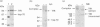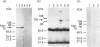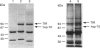Abstract
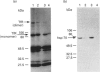
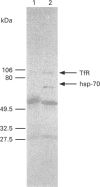
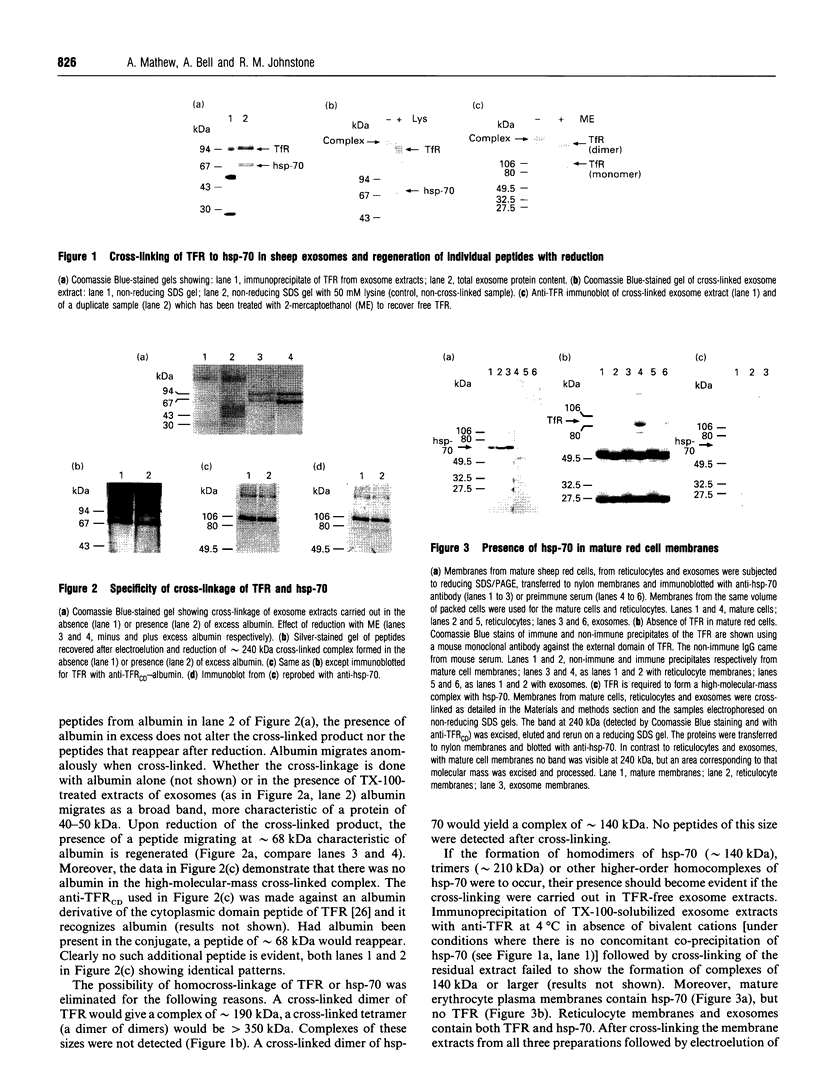
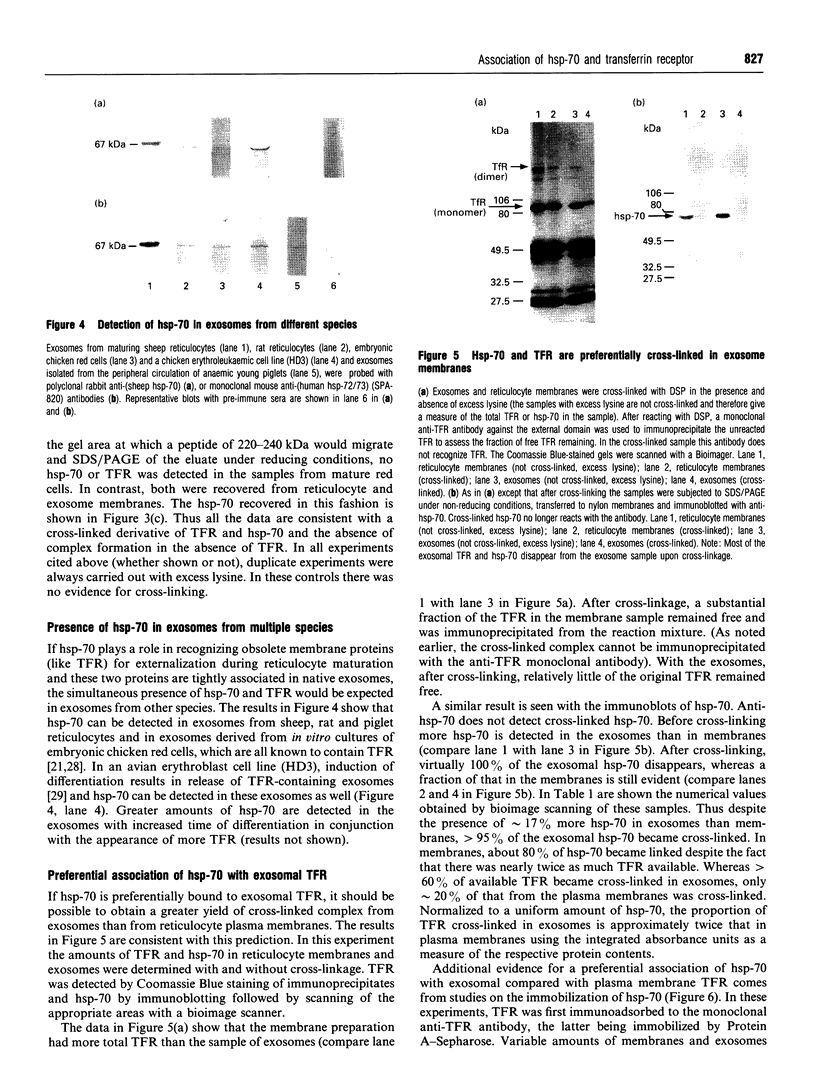
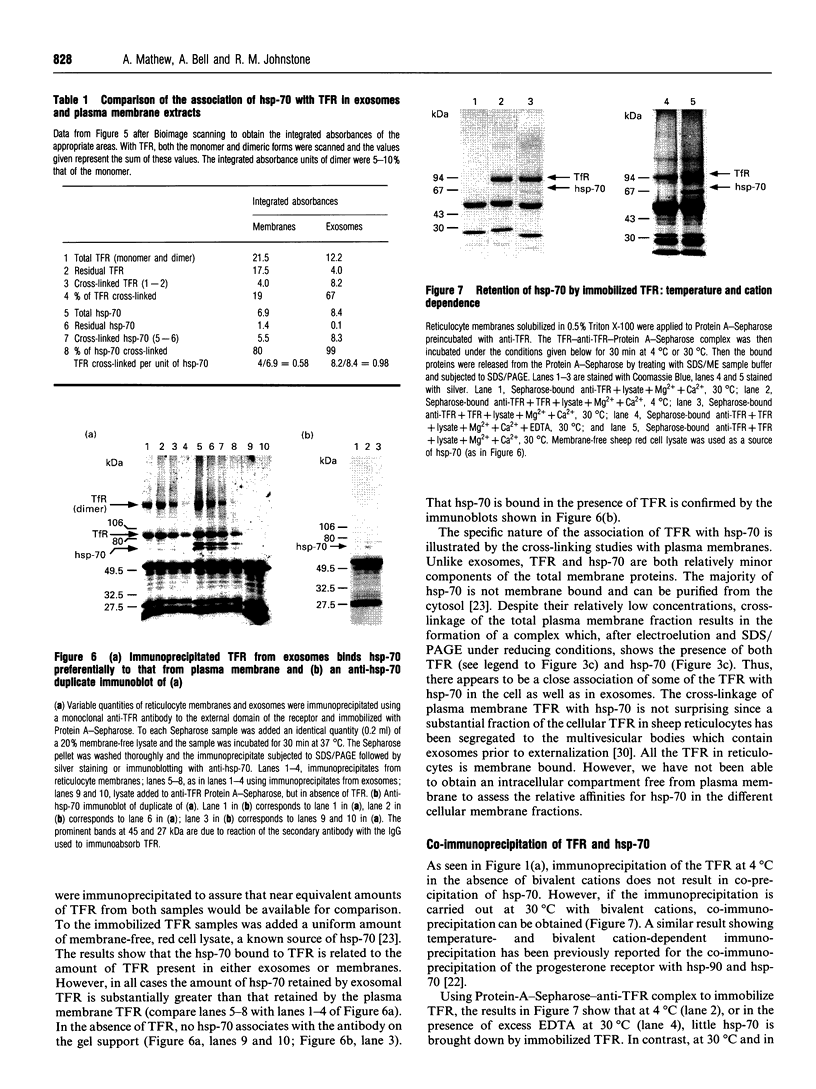
The presence of the heat shock protein (hsp-70) has been detected in exocytosed vesicles (which are named exosomes) from mammalian and avian immature red cells (i.e. reticulocytes) as well as from a differentiating avian erythroleukaemic cell line. The close, but non-covalent, association of hsp-70 with the transferrin receptor (TFR) in exosomes is demonstrated by: (1) the ability to cross-link hsp-70 to TFR; (2) the co-immunoprecipitation of hsp-70 and TFR with an antibody against TFR, and the co-immunoprecipitation of TFR and hsp-70 with antibody against hsp-70; and (3) the retention of TFR by hsp-70 bound to ATP-agarose and the simultaneous elution of both proteins by excess ATP. Semi-quantitative analysis of the relative efficiency of cross-linking of these proteins in exosomes versus plasma membranes shows that TFR in exosomes is preferentially bound to hsp-70. From an analysis of the relative amounts of hsp-70 and TFR regenerated from the cross-linked complex, the ratio of TFR monomer bound to hsp-70 is approximately 1.5 to 1. Given the presence of hsp-70 in exosomes from several species and its close association with TFR (the major protein lost during reticulocyte maturation) it is proposed that hsp-70 plays a role in exosome formation and/or release in immature red cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M. A., Johnstone R. M. Protein kinase C does not phosphorylate the externalized form of the transferrin receptor. Biochem J. 1987 Feb 15;242(1):151–161. doi: 10.1042/bj2420151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam M., Wu C., Turbide C., Larrick J., Johnstone R. M. Evidence for a pool of non-recycling transferrin receptors in peripheral sheep reticulocytes. J Cell Physiol. 1986 Apr;127(1):8–16. doi: 10.1002/jcp.1041270103. [DOI] [PubMed] [Google Scholar]
- Ahn J., Johnstone R. M. Origin of a soluble truncated transferrin receptor. Blood. 1993 May 1;81(9):2442–2451. [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Benderoff S., Johnstone R. M., Blostein R. Electrogenic sodium-dependent glycine transport in sheep reticulocytes. Can J Biochem. 1978 Jun;56(6):545–551. doi: 10.1139/o78-083. [DOI] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Davis J. Q., Bennett V. Human erythrocyte clathrin and clathrin-uncoating protein. J Biol Chem. 1985 Nov 25;260(27):14850–14856. [PubMed] [Google Scholar]
- Davis J. Q., Dansereau D., Johnstone R. M., Bennett V. Selective externalization of an ATP-binding protein structurally related to the clathrin-uncoating ATPase/heat shock protein in vesicles containing terminal transferrin receptors during reticulocyte maturation. J Biol Chem. 1986 Nov 25;261(33):15368–15371. [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Flynn G. C., Chappell T. G., Rothman J. E. Peptide binding and release by proteins implicated as catalysts of protein assembly. Science. 1989 Jul 28;245(4916):385–390. doi: 10.1126/science.2756425. [DOI] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Grdisa M., Mathew A., Johnstone R. M. Expression and loss of the transferrin receptor in growing and differentiating HD3 cells. J Cell Physiol. 1993 May;155(2):349–357. doi: 10.1002/jcp.1041550216. [DOI] [PubMed] [Google Scholar]
- Harding C., Heuser J., Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984 Nov;35(2):256–263. [PubMed] [Google Scholar]
- Johnstone R. M., Adam M., Hammond J. R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987 Jul 5;262(19):9412–9420. [PubMed] [Google Scholar]
- Johnstone R. M., Adam M., Turbide C., Larrick J. Phosphorylation of the transferrin receptor in isolated sheep reticulocyte plasma membranes. Can J Biochem Cell Biol. 1984 Sep;62(9):927–934. doi: 10.1139/o84-119. [DOI] [PubMed] [Google Scholar]
- Johnstone R. M., Bianchini A., Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989 Oct;74(5):1844–1851. [PubMed] [Google Scholar]
- Johnstone R. M., Mathew A., Mason A. B., Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991 Apr;147(1):27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Palleros D. R., Welch W. J., Fink A. L. Interaction of hsp70 with unfolded proteins: effects of temperature and nucleotides on the kinetics of binding. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5719–5723. doi: 10.1073/pnas.88.13.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. T., Blostein R., Johnstone R. M. Loss of the transferrin receptor during the maturation of sheep reticulocytes in vitro. An immunological approach. Biochem J. 1983 Jan 15;210(1):37–47. doi: 10.1042/bj2100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. T., Johnstone R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983 Jul;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Pan B. T., Johnstone R. Selective externalization of the transferrin receptor by sheep reticulocytes in vitro. Response to ligands and inhibitors of endocytosis. J Biol Chem. 1984 Aug 10;259(15):9776–9782. [PubMed] [Google Scholar]
- Pan B. T., Teng K., Wu C., Adam M., Johnstone R. M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985 Sep;101(3):942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins: the search for functions. J Cell Biol. 1986 Aug;103(2):321–325. doi: 10.1083/jcb.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer E., Angst W., Lutz H. U. Glycoprotein topology on intact human red blood cells reevaluated by cross-linking following amino group supplementation. Biochemistry. 1982 Dec 21;21(26):6807–6818. doi: 10.1021/bi00269a029. [DOI] [PubMed] [Google Scholar]
- Smith D. F., Stensgard B. A., Welch W. J., Toft D. O. Assembly of progesterone receptor with heat shock proteins and receptor activation are ATP mediated events. J Biol Chem. 1992 Jan 15;267(2):1350–1356. [PubMed] [Google Scholar]
- Sullivan A. L., Grasso J. A., Weintraub L. R. Micropinocytosis of transferrin by developing red cells: an electron-microscopic study utilizing ferritin-conjugated transferrin and ferritin-conjugated antibodies to transferrin. Blood. 1976 Jan;47(1):133–143. [PubMed] [Google Scholar]
- Terlecky S. R., Chiang H. L., Olson T. S., Dice J. F. Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J Biol Chem. 1992 May 5;267(13):9202–9209. [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M. J., Stahl P. D. The small GTP-binding proteins Rab4 and ARF are associated with released exosomes during reticulocyte maturation. Eur J Cell Biol. 1993 Apr;60(2):261–267. [PubMed] [Google Scholar]
- White S., Miller K., Hopkins C., Trowbridge I. S. Monoclonal antibodies against defined epitopes of the human transferrin receptor cytoplasmic tail. Biochim Biophys Acta. 1992 Jul 22;1136(1):28–34. doi: 10.1016/0167-4889(92)90081-l. [DOI] [PubMed] [Google Scholar]
- Yoshimori T., Shimonishi Y., Uchida T. Binding properties of monoclonal antibody to the cytoplasmic domain of transferrin receptor. Cell Struct Funct. 1988 Aug;13(4):311–324. doi: 10.1247/csf.13.311. [DOI] [PubMed] [Google Scholar]