Abstract
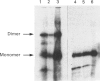
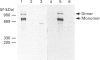
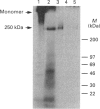
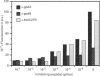
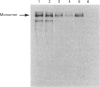
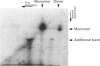
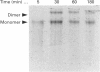
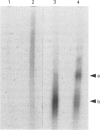
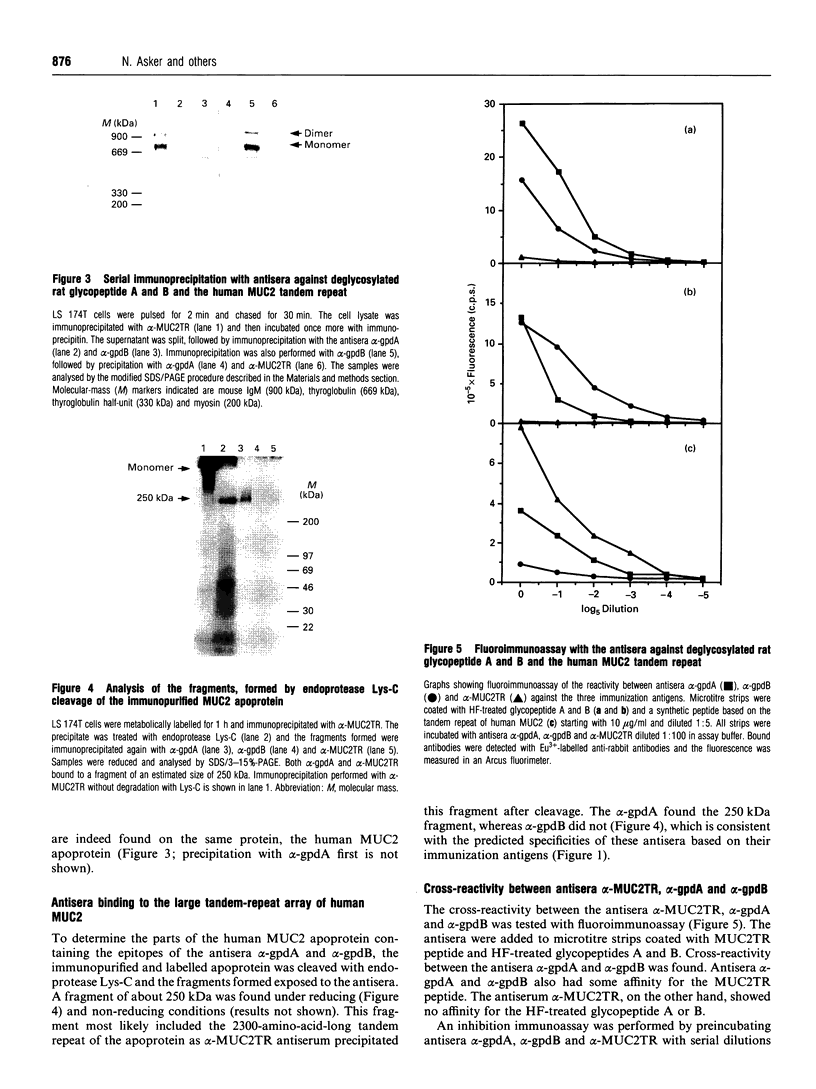
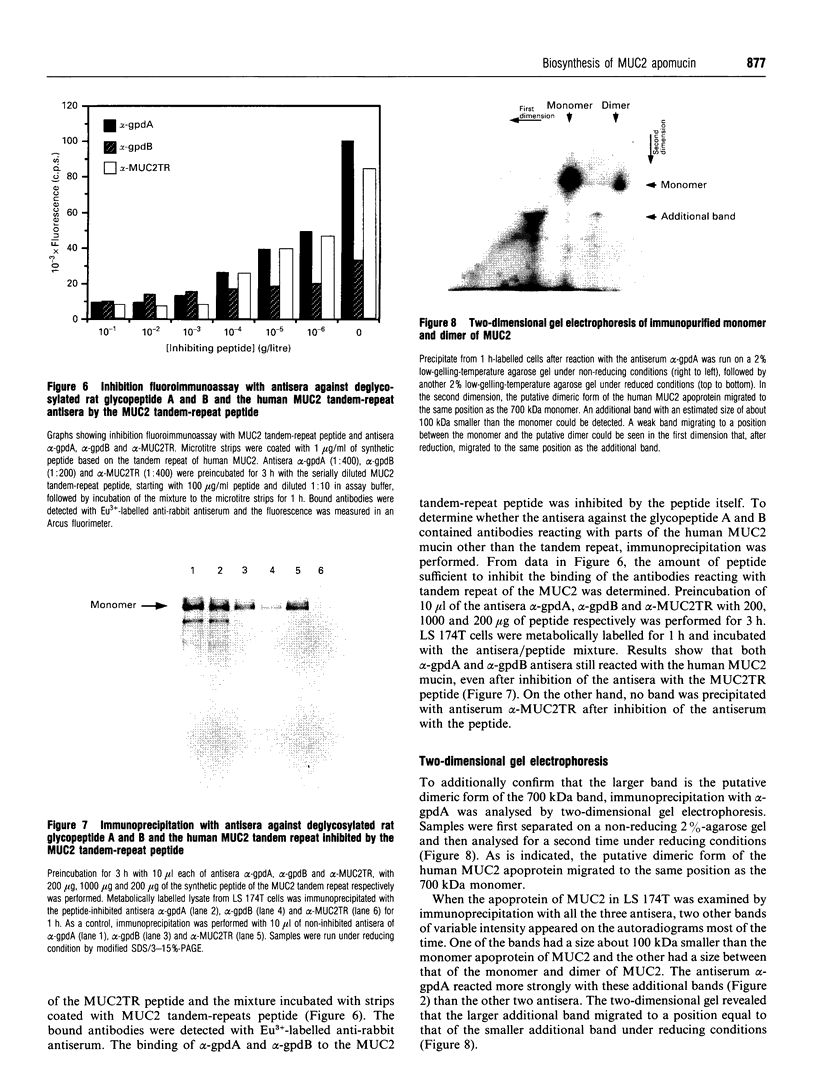
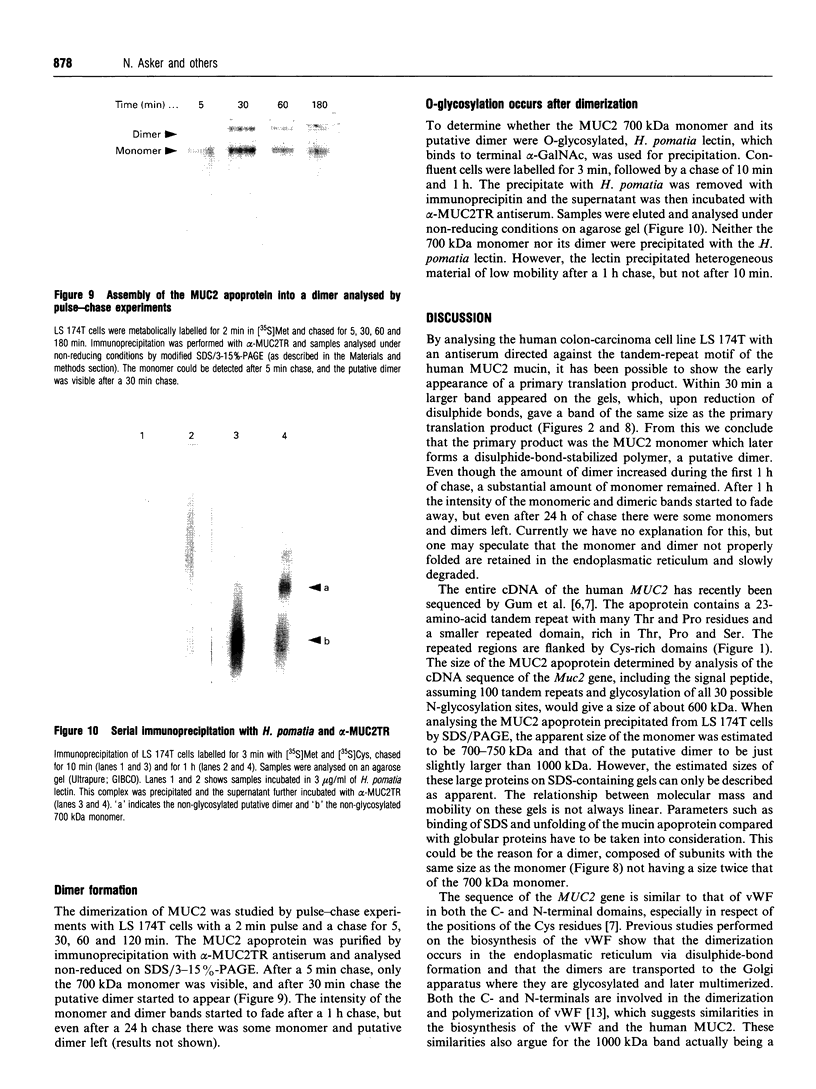
Rabbit antiserum against a synthetic peptide corresponding to a tandemly repeated amino acid sequence in the human intestinal mucin apoprotein MUC2 was used in immunoprecipitation to study the biosynthesis of MUC2 in the colon-carcinoma cell line LS 174T. Under non-reducing conditions, two bands were precipitated, the smaller with an apparent size of about 700 kDa on SDS/PAGE. When analysed by two-dimensional electrophoresis after reduction, the larger band migrated to the same position as the smaller band and was interpreted as a putative disulphide-bond-stabilized dimer. Pulse-chase experiments showed only the monomer after 5 min and the appearance of the putative dimer after 30 min. The MUC2 apoprotein was also precipitated by antisera against the HF-deglycosylated peptides of the two highly glycosylated domains of the 'insoluble' mucin complex of rat small intestine [Carlstedt, Herrmann, Karlsson, Sheehan, Fransson and Hansson (1993) J. Biol. Chem. 268, [18771-18781]. Endoprotease Lys-C cleavage of the immunopurified apoprotein gave a large fragment of about 250 kDa that was detected by both the antiserum against the MUC2 tandem repeat and one of the glycopeptide antisera. This supports the view that the 'insoluble' mucin of rat small intestine is encoded by the Muc2 gene, as recently indicated by a partial cDNA sequence [Hansson, Baeckström, Carlstedt and Klinga-Levan (1994) Biochem. Biophys. Res. Commun. 198, 181-190] and that parts of the apoprotein are conserved between the species. A lectin from the snail Helix pomatia that detects terminal alpha-GalNAc residues did not bind to the monomer or putative dimer, suggesting that O-glycosylation starts after dimerization. The results indicate that the biosynthetic pathway of the MUC2 mucin may be similar to that of the von Willebrand factor with which MUC2 shares sequence similarities at its C- and N-termini.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrd J. C., Lamport D. T., Siddiqui B., Kuan S. F., Erickson R., Itzkowitz S. H., Kim Y. S. Deglycosylation of mucin from LS174T colon cancer cells by hydrogen fluoride treatment. Biochem J. 1989 Jul 15;261(2):617–625. doi: 10.1042/bj2610617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Karlsson H., Sundler F., Fransson L. A. An insoluble mucin complex from rat small intestine. Adv Exp Med Biol. 1982;144:155–157. doi: 10.1007/978-1-4615-9254-9_22. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K., Corfield A. P., Gallagher J. T. Mucous glycoproteins: a gel of a problem. Essays Biochem. 1985;20:40–76. [PubMed] [Google Scholar]
- Dekker J., van der Ende A., Aelmans P. H., Strous G. J. Rat gastric mucin is synthesized and secreted exclusively as filamentous oligomers. Biochem J. 1991 Oct 1;279(Pt 1):251–256. doi: 10.1042/bj2790251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Rothe E. M., Lagace R. E., Kim Y. S. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem. 1992 Oct 25;267(30):21375–21383. [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Siddiki B., Kim Y. S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994 Jan 28;269(4):2440–2446. [PubMed] [Google Scholar]
- Hansson G. C., Baeckström D., Carlstedt I., Klinga-Levan K. Molecular cloning of a cDNA coding for a region of an apoprotein from the 'insoluble' mucin complex of rat small intestine. Biochem Biophys Res Commun. 1994 Jan 14;198(1):181–190. doi: 10.1006/bbrc.1994.1026. [DOI] [PubMed] [Google Scholar]
- Hemmilä I., Dakubu S., Mukkala V. M., Siitari H., Lövgren T. Europium as a label in time-resolved immunofluorometric assays. Anal Biochem. 1984 Mar;137(2):335–343. doi: 10.1016/0003-2697(84)90095-2. [DOI] [PubMed] [Google Scholar]
- Ho S. B., Niehans G. A., Lyftogt C., Yan P. S., Cherwitz D. L., Gum E. T., Dahiya R., Kim Y. S. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993 Feb 1;53(3):641–651. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCool D. J., Forstner J. F., Forstner G. G. Synthesis and secretion of mucin by the human colonic tumour cell line LS180. Biochem J. 1994 Aug 15;302(Pt 1):111–118. doi: 10.1042/bj3020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson S., Lundblad A., Hansson G. C., Carlstedt I. Mucins obtained from patients with enterocutaneous urinary diversions. Scand J Clin Lab Invest. 1988 Nov;48(7):633–640. [PubMed] [Google Scholar]
- Ohara S., Byrd J. C., Gum J. R., Jr, Kim Y. S. Biosynthesis of two distinct types of mucin in HM3 human colon cancer cells. Biochem J. 1994 Feb 1;297(Pt 3):509–516. doi: 10.1042/bj2970509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Dohrman A. F., Gallup M., Tsuda T., Kai H., Gum J. R., Jr, Kim Y. S., Basbaum C. B. Molecular cloning of the amino-terminal region of a rat MUC 2 mucin gene homologue. Evidence for expression in both intestine and airway. J Biol Chem. 1994 Jul 8;269(27):17833–17840. [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Ware J. von Willebrand factor. FASEB J. 1993 Feb 1;7(2):308–316. doi: 10.1096/fasebj.7.2.8440408. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Toribara N. W., Gum J. R., Jr, Culhane P. J., Lagace R. E., Hicks J. W., Petersen G. M., Kim Y. S. MUC-2 human small intestinal mucin gene structure. Repeated arrays and polymorphism. J Clin Invest. 1991 Sep;88(3):1005–1013. doi: 10.1172/JCI115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. doi: 10.1146/annurev.cb.06.110190.001245. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Lawrence S. O., Ohlsson-Wilhelm B. M., Fay P. J., Marder V. J. Topology and order of formation of interchain disulfide bonds in von Willebrand factor. Blood. 1987 Jan;69(1):27–32. [PubMed] [Google Scholar]
- Xu G., Huan L. J., Khatri I. A., Wang D., Bennick A., Fahim R. E., Forstner G. G., Forstner J. F. cDNA for the carboxyl-terminal region of a rat intestinal mucin-like peptide. J Biol Chem. 1992 Mar 15;267(8):5401–5407. [PubMed] [Google Scholar]