daycare centres associated with clustering of major enteropathogens. Epidemiol Infect. 2016;144(12):2527–39. 10.1017/S0950268816001011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pijnacker R, Mughini-Gras L, Heusinkveld M, Roelfsema J, van Pelt W, Kortbeek T. Different risk factors for infection with Giardia lamblia assemblages a and B in children attending day-care centres. Eur J Clin Microbiol Infect Dis. 2016;35(12):2005–13. 10.1007/s10096-016-2753-2 [DOI] [PubMed] [Google Scholar]
- 65.Kuhn KG, Nielsen EM, Mølbak K, Ethelberg S. Determinants of sporadic Campylobacter infections in Denmark: a nationwide case-control study among children and young adults. Clin Epidemiol. 2018;10:1695–707. 10.2147/CLEP.S177141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coles CL, Levy A, Dagan R, Deckelbaum RJ, Fraser D. Risk factors for the initial symptomatic giardia infection in a cohort of young arab-bedouin children. Ann Trop Paediatr. 2009;29(4):291–300. 10.1179/027249309X12547917869041 [DOI] [PubMed] [Google Scholar]
- 67.Boqvist S, Pettersson H, Svensson A, Andersson Y. Sources of sporadic Yersinia enterocolitica infection in children in Sweden, 2004: a case-control study. Epidemiol Infect. 2009;137(6):897–905. 10.1017/S0950268808001209 [DOI] [PubMed] [Google Scholar]
- 68.Mellou K, Sourtzi P, Tsakris A, Saroglou G, Velonakis E. Risk factors for sporadic Campylobacter jejuni infections in children in a Greek region. Epidemiol Infect. 2010;138(12):1719–25. 10.1017/S0950268810001196 [DOI] [PubMed] [Google Scholar]
- 69.Rosner BM, Stark K, Höhle M, Werber D. Risk factors for sporadic Yersinia enterocolitica infections, Germany 2009–2010. Epidemiol Infect. 2012;140(10):1738–47. 10.1017/S0950268811002664 [DOI] [PubMed] [Google Scholar]
- 70.Balaj M, York HW, Sripada K, Besnier E, Vonen HD, Aravkin A, et al. Parental education and inequalities in child mortality: a global systematic review and meta-analysis. Lancet. 2021;398(10300):608–20. 10.1016/S0140-6736(21)00534-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wamani H, Tylleskär T, Astrøm AN, Tumwine JK, Peterson S. Mothers’ education but not fathers’ education, household assets or land ownership is the best predictor of child health inequalities in rural Uganda. Int J Equity Health. 2004;3(1):9. 10.1186/1475-9276-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quihui L, Valencia ME, Crompton DW, Phillips S, Hagan P, Morales G, Díaz-Camacho SP. Role of the employment status and education of mothers in the prevalence of intestinal parasitic infections in Mexican rural schoolchildren. BMC Public Health. 2006;6:225. 10.1186/1471-2458-6-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nematian J, Nematian E, Gholamrezanezhad A, Asgari AA. Prevalence of intestinal parasitic infections and their relation with socio-economic factors and hygienic habits in Tehran primary school students. Acta Trop. 2004;92(3):179–86. 10.1016/j.actatropica.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 74.Vikram K, Vanneman R, Desai S. Linkages between maternal education and childhood immunization in India. Soc Sci Med. 2012;75(2):331–9. 10.1016/j.socscimed.2012.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desmennu AT, Oluwasanu MM, John-Akinola YO, Opeyemi O, Ayo AS. Maternal Education and Diarrhea among children aged 0–24 months in Nigeria. Afr J Reprod Health. 2017;21(3):27–36. 10.29063/ajrh2017/v21i3.2 [DOI] [PubMed] [Google Scholar]
- 76.Diaz-Quijano FA, Martínez-Vega RA, Rodriguez-Morales AJ, Rojas-Calero RA, Luna-González ML, Díaz-Quijano RG. Association between the level of education and knowledge, attitudes and practices regarding dengue in the Caribbean region of Colombia. BMC Public Health. 2018;18(1):1–10. 10.1186/s12889-018-5055-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adesegun OA, Binuyo T, Adeyemi O, Ehioghae O, Rabor DF, Amusan O, et al. The COVID-19 crisis in sub-saharan Africa: knowledge, attitudes, and practices of the Nigerian public. Am J Trop Med Hyg. 2020;103(5):1997. 10.4269/ajtmh.20-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogboghodo EO, Osaigbovo II, Obaseki DE, Okwara OHN, Omo-Ikirodah OT, Adio F, Ehinze ES. Community mitigation strategies for coronavirus disease 2019: an assessment of knowledge and adherence amongst residents of Benin City, Edo State, Nigeria. Nigerian Postgrad Med J. 2021;28(1):14–21. 10.4103/npmj.npmj_321_20 [DOI] [PubMed] [Google Scholar]
- 79.da Penha JC, do Nascimento LA, de Sabino LMM, da Rocha Mendes ER, da Rocha SS, Roubert ESC, et al. Effects of educational interventions on maternal self-efficacy and childhood diarrhea: a randomized clinical trial. Matern Child Health J. 2022;26(7):1507–15. 10.1007/s10995-022-03408-3 [DOI] [PubMed] [Google Scholar]
- 80.Keystone J, Krajden S, Warren M. Person-to-person transmission of Giardia lamblia in day-care nurseries. Can Med Assoc J. 1978;119(3):241. [PMC free article] [PubMed] [Google Scholar]
- 81.Black RE, Dykes AC, Sinclair SP, Wells JG. Giardiasis in day-care centers: evidence of person-to-person transmission. Pediatrics. 1977;60(4):486–91. 10.1542/peds.60.4.486 [DOI] [PubMed] [Google Scholar]
- 82.Berkowicz J, Lee A. Person-to-person transmission of Campylobacter pylori. 1987. [DOI] [PubMed]
- 83.Mitchell HM, Lee A, Carrick J. Increased incidence of Campylobacter pylori infection in gastroenterologists: further evidence to support person-to-person transmission of C. Pylori. Scand J Gastroenterol. 1989;24(4):396–400. 10.3109/00365528909093065 [DOI] [PubMed] [Google Scholar]
- 84.Kotch JB, Isbell P, Weber DJ, Nguyen V, Savage E, Gunn E, et al. Hand-washing and Diapering Equipment Reduces Disease among Children in Out-of-Home Child Care Centers. Pediatrics. 2007;120(1):e29–36. 10.1542/peds.2005-0760 [DOI] [PubMed] [Google Scholar]
- 85.Thompson S. Infectious diarrhoea in children: controlling transmission in the child care setting. J Paediatr Child Health. 1994;30(3):210–9. 10.1111/j.1440-1754.1994.tb00621.x [DOI] [PubMed] [Google Scholar]
- 86.Bartlett AV, Moore M, Gary GW, Starko KM, Erben JJ, Meredith BA. Diarrheal illness among infants and toddlers in day care centers. I. Epidemiology and pathogens. J Pediatr. 1985;107(4):495–502. 10.1016/S0022-3476(85)80004-4 [DOI] [PubMed] [Google Scholar]
- 87.Boone SA, Gerba CP. The occurrence of influenza a virus on household and day care center fomites. J Infect. 2005;51(2):103–9. 10.1016/j.jinf.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 88.Augustine JM, Crosnoe RL, Gordon R. Early Child Care and illness among preschoolers. J Health Soc Behav. 2013;54(3):315–34. 10.1177/0022146513496106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor M, Adams CL, Ellis A. Gatekeepers of health: a qualitative assessment of child care centre staff’s perspectives, practices and challenges to enteric illness prevention and management in child care centres. BMC Public Health. 2008;8(1):212. 10.1186/1471-2458-8-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dalgaard NT, Bondebjerg A, Svinth L. Caregiver/child ratio and group size in Scandinavian early childhood education and care (ECEC): a systematic review of qualitative research. Nordic Psychol. 2022:1–32.
- 91.Kahan E, Gross S, Cohen HA. Exclusion of ill children from child-care centers in Israel. Patient Educ Couns. 2005;56(1):93–7. 10.1016/j.pec.2003.12.012 [DOI] [PubMed] [Google Scholar]
- 92.Enserink R, Ypma R, Donker GA, Smit HA, van Pelt W. Infectious disease burden related to child day care in the Netherlands. Pediatr Infect Dis J. 2013;32(8):e334–40. 10.1097/INF.0b013e318290601e [DOI] [PubMed] [Google Scholar]
- 93.Furber SE. Exclusion of Sick Children from Child Care services. Australasian J Early Child. 1997;22(3):19–23. 10.1177/183693919702200305 [DOI] [Google Scholar]
- 94.Sticher B, Bielicki J, Berger C. Temporary exclusion of ill children from childcare centres in Switzerland: practice, problems and potential solutions. BMC Health Serv Res. 2018;18:1–8. 10.1186/s12913-018-2831-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yin JK, Salkeld G, Lambert SB, Dierig A, Heron L, Leask J, et al. Estimates and determinants of economic impacts from influenza-like illnesses caused by respiratory viruses in a ustralian children attending childcare: a cohort study. Influenza Other Respir Viruses. 2013;7(6):1103–12. 10.1111/irv.12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.El-Saadi DM. Managing infectious disease risks in long day care services: how well does this happen. Queensland University of Technology; 2020.
- 97.Matsuo J, Nakashio S. Prevalence of fecal contamination in sandpits in public parks in Sapporo City, Japan. Vet Parasitol. 2005;128(1):115–9. 10.1016/j.vetpar.2004.11.008 [DOI] [PubMed] [Google Scholar]
- 98.Podewils LJ, Zanardi Blevins L, Hagenbuch M, Itani D, Burns A, Otto C, et al. Outbreak of norovirus illness associated with a swimming pool. Epidemiol Infect. 2007;135(5):827–33. 10.1017/S0950268806007370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thompson RCA. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int J Parasitol. 2000;30(12):1259–67. 10.1016/S0020-7519(00)00127-2 [DOI] [PubMed] [Google Scholar]
- 100.Mahmud MA, Chappell C, Hossain MM, Habib M, Dupont HL. Risk factors for development of First Symptomatic Giardia infection among infants of a birth cohort in rural Egypt. Am J Trop Med Hyg. 1995;53(1):84–8. 10.4269/ajtmh.1995.53.84 [DOI] [PubMed] [Google Scholar]
- 101.Robertson ID, Irwin PJ, Lymbery AJ, Thompson RCA. The role of companion animals in the emergence of parasitic zoonoses. Int J Parasitol. 2000;30(12):1369–77. 10.1016/S0020-7519(00)00134-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the study’s conclusions can be found within the supplementary files, while all other data is presented in the results section.