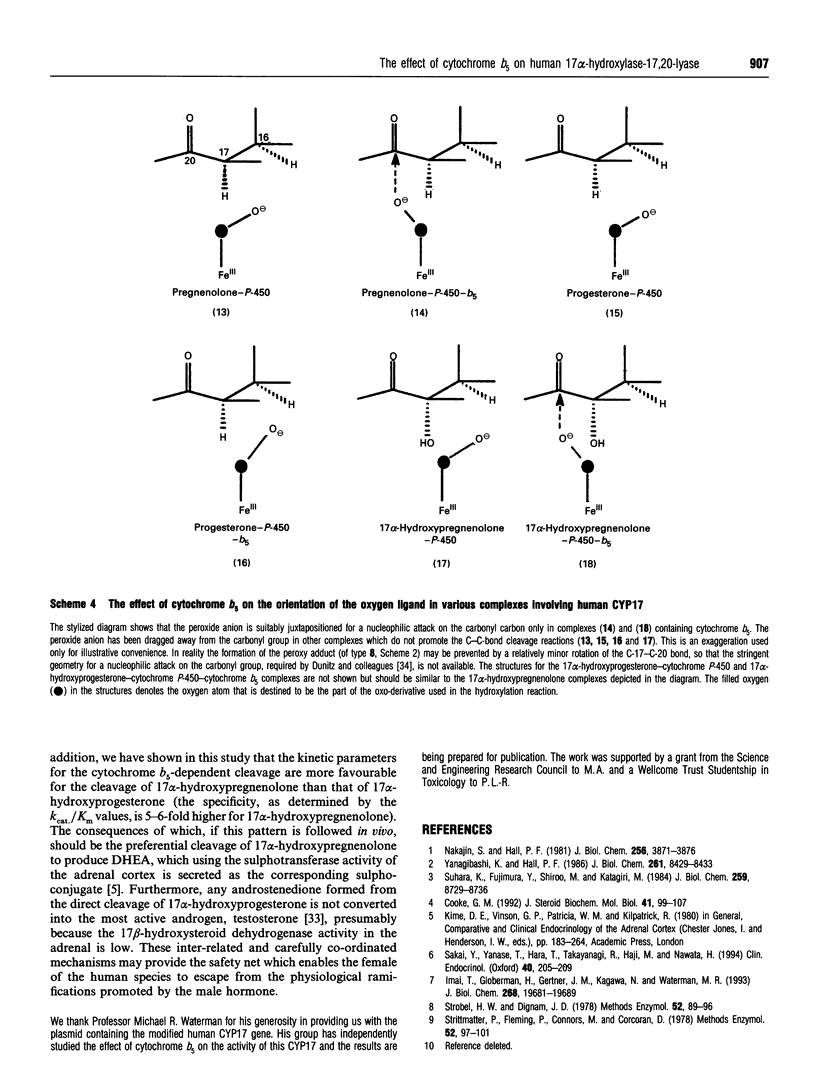
Abstract
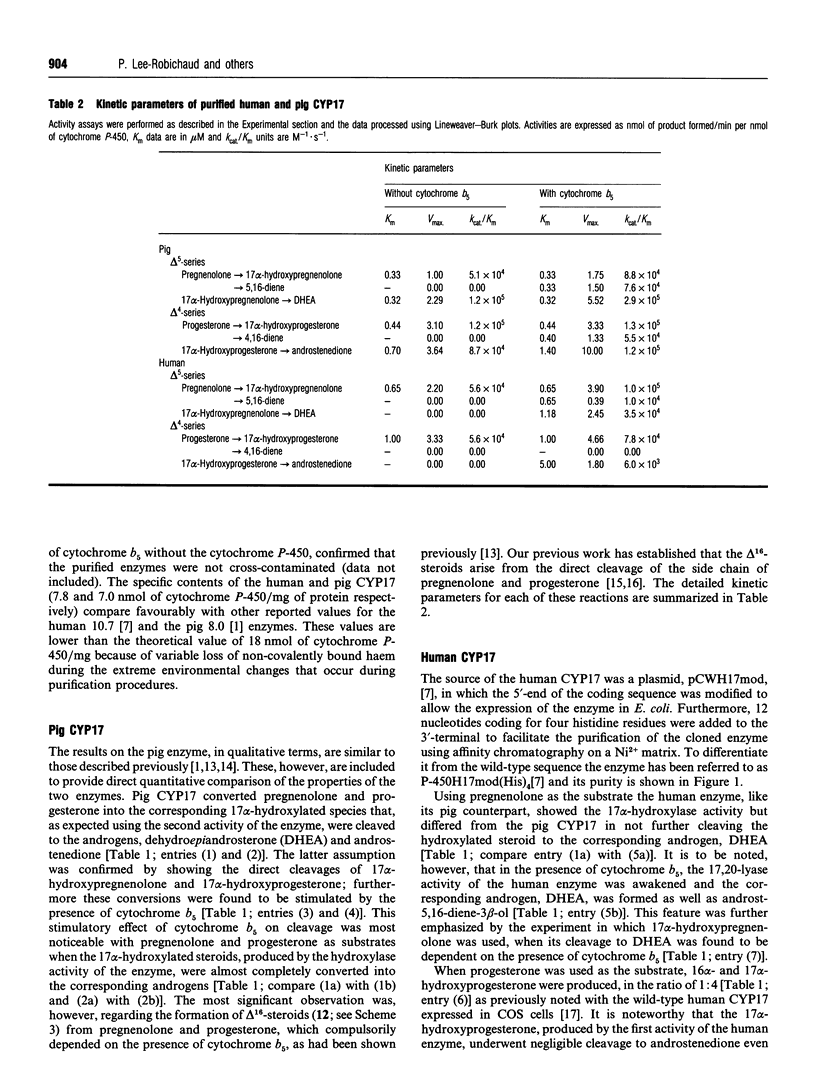
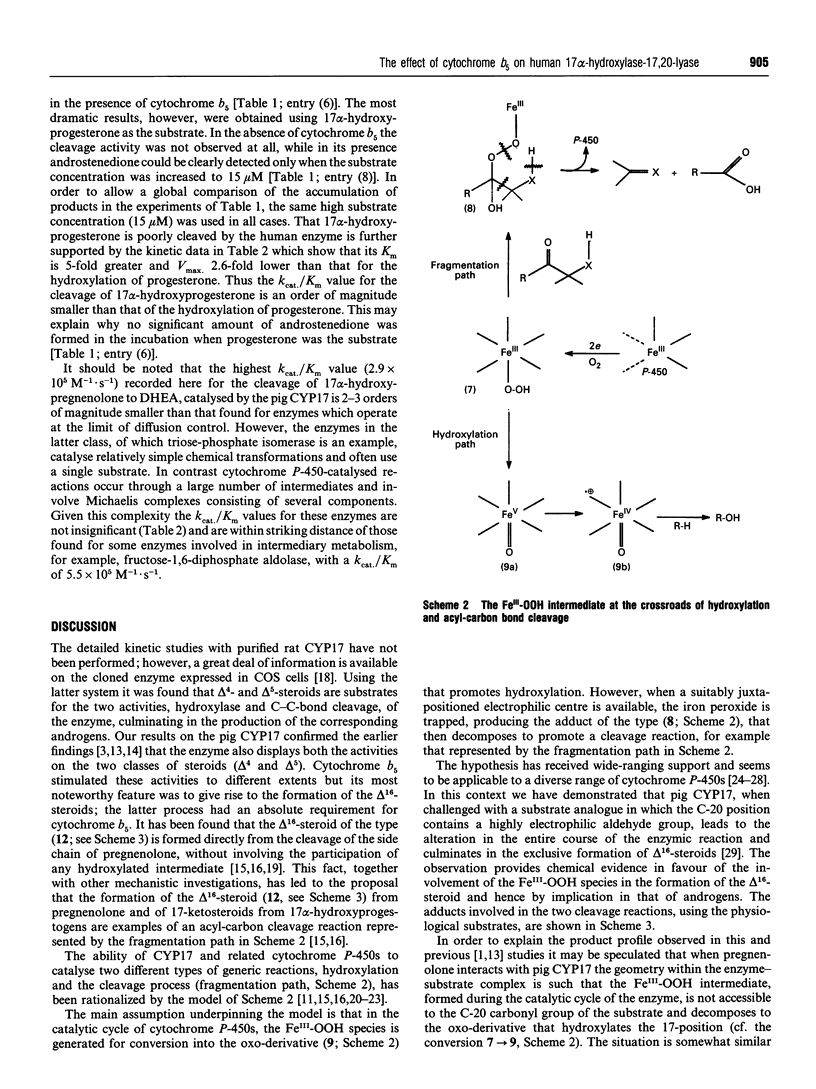
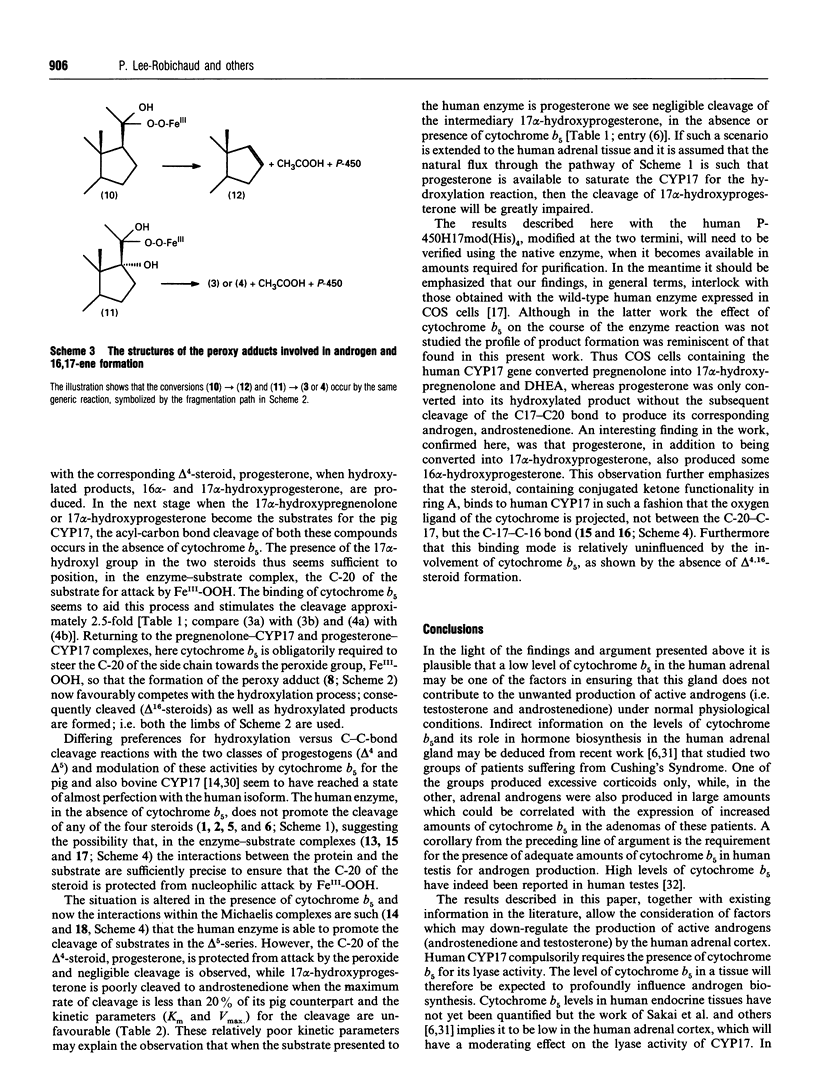
Using NADPH-cytochrome P-450 reductase as electron donor the homogeneous pig 17 alpha-hydroxylase-17,20-lyase (CYP17) was shown to catalyse the conversion of delta 5, as well as delta 4, steroids (pregnenolone and progesterone respectively) predominantly into the corresponding 17 alpha-hydroxylated products. The latter were then cleaved by the lyase (desmolase) activity of the enzyme into androgens. Cytochrome b5 stimulated both these activities, but its most noticeable effect was on the formation of delta 16-steroids, which compulsorily required the presence of cytochrome b5. These results on the pig enzyme confirm the original findings [Nakajin, Takahashi, Shinoda and Hall (1985) Biochem. Biophys. Res. Commun. 132, 708-713]. The human CYP17 expressed in Escherichia coli [Imai, Globerman, Gertner, Kagawa and Waterman (1993) J. Biol. Chem. 268, 19681-19689] was also purified to homogeneity and was found to catalyse the hydroxylation of pregnenolone and progesterone without requiring cytochrome b5. Like the pig CYP17, the human CYP17 also catalysed the cytochrome b5-dependent direct cleavage of pregnenolone into the delta 5,16-steroid, but unlike it the human enzyme did not cleave progesterone at all. 17 alpha-Hydroxypregnenolone was, however, cleaved into the corresponding androgen but only in the presence of cytochrome b5. 17 alpha-Hydroxyprogesterone was a poor substrate for the human CYP17; although it was converted into androstenedione in the presence of cytochrome b5 its K(m) was 5 times higher and Vmax. 2.6 times lower than those for the hydroxylation of progesterone. The endocrinological and mechanistic implications of these results are discussed.
Full text
PDF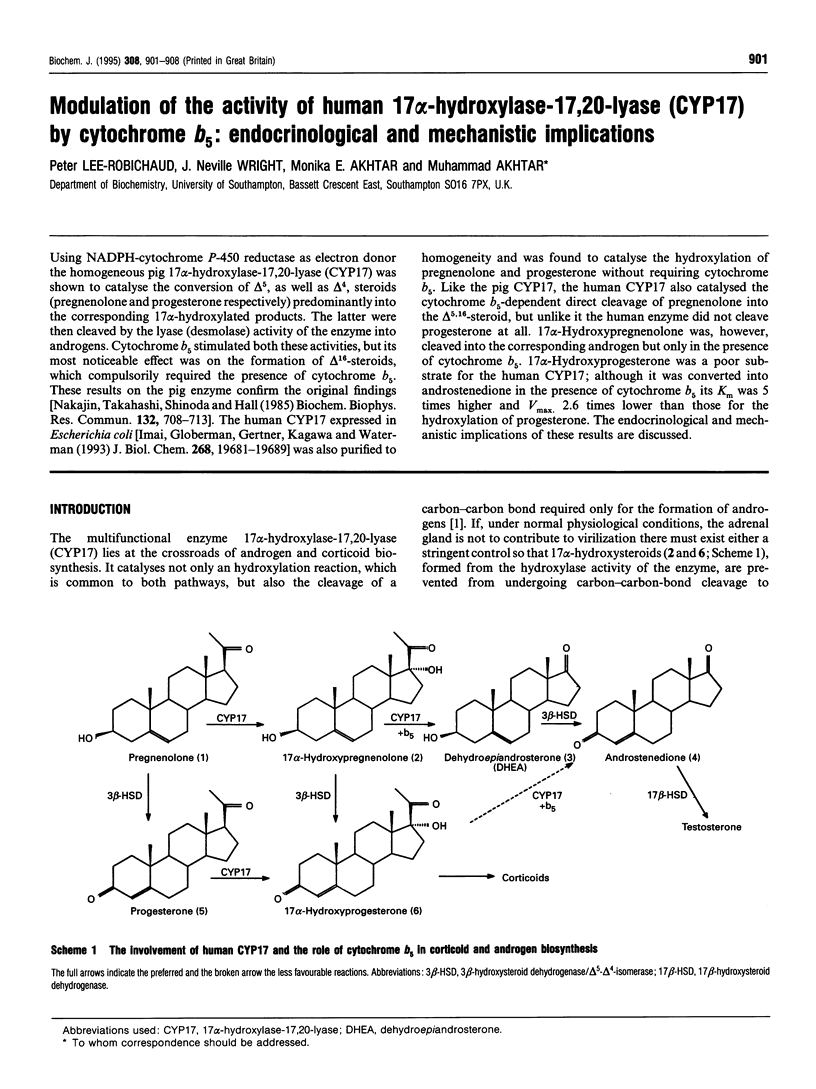



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Corina D., Miller S., Shyadehi A. Z., Wright J. N. Mechanism of the acyl-carbon cleavage and related reactions catalyzed by multifunctional P-450s: studies on cytochrome P-450(17)alpha. Biochemistry. 1994 Apr 12;33(14):4410–4418. doi: 10.1021/bi00180a039. [DOI] [PubMed] [Google Scholar]
- Akhtar M., Njar V. C., Wright J. N. Mechanistic studies on aromatase and related C-C bond cleaving P-450 enzymes. J Steroid Biochem Mol Biol. 1993 Mar;44(4-6):375–387. doi: 10.1016/0960-0760(93)90241-n. [DOI] [PubMed] [Google Scholar]
- Akhtar M., Wright J. N. A unified mechanistic view of oxidative reactions catalysed by P-450 and related Fe-containing enzymes. Nat Prod Rep. 1991 Dec;8(6):527–551. doi: 10.1039/np9910800527. [DOI] [PubMed] [Google Scholar]
- Cooke G. M. Phospholipases modulate immature pig testicular androgen and 16-androstene biosynthetic pathways in vitro. J Steroid Biochem Mol Biol. 1992 Jan;41(1):99–107. doi: 10.1016/0960-0760(92)90230-g. [DOI] [PubMed] [Google Scholar]
- Imai T., Globerman H., Gertner J. M., Kagawa N., Waterman M. R. Expression and purification of functional human 17 alpha-hydroxylase/17,20-lyase (P450c17) in Escherichia coli. Use of this system for study of a novel form of combined 17 alpha-hydroxylase/17,20-lyase deficiency. J Biol Chem. 1993 Sep 15;268(26):19681–19689. [PubMed] [Google Scholar]
- Koh Y., Buczko E., Dufau M. L. Requirement of phenylalanine 343 for the preferential delta 4-lyase versus delta 5-lyase activity of rat CYP17. J Biol Chem. 1993 Aug 25;268(24):18267–18271. [PubMed] [Google Scholar]
- Kremers P., Denoel J., Lapiere C. L. Synthesis and study of the labeling of pregnenolone and progesterone specifically tritiated at the 17 position. Steroids. 1974 Apr;23(4):603–613. doi: 10.1016/0039-128x(74)90011-7. [DOI] [PubMed] [Google Scholar]
- Marletta M. A. Approaches toward selective inhibition of nitric oxide synthase. J Med Chem. 1994 Jun 24;37(13):1899–1907. doi: 10.1021/jm00039a001. [DOI] [PubMed] [Google Scholar]
- Mason J. I., Estabrook R. W., Purvis J. L. Testicular cytochrome P-450 and iron-sulfur protein as related to steroid metabolism. Ann N Y Acad Sci. 1973;212:406–419. doi: 10.1111/j.1749-6632.1973.tb47610.x. [DOI] [PubMed] [Google Scholar]
- Meadus W. J., Mason J. I., Squires E. J. Cytochrome P450c17 from porcine and bovine adrenal catalyses the formation of 5,16-androstadien-3 beta-ol from pregnenolone in the presence of cytochrome b5. J Steroid Biochem Mol Biol. 1993 Nov;46(5):565–572. doi: 10.1016/0960-0760(93)90183-w. [DOI] [PubMed] [Google Scholar]
- Nakajin S., Hall P. F. Microsomal cytochrome P-450 from neonatal pig testis. Purification and properties of A C21 steroid side-chain cleavage system (17 alpha-hydroxylase-C17,20 lyase). J Biol Chem. 1981 Apr 25;256(8):3871–3876. [PubMed] [Google Scholar]
- Nakajin S., Takahashi M., Shinoda M., Hall P. F. Cytochrome b5 promotes the synthesis of delta 16-C19 steroids by homogeneous cytochrome P-450 C21 side-chain cleavage from pig testis. Biochem Biophys Res Commun. 1985 Oct 30;132(2):708–713. doi: 10.1016/0006-291x(85)91190-8. [DOI] [PubMed] [Google Scholar]
- Roberts E. S., Vaz A. D., Coon M. J. Catalysis by cytochrome P-450 of an oxidative reaction in xenobiotic aldehyde metabolism: deformylation with olefin formation. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8963–8966. doi: 10.1073/pnas.88.20.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y., Yanase T., Hara T., Takayanagi R., Haji M., Nawata H. In-vitro evidence for the regulation of 17,20-lyase activity by cytochrome b5 in adrenocortical adenomas from patients with Cushing's syndrome. Clin Endocrinol (Oxf) 1994 Feb;40(2):205–209. doi: 10.1111/j.1365-2265.1994.tb02469.x. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Yanase T., Takayanagi R., Nakao R., Nishi Y., Haji M., Nawata H. High expression of cytochrome b5 in adrenocortical adenomas from patients with Cushing's syndrome associated with high secretion of adrenal androgens. J Clin Endocrinol Metab. 1993 May;76(5):1286–1290. doi: 10.1210/jcem.76.5.8496319. [DOI] [PubMed] [Google Scholar]
- Shet M. S., Fisher C. W., Arlotto M. P., Shackleton C. H., Holmans P. L., Martin-Wixtrom C. A., Saeki Y., Estabrook R. W. Purification and enzymatic properties of a recombinant fusion protein expressed in Escherichia coli containing the domains of bovine P450 17A and rat NADPH-P450 reductase. Arch Biochem Biophys. 1994 Jun;311(2):402–417. doi: 10.1006/abbi.1994.1255. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Nakada F. Formation of [17-2H]androsta-5,16-dien-3beta-ol from [17,21,21,21]pregnenolone by the microsomal fraction of boar testis. Biochim Biophys Acta. 1976 Dec 20;450(3):441–449. [PubMed] [Google Scholar]
- Strittmatter P., Fleming P., Connors M., Corcoran D. Purification of cytochrome b5. Methods Enzymol. 1978;52:97–101. doi: 10.1016/s0076-6879(78)52010-7. [DOI] [PubMed] [Google Scholar]
- Strobel H. W., Dignam J. D. Purification and properties of NADPH-cytochrome P-450 reductase. Methods Enzymol. 1978;52:89–96. doi: 10.1016/s0076-6879(78)52009-0. [DOI] [PubMed] [Google Scholar]
- Suhara K., Fujimura Y., Shiroo M., Katagiri M. Multiple catalytic properties of the purified and reconstituted cytochrome P-450 (P-450sccII) system of pig testis microsomes. J Biol Chem. 1984 Jul 25;259(14):8729–8736. [PubMed] [Google Scholar]
- Swart P., Swart A. C., Waterman M. R., Estabrook R. W., Mason J. I. Progesterone 16 alpha-hydroxylase activity is catalyzed by human cytochrome P450 17 alpha-hydroxylase. J Clin Endocrinol Metab. 1993 Jul;77(1):98–102. doi: 10.1210/jcem.77.1.8325965. [DOI] [PubMed] [Google Scholar]
- Swinney D. C., Mak A. Y. Androgen formation by cytochrome P450 CYP17. Solvent isotope effect and pL studies suggest a role for protons in the regulation of oxene versus peroxide chemistry. Biochemistry. 1994 Mar 1;33(8):2185–2190. doi: 10.1021/bi00174a027. [DOI] [PubMed] [Google Scholar]
- WIELAND R. G., DECOURCY C., LEVY R. P., ZALA A. P., HIRSCHMANN H. C-19-O-2 STEROIDS AND SOME OF THEIR PRECURSORS IN BLOOD FROM NORMAL HUMAN ADRENALS. J Clin Invest. 1965 Jan;44:159–168. doi: 10.1172/JCI105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagibashi K., Hall P. F. Role of electron transport in the regulation of the lyase activity of C21 side-chain cleavage P-450 from porcine adrenal and testicular microsomes. J Biol Chem. 1986 Jun 25;261(18):8429–8433. [PubMed] [Google Scholar]



