Abstract
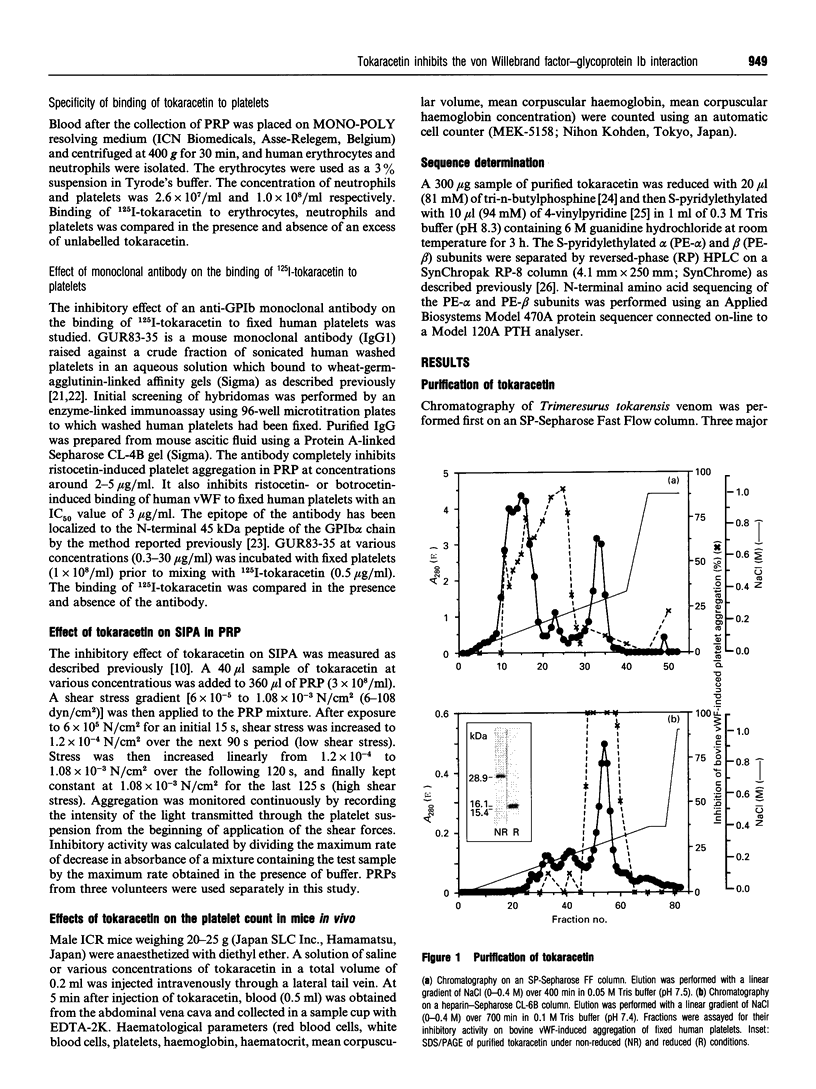
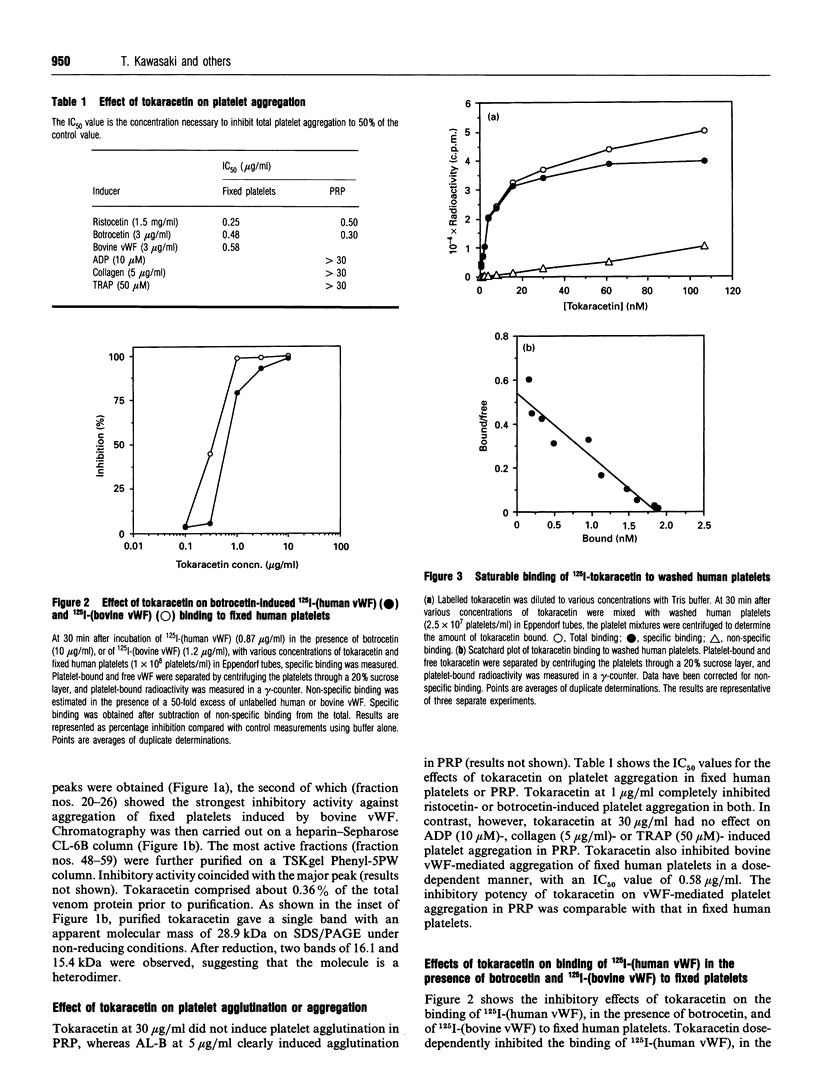
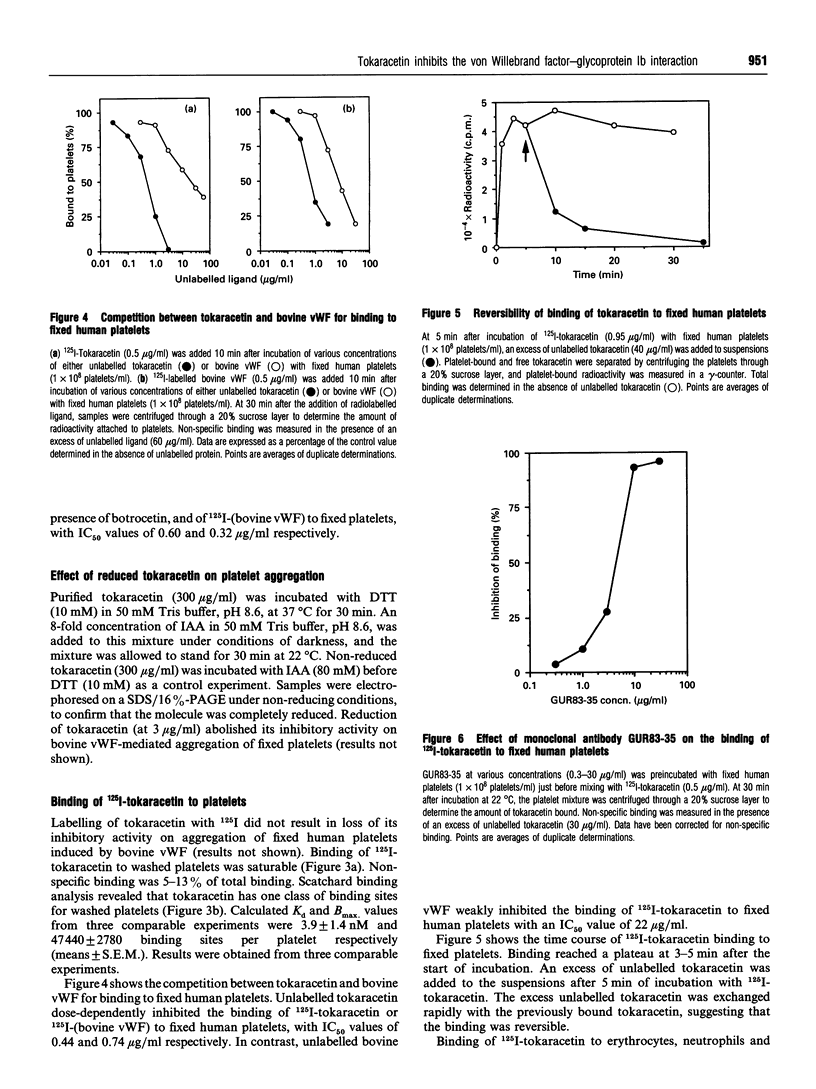
A new platelet antagonist, tokaracetin, was isolated from the venom of Trimeresurus tokarensis by ion-exchange chromatography, heparin-Sepharose chromatography and hydrophobic HPLC. The purified protein showed an apparent molecular mass on SDS/PAGE of 28.9 kDa under non-reducing conditions. On reduction, 16.1 and 15.4 kDa subunits were observed, suggesting that the molecule is a heterodimer. Tokaracetin inhibited the binding of 125I-labelled bovine von Willebrand factor (vWF) and 125I-labelled human vWF in the presence of botrocetin to fixed human platelets. It did not block ADP-, collagen- or thrombin receptor agonist peptide-induced platelet aggregation in human platelet-rich plasma (PRP), or induce platelet agglutination in PRP. On reduction, tokaracetin lost its inhibitory activity on the agglutination of fixed human platelets by bovine vWF. 125I-Tokaracetin specifically bound to washed human platelets with high affinity (Kd 3.9 +/- 1.4 nM) at 47,440 +/- 2780 binding sites per platelet. Binding of tokaracetin to fixed human platelets was reversible, and was inhibited by monoclonal antibody GUR83-35, which is directed against the N-terminal vWF-binding domain of human glycoprotein Ib (GPIb). Tokaracetin completely inhibited vWF-dependent shear-induced platelet aggregation in PRP at 3 micrograms/ml. The N-terminal amino acid sequences of tokaracetin subunits showed a high degree of identity with those of alboaggregin-B. These results suggest that this new platelet antagonist may be a useful tool in the development of specific inhibitors of the vWF-GPIb interaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhshi M. R., Kirby E. P. Proteolytic and immunologic comparison of human and bovine von Willebrand factor. Thromb Haemost. 1990 Jun 28;63(3):517–523. [PubMed] [Google Scholar]
- Becker B. H., Miller J. L. Effects of an antiplatelet glycoprotein Ib antibody on hemostatic function in the guinea pig. Blood. 1989 Aug 1;74(2):690–694. [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983 Jan;61(1):99–110. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fujimura Y., Titani K., Usami Y., Suzuki M., Oyama R., Matsui T., Fukui H., Sugimoto M., Ruggeri Z. M. Isolation and chemical characterization of two structurally and functionally distinct forms of botrocetin, the platelet coagglutinin isolated from the venom of Bothrops jararaca. Biochemistry. 1991 Feb 19;30(7):1957–1964. doi: 10.1021/bi00221a032. [DOI] [PubMed] [Google Scholar]
- Fujimura Y., Usami Y., Titani K., Niinomi K., Nishio K., Takase T., Yoshioka A., Fukui H. Studies on anti-von Willebrand factor (vWF) monoclonal antibody NMC-4, which inhibits both ristocetin- and botrocetin-induced vWF binding to platelet glycoprotein Ib. Blood. 1991 Jan 1;77(1):113–120. [PubMed] [Google Scholar]
- Goto S., Ikeda Y., Murata M., Handa M., Takahashi E., Yoshioka A., Fujimura Y., Fukuyama M., Handa S., Ogawa S. Epinephrine augments von Willebrand factor-dependent shear-induced platelet aggregation. Circulation. 1992 Dec;86(6):1859–1863. doi: 10.1161/01.cir.86.6.1859. [DOI] [PubMed] [Google Scholar]
- Handa M., Titani K., Holland L. Z., Roberts J. R., Ruggeri Z. M. The von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Characterization by monoclonal antibodies and partial amino acid sequence analysis of proteolytic fragments. J Biol Chem. 1986 Sep 25;261(27):12579–12585. [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Neurath H., Walsh K. A. Determination of the amino acid sequence of porcine trypsin by sequenator aalysis. Biochemistry. 1973 Aug 14;12(17):3146–3153. doi: 10.1021/bi00741a002. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Handa M., Kawano K., Kamata T., Murata M., Araki Y., Anbo H., Kawai Y., Watanabe K., Itagaki I. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. J Clin Invest. 1991 Apr;87(4):1234–1240. doi: 10.1172/JCI115124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri Y., Hayashi Y., Yamamoto K., Tanoue K., Kosaki G., Yamazaki H. Localization of von Willebrand factor and thrombin-interactive domains on human platelet glycoprotein Ib. Thromb Haemost. 1990 Feb 19;63(1):122–126. [PubMed] [Google Scholar]
- Katayama M., Handa M., Ambo H., Araki Y., Hirai S., Kato I., Kawai Y., Watanabe K., Ikeda Y. A monoclonal antibody-based enzyme immunoassay for human GMP-140/P-selectin. J Immunol Methods. 1992 Aug 30;153(1-2):41–48. doi: 10.1016/0022-1759(92)90303-b. [DOI] [PubMed] [Google Scholar]
- Katayama M., Handa M., Araki Y., Ambo H., Kawai Y., Watanabe K., Ikeda Y. Soluble P-selectin is present in normal circulation and its plasma level is elevated in patients with thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome. Br J Haematol. 1993 Aug;84(4):702–710. doi: 10.1111/j.1365-2141.1993.tb03149.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kaku S., Kohinata T., Sakai Y., Taniuchi Y., Kawamura K., Yano S., Takenaka T., Fujimura Y. Inhibition by aurintricarboxylic acid of von Willebrand factor binding to platelet GPIb, platelet retention, and thrombus formation in vivo. Am J Hematol. 1994 Sep;47(1):6–15. doi: 10.1002/ajh.2830470103. [DOI] [PubMed] [Google Scholar]
- Kirby E. P. The agglutination of human platelets by bovine factor VIII: R. J Lab Clin Med. 1982 Dec;100(6):963–976. [PubMed] [Google Scholar]
- Macfarlane D. E., Stibbe J., Kirby E. P., Zucker M. B., Grant R. A., McPherson J. Letter: A method for assaying von Willebrand factor (ristocetin cofactor). Thromb Diath Haemorrh. 1975 Sep 30;34(1):306–308. [PubMed] [Google Scholar]
- Mascelli M. A., Edgington T. S., Kirby E. P. Characterization of a fragment of bovine von Willebrand factor that binds to platelets. Biochemistry. 1986 Oct 7;25(20):6325–6335. doi: 10.1021/bi00368a074. [DOI] [PubMed] [Google Scholar]
- Nishio K., Fujimura Y., Nishida S., Takeda I., Yoshioka A., Fukui H., Tomiyama Y., Kurata Y. Antiplatelet glycoprotein Ib monoclonal antibody (OP-F1) totally abolishes ristocetin-induced von Willebrand factor binding, but has minimal effect on the botrocetin-induced binding. Haemostasis. 1991;21(6):353–359. doi: 10.1159/000216249. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Didry D., Rosa J. P. Molecular defects of platelets in Bernard-Soulier syndrome. Blood Cells. 1983;9(2):333–358. [PubMed] [Google Scholar]
- Peng M., Lu W., Beviglia L., Niewiarowski S., Kirby E. P. Echicetin: a snake venom protein that inhibits binding of von Willebrand factor and alboaggregins to platelet glycoprotein Ib. Blood. 1993 May 1;81(9):2321–2328. [PubMed] [Google Scholar]
- Peng M., Lu W., Kirby E. P. Alboaggregin-B: a new platelet agonist that binds to platelet membrane glycoprotein Ib. Biochemistry. 1991 Dec 10;30(49):11529–11536. doi: 10.1021/bi00113a007. [DOI] [PubMed] [Google Scholar]
- Peng M., Lu W., Kirby E. P. Characterization of three alboaggregins purified from Trimeresurus albolabris venom. Thromb Haemost. 1992 Jun 1;67(6):702–707. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Platelets and von Willebrand disease. Semin Hematol. 1985 Jul;22(3):203–218. [PubMed] [Google Scholar]
- Rüegg U. T., Rudinger J. Reductive cleavage of cystine disulfides with tributylphosphine. Methods Enzymol. 1977;47:111–116. doi: 10.1016/0076-6879(77)47012-5. [DOI] [PubMed] [Google Scholar]
- Sakariassen K. S., Bolhuis P. A., Sixma J. J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979 Jun 14;279(5714):636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Scott J. P., Montgomery R. R., Retzinger G. S. Dimeric ristocetin flocculates proteins, binds to platelets, and mediates von Willebrand factor-dependent agglutination of platelets. J Biol Chem. 1991 May 5;266(13):8149–8155. [PubMed] [Google Scholar]
- Strony J., Phillips M., Brands D., Moake J., Adelman B. Aurintricarboxylic acid in a canine model of coronary artery thrombosis. Circulation. 1990 Mar;81(3):1106–1114. doi: 10.1161/01.cir.81.3.1106. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Nishioka J., Hashimoto S. Identical binding site on human platelets for von Willebrand factor and bovine platelet aggregating factor. Thromb Res. 1980 Jan 1;17(1-2):215–223. doi: 10.1016/0049-3848(80)90308-4. [DOI] [PubMed] [Google Scholar]
- Usami Y., Fujimura Y., Suzuki M., Ozeki Y., Nishio K., Fukui H., Titani K. Primary structure of two-chain botrocetin, a von Willebrand factor modulator purified from the venom of Bothrops jararaca. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):928–932. doi: 10.1073/pnas.90.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida E., Fujimura Y., Miura S., Sugimoto M., Fukui H., Narita N., Usami Y., Suzuki M., Titani K. Alboaggregin-B and botrocetin, two snake venom proteins with highly homologous amino acid sequences but totally distinct functions on von Willebrand factor binding to platelets. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1386–1392. doi: 10.1006/bbrc.1993.1371. [DOI] [PubMed] [Google Scholar]