Abstract
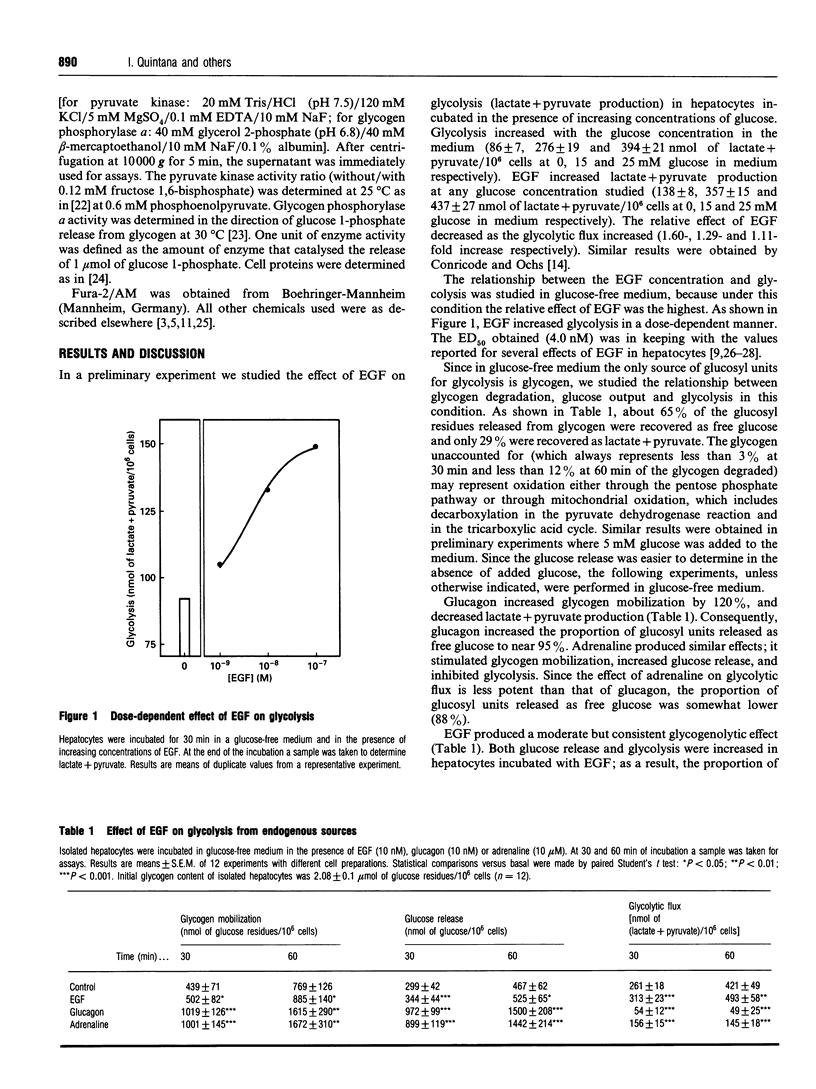
We have studied the relationship between the effect of epidermal growth factor (EGF) on glycogen metabolism and its effect on glycolysis, in rat hepatocyte suspensions. Although 10 nM glucagon or 10 microM adrenaline increased glycogen degradation by more than 120%, 10 nM EGF increased glycogenolysis by less than 20% in hepatocytes incubated in glucose-free medium. Both glucagon and adrenaline increased phosphorylase a activity by more than 130%; EGF increased this activity by about 30%. Under basal conditions, 65% of the glucosyl residues were released as free glucose and about 30% ended up as C3 molecules (lactate and pyruvate). Both glucagon and adrenaline decreased the proportion of glucosyl units that rendered glycolysis end-products (to 2% for glucagon and 6% for adrenaline) and increased the proportion that ended up as free glucose (to 94% and 88% of the glucosyl residues for glucagon and adrenaline respectively). EGF increased the production of both free glucose and lactate+pyruvate, but the proportion of glucosyl residues that ended up as free glucose or glycolysis end-products was unchanged. In glycogen-depleted hepatocytes incubated in the presence of 25 mM glucose, EGF affected neither glycogen deposition nor glycolysis. EGF increased cytosolic free Ca2+, and neomycin decreased both the Ca2+ signal and the glycogenolytic effect. In conclusion, our results indicate that the effect of EGF on glycolysis is secondary to the Ca(2+)-mediated stimulation of glycogenolysis in rat hepatocyte suspensions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J. B., James M. E., Foster J. L. Adrenergic control of glucose output and adenosine 3':5'-monophosphate levels in hepatocytes from juvenile and adult rats. J Biol Chem. 1979 Aug 25;254(16):7579–7584. [PubMed] [Google Scholar]
- Bosch F., Bouscarel B., Slaton J., Blackmore P. F., Exton J. H. Epidermal growth factor mimics insulin effects in rat hepatocytes. Biochem J. 1986 Nov 1;239(3):523–530. doi: 10.1042/bj2390523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik L. T., Mukhopadhyay A. K. Epidermal growth factor, a modulator of luteal adenylate cyclase. Characterization of epidermal growth factor receptors and its interaction with adenylate cyclase system in bovine luteal cell membrane. J Biol Chem. 1991 Jul 25;266(21):13908–13913. [PubMed] [Google Scholar]
- Budnik L. T., Mukhopadhyay A. K. Pertussis toxin can distinguish the augmentary effect elicited by epidermal growth factor from that of phorbol ester on luteal adenylate cyclase activity. Endocrinology. 1993 Jul;133(1):265–270. doi: 10.1210/endo.133.1.8319575. [DOI] [PubMed] [Google Scholar]
- Burgaya F., Peinado J., Vilaró S., Llobera M., Ramírez I. Lipoprotein lipase activity in neonatal-rat liver cell types. Biochem J. 1989 Apr 1;259(1):159–166. doi: 10.1042/bj2590159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Chowdhury M. H., Agius L. Epidermal growth factor counteracts the glycogenic effect of insulin in parenchymal hepatocyte cultures. Biochem J. 1987 Oct 15;247(2):307–314. doi: 10.1042/bj2470307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly P. A., Botelho L. H., Sisk R. B., Garrison J. C. A study of the mechanism of glucagon-induced protein phosphorylation in isolated rat hepatocytes using (Sp)-cAMPS and (Rp)-cAMPS, the stimulatory and inhibitory diastereomers of adenosine cyclic 3',5'-phosphorothioate. J Biol Chem. 1987 Mar 25;262(9):4324–4332. [PubMed] [Google Scholar]
- Conricode K. M., Ochs R. S. Epidermal growth factor and 12-O-tetradecanoylphorbol 13-acetate stimulate lactate production and the pentose phosphate pathway in freshly isolated rat hepatocytes. J Biol Chem. 1990 Dec 5;265(34):20931–20937. [PubMed] [Google Scholar]
- García-Sáinz J. A., Tussié-Luna M. I., Hernăndez-Sotomayor S. M. Insulin-like effect of epidermal growth factor in isolated rat hepatocytes. Modulation of the alpha-1-adrenergic stimulation of ureagenesis. Biochim Biophys Acta. 1986 Nov 28;889(2):266–269. doi: 10.1016/0167-4889(86)90113-8. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haystead T. A., Hardie D. G. Both insulin and epidermal growth factor stimulate lipogenesis and acetyl-CoA carboxylase activity in isolated adipocytes. Importance of homogenization procedure in avoiding artefacts in acetyl-CoA carboxylase assay. Biochem J. 1986 Mar 1;234(2):279–284. doi: 10.1042/bj2340279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Crofts J. N., Auld A. M., Read L. C., Barritt G. J. Evidence that a pertussis-toxin-sensitive substrate is involved in the stimulation by epidermal growth factor and vasopressin of plasma-membrane Ca2+ inflow in hepatocytes. Biochem J. 1987 Dec 15;248(3):911–918. doi: 10.1042/bj2480911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. P., Crofts J. N., Auld A. M., Read L. C., Barritt G. J. Evidence that a pertussis-toxin-sensitive substrate is involved in the stimulation by epidermal growth factor and vasopressin of plasma-membrane Ca2+ inflow in hepatocytes. Biochem J. 1987 Dec 15;248(3):911–918. doi: 10.1042/bj2480911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Connelly P. A., Sisk R. B., Pobiner B. F., Hewlett E. L., Garrison J. C. Pertussis toxin or phorbol 12-myristate 13-acetate can distinguish between epidermal growth factor- and angiotensin-stimulated signals in hepatocytes. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2032–2036. doi: 10.1073/pnas.83.7.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Garrison J. C. Epidermal growth factor and angiotensin II stimulate formation of inositol 1,4,5- and inositol 1,3,4-trisphosphate in hepatocytes. Differential inhibition by pertussis toxin and phorbol 12-myristate 13-acetate. J Biol Chem. 1987 Dec 25;262(36):17285–17293. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leoni S., Spagnuolo S., Marino M., Terenzi F., Massimi M., Conti Devirgiliis L. Different signal transduction by epidermal growth factor may be responsible for the difference in modulation of amino acid transport between fetal and adult hepatocytes. J Cell Physiol. 1993 Jun;155(3):549–555. doi: 10.1002/jcp.1041550313. [DOI] [PubMed] [Google Scholar]
- Liang M. N., Garrison J. C. The epidermal growth factor receptor is coupled to a pertussis toxin-sensitive guanine nucleotide regulatory protein in rat hepatocytes. J Biol Chem. 1991 Jul 15;266(20):13342–13349. [PubMed] [Google Scholar]
- Liang M., Garrison J. C. Epidermal growth factor activates phospholipase C in rat hepatocytes via a different mechanism from that in A431 or rat1hER cells. Mol Pharmacol. 1992 Nov;42(5):743–752. [PubMed] [Google Scholar]
- Missale C., Boroni F., Castelletti L., Dal Toso R., Gabellini N., Sigala S., Spano P. Lack of coupling of D-2 receptors to adenylate cyclase in GH-3 cells exposed to epidermal growth factor. Possible role of a differential expression of Gi protein subtypes. J Biol Chem. 1991 Dec 5;266(34):23392–23398. [PubMed] [Google Scholar]
- Moreno F., Pastor-Anglada M., Hollenberg M. D., Soley M. Effects of epidermal growth factor (urogastrone) on gluconeogenesis, glucose oxidation, and glycogen synthesis in isolated rat hepatocytes. Biochem Cell Biol. 1989 Oct;67(10):724–729. doi: 10.1139/o89-108. [DOI] [PubMed] [Google Scholar]
- Moule S. K., Edgell N. J., Borthwick A. C., Denton R. M. Coenzyme A is a potent inhibitor of acetyl-CoA carboxylase from rat epididymal fat-pads. Biochem J. 1992 Apr 1;283(Pt 1):35–38. doi: 10.1042/bj2830035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moule S. K., McGivan J. D. Epidermal growth factor stimulates the phosphorylation of pyruvate kinase in freshly isolated rat hepatocytes. FEBS Lett. 1991 Mar 11;280(1):37–40. doi: 10.1016/0014-5793(91)80198-c. [DOI] [PubMed] [Google Scholar]
- Moule S. K., McGivan J. F. Epidermal-growth-factor stimulation of gluconeogenesis in isolated rat hepatocytes involves the inactivation of pyruvate kinase. Biochem J. 1988 Oct 1;255(1):361–364. [PMC free article] [PubMed] [Google Scholar]
- Nair B. G., Parikh B., Milligan G., Patel T. B. Gs alpha mediates epidermal growth factor-elicited stimulation of rat cardiac adenylate cyclase. J Biol Chem. 1990 Dec 5;265(34):21317–21322. [PubMed] [Google Scholar]
- Nair B. G., Rashed H. M., Patel T. B. Epidermal growth factor stimulates rat cardiac adenylate cyclase through a GTP-binding regulatory protein. Biochem J. 1989 Dec 1;264(2):563–571. doi: 10.1042/bj2640563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peak M., Agius L. Inhibition of glycogen synthesis by epidermal growth factor in hepatocytes. The role of cell density and pertussis toxin-sensitive GTP-binding proteins. Eur J Biochem. 1994 Apr 1;221(1):529–536. doi: 10.1111/j.1432-1033.1994.tb18765.x. [DOI] [PubMed] [Google Scholar]
- Rashed S. M., Patel T. B. Regulation of hepatic energy metabolism by epidermal growth factor. Eur J Biochem. 1991 May 8;197(3):805–813. doi: 10.1111/j.1432-1033.1991.tb15975.x. [DOI] [PubMed] [Google Scholar]
- Rashed S. M., Patel T. B. Regulation of hepatic energy metabolism by epidermal growth factor. Eur J Biochem. 1991 May 8;197(3):805–813. doi: 10.1111/j.1432-1033.1991.tb15975.x. [DOI] [PubMed] [Google Scholar]
- Severson D. L., Carroll R., Kryski A., Jr, Ramírez I. Short-term incubation of cardiac myocytes with isoprenaline has no effect on heparin-releasable or cellular lipoprotein lipase activity. Biochem J. 1987 Nov 15;248(1):289–292. doi: 10.1042/bj2480289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C., Galan X., Peinado-Onsurbe J., Quintana I., Llobera M., Soley M., Ramírez I. Epidermal growth factor interferes with the effect of adrenaline on glucose production and on hepatic lipase secretion in rat hepatocytes. Regul Pept. 1993 Mar 5;44(1):11–16. doi: 10.1016/0167-0115(93)90125-r. [DOI] [PubMed] [Google Scholar]
- Soler C., Poveda B., Pastor-Anglada M., Soley M. Effect of epidermal growth factor (EGF) on gluconeogenesis in isolated rat hepatocytes. Dependency on the red-ox state of the substrate. Biochim Biophys Acta. 1991 Jan 31;1091(2):193–196. doi: 10.1016/0167-4889(91)90061-2. [DOI] [PubMed] [Google Scholar]
- Soler C., Soley M. Rapid and delayed effects of epidermal growth factor on gluconeogenesis. Biochem J. 1993 Sep 15;294(Pt 3):865–872. doi: 10.1042/bj2940865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soley M., Hollenberg M. D. Epidermal growth factor (urogastrone)-stimulated gluconeogenesis in isolated mouse hepatocytes. Arch Biochem Biophys. 1987 May 15;255(1):136–146. doi: 10.1016/0003-9861(87)90303-1. [DOI] [PubMed] [Google Scholar]
- Tebar F., Ramírez I., Soley M. Epidermal growth factor modulates the lipolytic action of catecholamines in rat adipocytes. Involvement of a Gi protein. J Biol Chem. 1993 Aug 15;268(23):17199–17204. [PubMed] [Google Scholar]
- Wilson M. D., Rudel L. L. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J Lipid Res. 1994 Jun;35(6):943–955. [PubMed] [Google Scholar]
- Yang L. J., Baffy G., Rhee S. G., Manning D., Hansen C. A., Williamson J. R. Pertussis toxin-sensitive Gi protein involvement in epidermal growth factor-induced activation of phospholipase C-gamma in rat hepatocytes. J Biol Chem. 1991 Nov 25;266(33):22451–22458. [PubMed] [Google Scholar]
- Yang L., Camoratto A. M., Baffy G., Raj S., Manning D. R., Williamson J. R. Epidermal growth factor-mediated signaling of G(i)-protein to activation of phospholipases in rat-cultured hepatocytes. J Biol Chem. 1993 Feb 15;268(5):3739–3746. [PubMed] [Google Scholar]


