Abstract
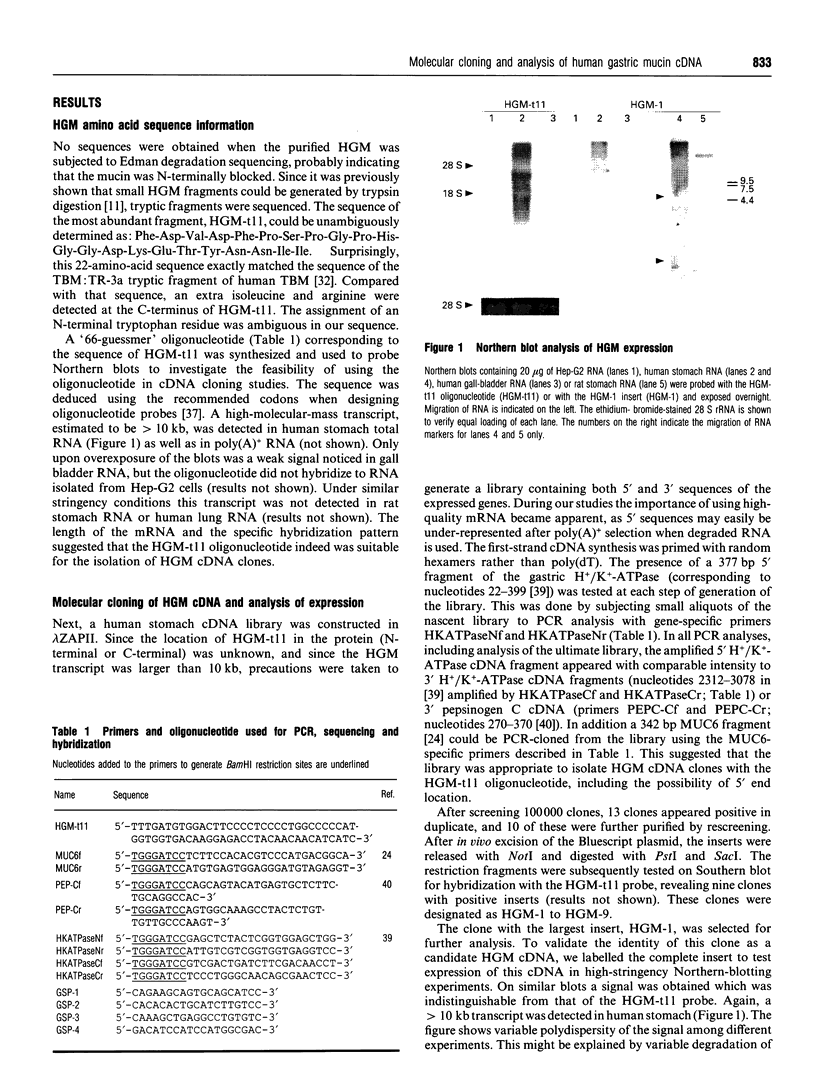
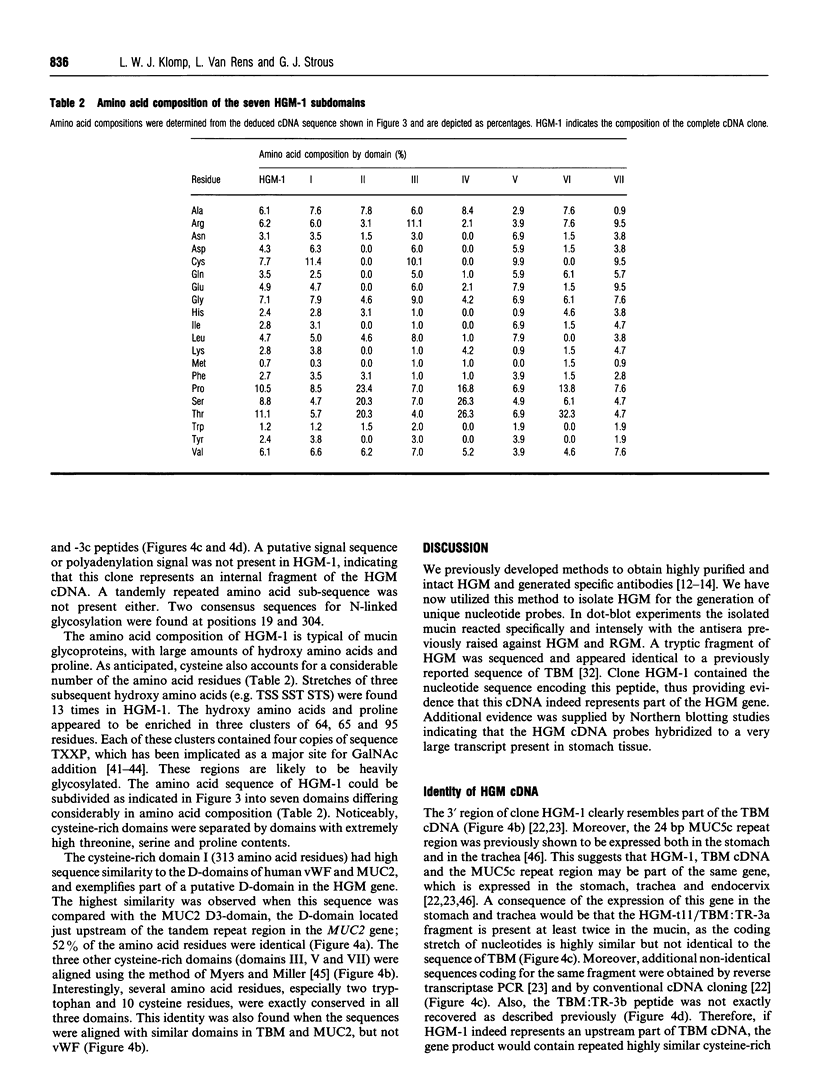
Human gastric mucin was isolated by successive CsCl-gradient ultracentrifugation in the presence of guanidinium hydrochloride to prevent degradation of the polypeptide moieties of the molecules. The amino acid sequence of a tryptic fragment of this molecule was identical to that of a tryptic fragment of tracheobronchial mucin. An oligonucleotide based on this sequence hybridized specifically to human stomach mRNA and was subsequently used to screen a human stomach lambda ZAPII cDNA library. The largest of 10 positive clones encoded 850 amino acid residues, including the tryptic fragment, with high amounts of threonine, serine and proline residues. Interestingly, cysteine accounted for almost 8% of the amino acid residues. The 3' part of the sequence was very similar but not identical to the 3' region of human tracheobronchial cDNA. No tandem repeated sequences were present and the deduced polypeptide sequence contained two potential N-linked glycosylation sites. Four cysteine-rich clusters were detected, one of which was apparently homologous to the D-domains present in other mucins and in von Willebrand factor. The arrangement of the cysteines in three other cysteine-rich clusters was conserved in the human gastric mucin cDNA in a similar fashion as in two domains in the MUC2 gene product. The cysteine-rich domains were separated by short stretches of non-repetitive amino acid residues with a very high content of threonine and serine residues. These data suggest that the encoded polypeptide of this clone may be involved in disulphide-bond-mediated oligomerization of the mucin, and provide new insights into the molecular organization of mammalian apomucins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubert J. P., Porchet N., Crepin M., Duterque-Coquillaud M., Vergnes G., Mazzuca M., Debuire B., Petitprez D., Degand P. Evidence for different human tracheobronchial mucin peptides deduced from nucleotide cDNA sequences. Am J Respir Cell Mol Biol. 1991 Aug;5(2):178–185. doi: 10.1165/ajrcmb/5.2.178. [DOI] [PubMed] [Google Scholar]
- Audie J. P., Janin A., Porchet N., Copin M. C., Gosselin B., Aubert J. P. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993 Oct;41(10):1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A. K., Woitach J. T., Davidson E. A., Bhavanandan V. P. Cloning and cDNA sequence of a bovine submaxillary gland mucin-like protein containing two distinct domains. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6798–6802. doi: 10.1073/pnas.87.17.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dekker J., Aelmans P. H., Strous G. J. The oligomeric structure of rat and human gastric mucins. Biochem J. 1991 Jul 15;277(Pt 2):423–427. doi: 10.1042/bj2770423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Strous G. J. Covalent oligomerization of rat gastric mucin occurs in the rough endoplasmic reticulum, is N-glycosylation-dependent, and precedes initial O-glycosylation. J Biol Chem. 1990 Oct 25;265(30):18116–18122. [PubMed] [Google Scholar]
- Dekker J., Van Beurden-Lamers W. M., Oprins A., Strous G. J. Isolation and structural analysis of rat gastric mucus glycoprotein suggests a homogeneous protein backbone. Biochem J. 1989 Jun 15;260(3):717–723. doi: 10.1042/bj2600717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J., Van Beurden-Lamers W. M., Strous G. J. Biosynthesis of gastric mucus glycoprotein of the rat. J Biol Chem. 1989 Jun 25;264(18):10431–10437. [PubMed] [Google Scholar]
- Dekker J., van der Ende A., Aelmans P. H., Strous G. J. Rat gastric mucin is synthesized and secreted exclusively as filamentous oligomers. Biochem J. 1991 Oct 1;279(Pt 1):251–256. doi: 10.1042/bj2790251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989 May;7(5):514–520. [PubMed] [Google Scholar]
- Eckhardt A. E., Timpte C. S., Abernethy J. L., Zhao Y., Hill R. L. Porcine submaxillary mucin contains a cystine-rich, carboxyl-terminal domain in addition to a highly repetitive, glycosylated domain. J Biol Chem. 1991 May 25;266(15):9678–9686. [PubMed] [Google Scholar]
- Elhammer A. P., Poorman R. A., Brown E., Maggiora L. L., Hoogerheide J. G., Kézdy F. J. The specificity of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase as inferred from a database of in vivo substrates and from the in vitro glycosylation of proteins and peptides. J Biol Chem. 1993 May 15;268(14):10029–10038. [PubMed] [Google Scholar]
- Forstner J. F., Jabbal I., Qureshi R., Kells D. I., Forstner G. G. The role of disulphide bonds in human intestinal mucin. Biochem J. 1979 Sep 1;181(3):725–732. doi: 10.1042/bj1810725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Rothe E. M., Lagace R. E., Kim Y. S. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem. 1992 Oct 25;267(30):21375–21383. [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Siddiki B., Kim Y. S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994 Jan 28;269(4):2440–2446. [PubMed] [Google Scholar]
- Harding S. E., Rowe A. J., Creeth J. M. Further evidence for a flexible and highly expanded spheroidal model for mucus glycoproteins in solution. Biochem J. 1983 Mar 1;209(3):893–896. doi: 10.1042/bj2090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser F., Hoffmann W. P-domains as shuffled cysteine-rich modules in integumentary mucin C.1 (FIM-C.1) from Xenopus laevis. Polydispersity and genetic polymorphism. J Biol Chem. 1992 Dec 5;267(34):24620–24624. [PubMed] [Google Scholar]
- Hoffmann W. A new repetitive protein from Xenopus laevis skin highly homologous to pancreatic spasmolytic polypeptide. J Biol Chem. 1988 Jun 5;263(16):7686–7690. [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Klomp L. W., van Rens L., Strous G. J. Identification of a human gastric mucin precursor: N-linked glycosylation and oligomerization. Biochem J. 1994 Dec 15;304(Pt 3):693–698. doi: 10.1042/bj3040693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg M. J., Vos H. L., Gennissen A. M., Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990 Apr 5;265(10):5573–5578. [PubMed] [Google Scholar]
- Maeda M., Oshiman K., Tamura S., Futai M. Human gastric (H+ + K+)-ATPase gene. Similarity to (Na+ + K+)-ATPase genes in exon/intron organization but difference in control region. J Biol Chem. 1990 Jun 5;265(16):9027–9032. [PubMed] [Google Scholar]
- Mayadas T. N., Wagner D. D. Vicinal cysteines in the prosequence play a role in von Willebrand factor multimer assembly. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3531–3535. doi: 10.1073/pnas.89.8.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas T. N., Wagner D. D. von Willebrand factor biosynthesis and processing. Ann N Y Acad Sci. 1991;614:153–166. doi: 10.1111/j.1749-6632.1991.tb43700.x. [DOI] [PubMed] [Google Scholar]
- McCool D. J., Forstner J. F., Forstner G. G. Synthesis and secretion of mucin by the human colonic tumour cell line LS180. Biochem J. 1994 Aug 15;302(Pt 1):111–118. doi: 10.1042/bj3020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meezaman D., Charles P., Daskal E., Polymeropoulos M. H., Martin B. M., Rose M. C. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5). J Biol Chem. 1994 Apr 29;269(17):12932–12939. [PubMed] [Google Scholar]
- Myers E. W., Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988 Mar;4(1):11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Nishimori I., Johnson N. R., Sanderson S. D., Perini F., Mountjoy K., Cerny R. L., Gross M. L., Hollingsworth M. A. Influence of acceptor substrate primary amino acid sequence on the activity of human UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferase. Studies with the MUC1 tandem repeat. J Biol Chem. 1994 Jun 10;269(23):16123–16130. [PubMed] [Google Scholar]
- Porchet N., Nguyen V. C., Dufosse J., Audie J. P., Guyonnet-Duperat V., Gross M. S., Denis C., Degand P., Bernheim A., Aubert J. P. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991 Mar 15;175(2):414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- Probst J. C., Hauser F., Joba W., Hoffmann W. The polymorphic integumentary mucin B.1 from Xenopus laevis contains the short consensus repeat. J Biol Chem. 1992 Mar 25;267(9):6310–6316. [PubMed] [Google Scholar]
- Rijnboutt S., Aerts H. M., Geuze H. J., Tager J. M., Strous G. J. Mannose 6-phosphate-independent membrane association of cathepsin D, glucocerebrosidase, and sphingolipid-activating protein in HepG2 cells. J Biol Chem. 1991 Mar 15;266(8):4862–4868. [PubMed] [Google Scholar]
- Rose M. C., Kaufman B., Martin B. M. Proteolytic fragmentation and peptide mapping of human carboxyamidomethylated tracheobronchial mucin. J Biol Chem. 1989 May 15;264(14):8193–8199. [PubMed] [Google Scholar]
- Sheehan J. K., Carlstedt I. Electron microscopy of cervical-mucus glycoproteins and fragments therefrom. The use of colloidal gold to make visible 'naked' protein regions. Biochem J. 1990 Jan 1;265(1):169–177. doi: 10.1042/bj2650169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton-Inloes B. B., Titani K., Sadler J. E. cDNA sequences for human von Willebrand factor reveal five types of repeated domains and five possible protein sequence polymorphisms. Biochemistry. 1986 Jun 3;25(11):3164–3171. doi: 10.1021/bi00359a014. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Taggart R. T., Cass L. G., Mohandas T. K., Derby P., Barr P. J., Pals G., Bell G. I. Human pepsinogen C (progastricsin). Isolation of cDNA clones, localization to chromosome 6, and sequence homology with pepsinogen A. J Biol Chem. 1989 Jan 5;264(1):375–379. [PubMed] [Google Scholar]
- Thornton D. J., Davies J. R., Kraayenbrink M., Richardson P. S., Sheehan J. K., Carlstedt I. Mucus glycoproteins from 'normal' human tracheobronchial secretion. Biochem J. 1990 Jan 1;265(1):179–186. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribara N. W., Gum J. R., Jr, Culhane P. J., Lagace R. E., Hicks J. W., Petersen G. M., Kim Y. S. MUC-2 human small intestinal mucin gene structure. Repeated arrays and polymorphism. J Clin Invest. 1991 Sep;88(3):1005–1013. doi: 10.1172/JCI115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribara N. W., Roberton A. M., Ho S. B., Kuo W. L., Gum E., Hicks J. W., Gum J. R., Jr, Byrd J. C., Siddiki B., Kim Y. S. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993 Mar 15;268(8):5879–5885. [PubMed] [Google Scholar]
- Tytgat K. M., Büller H. A., Opdam F. J., Kim Y. S., Einerhand A. W., Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994 Nov;107(5):1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Hart M., Pannekoek H. Expression of variant von Willebrand factor (vWF) cDNA in heterologous cells: requirement of the pro-polypeptide in vWF multimer formation. EMBO J. 1987 Oct;6(10):2885–2890. doi: 10.1002/j.1460-2075.1987.tb02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. B., Gavel Y., von Heijne G. Amino acid distributions around O-linked glycosylation sites. Biochem J. 1991 Apr 15;275(Pt 2):529–534. doi: 10.1042/bj2750529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Huan L. J., Khatri I. A., Wang D., Bennick A., Fahim R. E., Forstner G. G., Forstner J. F. cDNA for the carboxyl-terminal region of a rat intestinal mucin-like peptide. J Biol Chem. 1992 Mar 15;267(8):5401–5407. [PubMed] [Google Scholar]




