Abstract
Introduction
Goeckerman therapy, which combines ultraviolet B (UVB) light with crude coal tar (CCT), remains highly effective for moderate-to-severe psoriasis. While it is rarely still used in the USA as effective biotherapeutics have become more readily available, it offers an alternative therapy in developing countries with limited access to newer medications. Moi Teaching & Referral Hospital (MTRH) in Eldoret, Kenya, in collaboration with UCSF, developed a modified Goeckerman regimen suitable for local healthcare needs, condensing the treatment into an intensive two-week program.
Case Report
A 55-year-old female with erythrodermic psoriasis traveled 350 kilometers to MTRH. After the diagnosis was confirmed, she underwent a nine-day inpatient treatment with narrow-band UVB phototherapy and topical medications under occlusion as a modified Goeckerman regimen.
Response to Treatment
Significant improvement was observed within three days, with full recovery in ten days. Follow-up one month later showed no active lesions, and her psoriasis remained controlled for four months with topical treatments.
Conclusion
The modified Goeckerman regimen at MTRH, in collaboration with UCSF, effectively treated severe psoriasis in a challenging healthcare context. This case highlights the potential for adapting established treatments to improve patient outcomes in developing countries with limited access to systemic therapies.
Keywords: Goeckerman therapy, erythrodermic psoriasis, UVB phototherapy, global health
Introduction
Goeckerman therapy was developed in 1925 and remains a highly effective treatment for moderate-to-severe psoriasis, including patients who have tried and failed other therapies.1,2 Traditional Goeckerman therapy combines exposure to ultraviolet B (UVB) light with the application of crude coal tar (CCT) until disease clearance, but for a minimum of six weeks.3 Currently, only a few institutions in the USA still employ Goeckerman therapy as injectable biologic therapies now offer a less time intensive, but extremely efficacious option for treating psoriasis.4 In developing countries, where access to newer psoriasis medications is limited, alternative therapies like the Goeckerman regimen could provide an effective treatment option outside biologics.5,6 Recognizing the need for accessible psoriasis treatments in developing countries, Moi Teaching & Referral Hospital (MTRH) in Eldoret, Kenya, developed a modified Goeckerman regimen in collaboration with the University of California San Francisco (UCSF) suitable for its healthcare context (Table 1).
Table 1.
Modified Goeckerman Regimen for Psoriasis Treatment, Detailing Cool-Down Procedures, Phototherapy, Tar Application Protocols, Daily Monitoring, and Discharge and Maintenance Plans
| Cool Down Procedure* | |
| Topical corticosteroids application: | |
| - Trunk/Extremities: Triamcinolone 0.1% ointment | |
| - Face/Axillae/Groin: Hydrocortisone 2.5% cream | |
| - Scalp: Clobetasol 0.05% lotion | |
| - Highly inflamed areas: Clobetasol 0.05% ointment | |
| Occlusion: | |
| - Trunk/Extremities: Cling film | |
| - Hands: Impermeable gloves | |
| - Feet: Shower caps inside socks | |
| - Scalp: Shower cap | |
| Phototherapy and Tar | The patient should shower daily before phototherapy sessions. |
| Set up a treatment room with plastic wrap, socks, UV eye protection, and gowns | |
| Decide on initial phototherapy dose using the Fitzpatrick skin type (Table 2) and increase the phototherapy dose based on patient response. | |
| Post-phototherapy, apply crude coal tar (CCT) in Aquaphor to affected areas. If CCT is not available, then an alternative type of tar or topical steroid under occlusion can be used. | |
| For scalp involvement, apply 4% tar shampoo or 20% liquid carbonis detergens (LCD) in Nutraderm. | |
| Start with the lowest CCT strength (2%), and increase strength as tolerated by the patient (up to 5%). | |
| Weekend Protocol: | As the phototherapy center is closed, instruct the patient to use 10% CCT in the morning and Mometasone cream/ointment at night. |
| Daily Monitoring | Assess the patient’s skin daily for sensations of burning, which can indicate the patient’s inability to tolerate light therapy, or worsening itch, which can indicate irritation from the tar, light, or plastic wrap. |
| Decrease phototherapy dose if any adverse reactions are detected. | |
| Continue the therapy for two weeks, then plan discharge. | |
| Discharge and Maintenance | Prescribe topical medications: Triamcinolone cream/ointment (twice daily for body), Clobetasol with or without vitamin D analog for recalcitrant lesions (twice daily), and 20% LCD in Aquaphor to the body (once daily), and 20% LCD in Nutraderm to the scalp (once daily). |
| If able, schedule for outpatient phototherapy 1–3x/week for at least one month (preferably 2–3 months). | |
| Follow-Up: | Schedule a physician appointment within 1–2 months post-discharge for monitoring. |
Notes: *If necessary, ask patient to do remotely at home for two weeks before the start of the program, as well as up to the first five days of the program, depending on the level of erythema.
This adaptation was specifically designed for patients who travel long distances for treatment, condensing the therapy into an intensive two-week program. The original Goeckerman regimen is administered for at least six weeks. It involves an initial evaluation, a possible cool-down phase with topical corticosteroids for erythema, followed by daily narrow band (most commonly used for psoriasis) or broadband UVB phototherapy and CCT (potentially compounded with salicylic acid) application.3 In contrast, the modified regimen streamlines the process to accommodate the logistical challenges faced by patients in remote areas. This includes an initial evaluation, a potential remote cool-down period before hospital admission, and a daily, condensed schedule of phototherapy and CCT or other topical applications based on available resources. We present a case of a patient with severe psoriasis successfully treated with a modified Goeckerman regimen at the MTRH.
Case Report
A 55-year-old female traveled 350 kilometers (217 miles) from her home to MTRH, Eldoret, Kenya, with a four-month history of progressive, painful skin redness, scaling, and severe pruritus covering most of her body (Figures 1–3). The lesions initially started with a single plaque on her left arm, then rapidly progressed, and within two months affected most of her skin. She reported associated chills, weight loss, edema, and difficulty maintaining body temperature. She was treated with topical and oral corticosteroids with minimal improvement. The patient had a medical history of treated hypertension and was also recently treated for Entamoeba histolytica infection with metronidazole (400 mg, TID) for ten days. The patient reported no history of dermatologic diseases and had no known drug allergies. Physical examination revealed widespread erythema, scaling, generalized edema, and skin exfoliation on Fitzpatrick skin type VI. A skin biopsy showed confluent parakeratosis and hypogranulosis, regular acanthosis, Munro’s microabscesses, and thin supra-papillary plates. The papillary blood vessels were dilated and surrounded by lymphocytic cell infiltrate. Based on both clinical presentation and histopathology, the patient was diagnosed with erythrodermic psoriasis.
Figure 1.
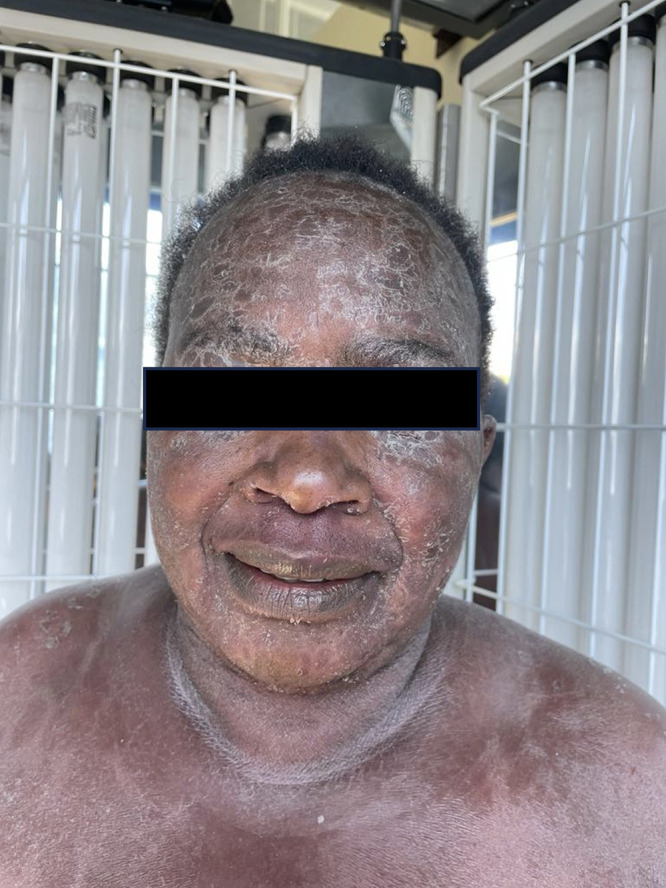
Widespread erythema, pronounced scaling around patient’s eyes and forehead.
Figure 2.
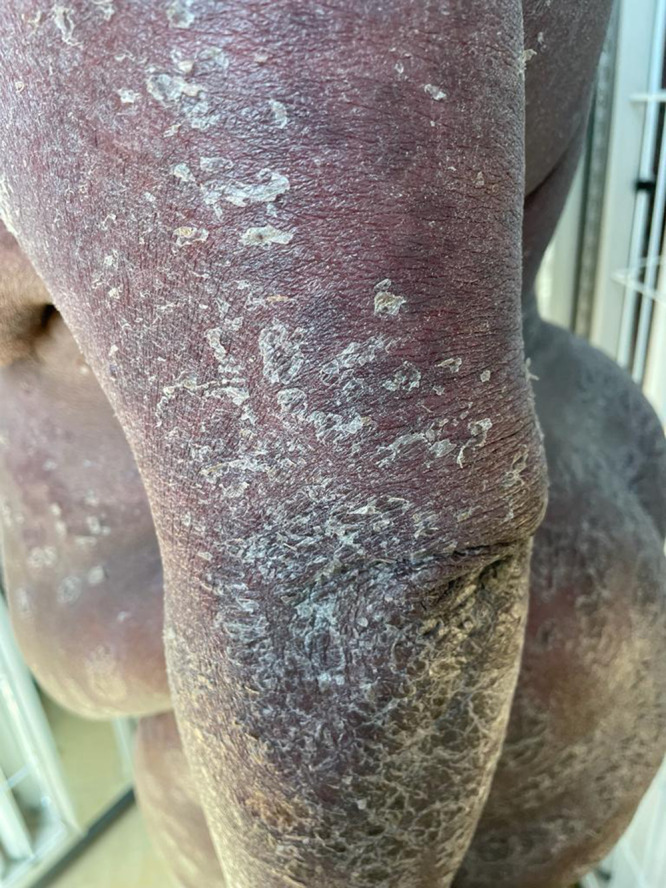
Extensive erythema and scaling on patient’s left arm, with patches of skin exfoliation visible against Fitzpatrick skin type 6.
Figure 3.
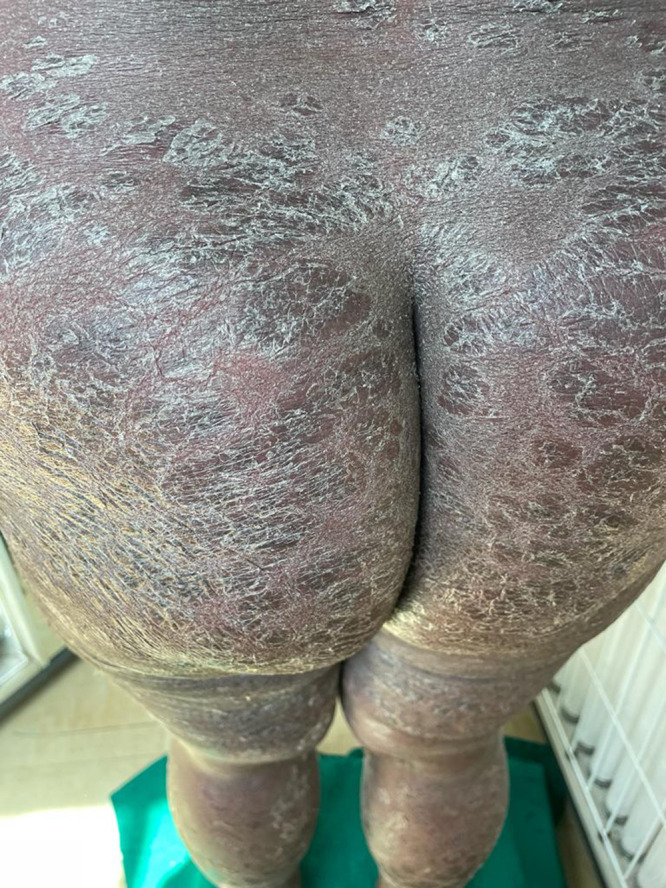
Severe erythema, scaling, and signs of skin exfoliation across patient’s lower back, buttocks, and legs.
The patient was admitted for inpatient care, and treatment included a series of narrow-band UVB phototherapy sessions and application of topical medication (clobetasol propionate ointment 0.001% and triamcinolone acetonide ointment 0.025%) under cling wrap occlusion to enhance treatment effectiveness. The treatment took place over nine days (Table 3), with phototherapy sessions administered on days 1, 3, 6, 8, and 9, adjusting the dosage based on the Fitzpatrick skin type (Table 2). The patient responded positively to the therapy within the first three days, showing significant improvements such as cessation of itching and better vision due to reduced psoriasis around the eyes. Adverse effects, such as burning, were monitored throughout the treatment course. Marked improvement was observed in 10 days.
Table 3.
Phototherapy Schedule and Adjunctive Topical Medications Used by the Treatment Team, Highlighting Progress and Adjustments
| Date | Treatment Details |
|---|---|
| Day 1 | First phototherapy session: 455 mJ over 2 minutes and 57 seconds |
| Day 3 | Second phototherapy session: 450 mJ over 2 minutes and 53 seconds; followed by 4 hours of topical medications and cling wrap, resulting in beginning scale shedding. |
| Day 4 | Topical medications and cling wrap reapplied for 4 hours. |
| Day 6 | Third phototherapy session: 600 mJ over 3 minutes and 42 seconds; followed by 4 hours of topical medications and cling wrap. |
| Day 7 | Topical medications and cling wrap application for 4 hours. |
| Day 8 | Fourth phototherapy session: 900 mJ over 5 minutes and 32 seconds, followed by 4 hours of topical medications and cling wrap. |
| Day 9 | Fifth phototherapy session: 950 mJ over 6 minutes and 38 seconds, followed by 4 hours of topical medications and cling wrap. |
Table 2.
Initial Dosing and Subsequent Increase Protocols for Narrow Band UVB (NBUVB) Phototherapy Based on the Fitzpatrick Skin Type
| Skin Type | NBUVB Initial Dose | Subsequent increase |
|---|---|---|
| I | 130 mJ | 15 mJ |
| II | 220 mJ | 25 mJ |
| III | 260 mJ | 40 mJ |
| IV | 330 mJ | 45 mJ |
| V | 350 mJ | 60 mJ |
| VI | 400 mJ | 65 mJ |
At discharge, the patient was prescribed LINOTAR 1% coal tar gel to use at home. After completing the treatment, the patient returned home and resumed her normal daily activities. A follow-up after one month revealed no active lesions and post-inflammatory hyperpigmented patches that were not causing discomfort, as seen in Figures 4 and 5. For maintenance, the patient intermittently continued the use of topical treatments (clobetasol, triamcinolone, and LINOTAR 1% coal tar gel). Her psoriasis remains under control, for at least four months since treatment, at the time of writing this report.
Figure 4.

Significant resolution of lesions on patient’s face, with restored skin integrity and evident post-inflammatory hyperpigmentation around the eyes, on the nose, and forehead.
Figure 5.

Significant resolution of lesions on patient’s arms two weeks post-treatment.
Discussion
The modified Goeckerman regimen, implemented at Kenya’s Moi Teaching & Referral Hospital in collaboration with UCSF, has demonstrated its efficacy in treating severe psoriasis within a challenging healthcare context. The classic Goeckerman regimen has largely been supplanted by newer therapies in the Western word, most notably biologics. However, there is less access to these medications in sub-Saharan Africa due to affordability and lack of infrastructure necessary to ensure biologics are kept at appropriate conditions, such as consistent and reliable refrigeration, to protect against breakdown of the medication.7 Though the modalities used in Goeckerman therapy do not require the stringent environmental conditions that biologics do, it does require the valuable resource of time in exchange for an impressively long remission period ranging from an average of nine and a half months to over one year.3 Of note, a recent report showed that 50% of psoriasis patients who discontinued biologics after achieving skin clearance were still disease-free or being managed by solely topical therapies after two years.8 This suggests that improving even temporary access to biologics until skin clearance could also provide long-lasting relief for many patients.
This case shows how in a both resource-limited and time-limited situation, the Goeckerman regimen may be shortened from six to two weeks and still be potentially effective. However, the modified regimen could have been even further enhanced with the application of CCT during treatment, such as in traditional Goeckerman therapy. Potential adverse effects include erythema from burns caused by UV therapy and skin irritation from coal tar or plastic wrap.3 These potential adverse effects are managed by daily assessments and lowering the phototherapy dose or CCT concentration if needed.
Conclusion
The successful management of a 55-year-old female patient who traveled a significant distance for treatment underscores the regimen’s potential as a viable and effective option for patients in developing countries, where access to systemic psoriasis therapies may be limited. This intensive regimen not only accommodated the logistical challenges faced by patients living in remote areas but also achieved remarkable clinical outcomes and improved the patient’s quality of life. This case highlights the importance of adapting established therapies to meet local healthcare needs and the potential for such modified treatments to bridge gaps in access to care.
Acknowledgments
This paper has been uploaded to the Journal of Investigative Dermatology as an abstract: https://www.sciencedirect.com/science/article/abs/pii/S0022202X24007620
Institutional Approval
Institutional approval from the Institutional Review Board was not obtained as it was not required per institution guidelines.
Statement of Consent
The study participant has provided written consent to participate as well as consent to publish the data and photographs.
Disclosure
T.B. is currently a principal investigator for studies being sponsored by Amgen, Castle, CorEvitas, Pfizer, and Regeneron. She has additional research funding from Novartis and Regeneron. She has served as an advisor for AbbVie, Arcutis, Boehringer-Ingelheim, Bristol Myers Squibb, Dermavant, Janssen, Leo, Lilly, Pfizer, Novartis, Sanofi, Sun, and UCB. W.L. has received research grant funding from Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. The authors report no other conflicts of interest in this work.
References
- 1.Serrao R, Davis MDP. Goeckerman treatment for remission of psoriasis refractory to biologic therapy. J Am Acad Dermatol. 2009;60(2):348–349. doi: 10.1016/j.jaad.2008.10.016 [DOI] [PubMed] [Google Scholar]
- 2.Goeckerman WH. Treatment of psoriasis: continued observations on the use of crude coal tar and ultraviolet light. Arch Derm Syphilol. 1931;1931:1. [Google Scholar]
- 3.Gupta R, Debbaneh M, Butler D, et al. The Goeckerman regimen for the treatment of moderate to severe psoriasis. J Vis Exp. 2013;77:e50509. doi: 10.3791/50509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu TH, Nakamura M, Farahnik B, et al. The patient’s guide to psoriasis treatment. part 4: goeckerman therapy. Dermatol Ther. 2016;6(3):333–339. doi: 10.1007/s13555-016-0132-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ly K, Chang AY, Kiprono SK, et al. Implementation of an ultraviolet phototherapy service at a national referral hospital in western Kenya: reflections on challenges and lessons learned. Dermatol Ther (Heidelb). 2020;10(1):107–117. doi: 10.1007/s13555-019-00342-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu YB, Briggs KT, Taraban MB, Brinson RG, Marino JP. Grand challenges in pharmaceutical research series: ridding the cold chain for biologics. Pharm Res. 2021;38(1):3–7. doi: 10.1007/s11095-021-03008-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah R, Dey D, Pietzonka T, et al. Determinants of use of biotherapeutics in sub-Saharan Africa. Trends Pharmacol Sci. 2021;42(2):75–84. doi: 10.1016/j.tips.2020.11.012 [DOI] [PubMed] [Google Scholar]
- 8.Nielsen ML, Thein D, Rasmussen MK, et al. Trajectories and prognosis after discontinuation of biologics due to remission in psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2023;88(6):1378–1381. doi: 10.1016/j.jaad.2023.01.029 [DOI] [PubMed] [Google Scholar]


