Abstract
Neoadjuvant HER2 Therapy: Beyond one-size-fits-all.
INTRODUCTION
Amplification of the human epidermal growth factor receptor 2 (HER2, ErbB2) gene leads to persistent activation of signaling pathways that enhance cell proliferation, resistance to apoptosis signals, heightened cell motility, and the induction of angiogenesis.1-3 HER2-positive breast cancer is known for its aggressive behavior and tendency to metastasize. The approval of trastuzumab, the first humanized anti-HER2 monoclonal antibody, revolutionized the treatment and prognosis of this subtype.4 As our understanding of tumor biology and HER2 signaling has improved, additional therapies targeting HER2 have been approved, including monoclonal antibodies, antibody-drug conjugates, and tyrosine kinase inhibitors.5
Neoadjuvant HER2-targeted therapy, also known as preoperative treatment, is now the preferred approach for patients with locally advanced breast cancer (clinical stage IIB-IIIC according to American Joint Committee on Cancer eighth edition criteria) and for operable tumors larger than 2 cm.6,7 The neoadjuvant approach in operable breast cancer is particularly attractive because of the high probability of pathological complete response (pCR) and the opportunity to tailor postoperative treatment on the basis of the pathological response at the time of surgery.8 It also increases the utilization of breast-conserving surgery and limits treatment to the axilla in patients who achieve clinical response.9 Moreover, the neoadjuvant setting allows for adaptive de-escalation strategies in the research realm.10 In addition to HER2, the estrogen receptor (ER) and progesterone receptor (PR) status of the tumor affects decision making in the neoadjuvant setting because ER/PR-negative tumors are more likely to achieve pCR.11,12
In this review, we provide a concise overview of neoadjuvant systemic therapy in early-stage HER2-positive breast cancer, with a focus on various chemotherapy backbones and de-escalation strategies.
KEY TRASTUZUMAB AND PERTUZUMAB TRIALS
Trastuzumab is a monoclonal antibody directed against domain IV of the extracellular region of the HER2 protein. Trastuzumab inhibits signaling, angiogenesis, and proliferation in HER2-overexpressing breast cancer cells. Baselga et al13 first reported the first clinical trial that showed efficacy of trastuzumab in patients with HER2-positive metastatic breast cancer. This was confirmed by Slamon et al14 in a pivotal randomized phase III trial. After the initial successful use of trastuzumab in the metastatic setting, clinical trials were rapidly launched in the adjuvant and neoadjuvant settings. In one of the first randomized preoperative studies of trastuzumab in combination with anthracycline- and taxane-based chemotherapy, Buzdar et al reported a pCR rate of 66%.15,16 The NOAH study, a randomized clinical trial of 235 women with HER2-positive locally advanced or inflammatory breast cancer demonstrated that the addition of trastuzumab significantly improved the pCR rate and event-free survival compared with chemotherapy alone.9 However, the multicenter randomized trial ACOSOG-Z1041 showed similar pCR rates in patients treated with preoperative trastuzumab in combination with taxane- and anthracycline-based chemotherapy compared with a sequential approach involving anthracycline-based chemotherapy followed by trastuzumab and paclitaxel (TH). Similar results were reported by the German Breast Group (GBG) GeparQuattro trial6 and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-41 study.17 These results support the prevailing practice of sequential chemotherapy and restricting HER2 therapy to the taxane component.18 Of note, in the NSABP B-41 trial the addition of lapatinib did not yield improved clinical outcomes, a finding that was also reported in other trials.19 Therefore, lapatinib has not progressed to the level of standard therapy in the neoadjuvant setting, and its use remains limited to the advanced stage.20 The development of trastuzumab biosimilars in the neoadjuvant and metastatic settings has improved access to trastuzumab therapy, which is classified as an essential medicine by the WHO.21-25
Pertuzumab is a monoclonal antibody that binds domain II of the extracellular region of the HER2 protein. This antibody was designed to disrupt the ligand-dependent heterodimerization of HER2 with other members of the HER family, most notably HER3.26 In 2004, Nahta et al27 reported a synergistic interaction between trastuzumab and pertuzumab (HP) in HER2-positive breast cancer cell lines. The HP combination resulted in the induction of programmed cell death (apoptosis). Importantly, apoptosis was not observed when either monoclonal antibody was administered individually to HER2-positive breast cancer cell lines in vitro. Subsequent experiments further substantiated the significance of the synergistic interaction of HP in animal models.28 As a result, a new paradigm emerged, wherein the use of two distinct antibodies targeting the same protein (ie, HER2) became an innovative approach in the treatment of patients with cancer. The early phase I/II clinical trials of HP therapy demonstrated the safety and efficacy of this novel combination in patients with metastatic HER2-positive breast cancer who had experienced disease progression despite prior trastuzumab therapy.29,30 These findings led to the pivotal CLEOPATRA trial, a randomized phase III study that established docetaxel and HP (THP) as the preferred first-line therapy for HER2-positive metastatic breast cancer.31
Two randomized phase II rials (NeoSphere and TRYPHAENA) provided compelling evidence of significant improvements in pCR rates by adding pertuzumab to trastuzumab-based chemotherapy in the neoadjuvant setting. In the NeoSphere trial, 417 patients with HER2-positive breast cancer were randomly assigned into four treatment arms: (1) docetaxel and trastuzumab; (2) THP; (3) HP; and (4) docetaxel and pertuzumab. The THP group demonstrated the highest pCR rate of 46% after 12 weeks of treatment, surpassing the other three groups.32,33 All patients received anthracycline-based chemotherapy postoperatively. In the TRYPHAENA trial, 225 patients with early HER2-positive breast cancer were randomly assigned to the following regimens in the neoadjuvant setting: ARM A: fluorouracil, epirubicin, cyclophosphamide (FEC) + HP followed by THP; ARM B: FEC ×3 followed by THP ×3; and ARM C: docetaxel, carboplatin and HP (TCHP) ×6. The trial demonstrated that the combination of HP and chemotherapy was well-tolerated, with a low incidence of symptomatic left ventricular systolic dysfunction (primary end point). The efficacy achieved with all regimens was encouraging with pCR rates of 57%-66%, notably those patients treated with six cycles of the non–anthracycline-containing regimen (TCHP) who achieved a pCR rate of 66% (Table 1).34
TABLE 1.
Pivotal HP Neoadjuvant Trials
| Characteristic | NeoSphere, Phase II (primary end point: pCR) | TRYPHAENA, Phase II (primary end point: cardiac safety) | |
|---|---|---|---|
| No. and type of patients | 417 treatment-naїve women with HER2-positive operable, locally advanced and inflammatory BC | 225 treatment-naїve women with HER2-positive operable, locally advanced, or inflammatory BC | |
| Random assignment | 1:1:1:1 | 1:1:1 | |
| Treatment arms | Group A: TH Group B: THP Group C: HP Group D: TP |
Group A: FEC H P × 3 → THP × 3 Group B: FEC × 3 → THP × 3 Group C: TCHP × 6 |
|
| pCR rate | Group A: 29.0% (20.6-38.5) Group B: 45.8% (36.1-55.7) Group C: 16.8% (10.3%-25.3%) Group D: 24% (15.8%-33.7%) |
Group A: 62% Group B: 57% Group C: 66% |
|
| Adverse events | Neutropenia (grade 3 or higher): Group A: 61/107 Group B: 48/107 Group C: 1/108 Group D: 52/94 |
Febrile neutropenia: Group A: 8/107 Group B: 9/107 Group C: 0/108 Group D: 7/94 |
B: 2.7% symptomatic LVSD 11 patients with declines in LVEF 10% or greater from baseline to <50%: Group A: 4 (5.6%) Group B: 4 (5.3%) Group C: 3 (3.9%) |
NOTE. The most effective regimens and the respective pCR rates are shown in bold.
Abbreviations: BC, breast cancer; C, carboplatin; FEC, fluorouracil, epirubicin, and cyclophosphamide; H, trastuzumab; HER2, human epidermal growth factor receptor 2; P, pertuzumab; pCR, pathological complete response; T, docetaxel.
Although NeoSphere and TRYPHAENA were phase II trials, the significant improvement in pCR rates led to the accelerated approval of pertuzumab as preoperative therapy for breast cancer by the US Food and Drug Administration (FDA) in 2013. This milestone was significant as it marked the first FDA approval of an anticancer drug using pCR as the primary end point. The conditional approval was made final when the APHINITY trial demonstrated an improvement in disease-free survival (DFS) rate for patients treated with THP compared with TH in the adjuvant setting.35
Patients undergoing neoadjuvant HER2-targeted therapy have the option of a subcutaneous (SQ) HP formulation. The phase III randomized FeDeriCa trial showed similar pharmacokinetic profiles between trastuzumab given intravenously or subcutaneously.36 In the PHranceSCa trial, patients preferred the HP SQ over the IV formulation in the adjuvant treatment of their HER2-positive locally advanced and inflammatory breast cancer.37
CHEMOTHERAPY BACKBONE
Both the TRYPHAENA and BERENICE trials demonstrated the cardiac safety of both dense-dose and standard anthracycline-containing regimens in combination with HP.34,38 Nevertheless, the application of anthracyclines in early-stage HER2-positive breast cancer has seen restricted use in recent years because of concerns about potential long-term cardiac toxicity.
Although the efficacy of HP is evident in early HER2-positive breast cancer, questions remain regarding the optimal chemotherapy backbone. The choice of taxane and the significance of carboplatin require consideration when designing the optimal therapeutic strategy for individual patients (Table 2).
TABLE 2.
Neoadjuvant Chemotherapy Backbone
| Trial | Phase | Treatment Arms | Survival | n | pCR, % |
|---|---|---|---|---|---|
| Single-agent docetaxel | |||||
| NeoSphere | II | TH | 5-year DFS, 81% | 107 | 29 |
| THP | 5-year DFS, 84% | 107 | 46 | ||
| TP | 5-year DFS, 75% | 96 | 24 | ||
| HP | 5-year DFS, 80% | 107 | 17 | ||
| Anthracycline → docetaxel v docetaxel/carboplatin | |||||
| TRYPHAENA | II | FECHP → THP | 3-year OS, 94% | 73 | 62 |
| FEC → THP | 3-year OS, 94% | 75 | 57 | ||
| TCHP | 3-year OS, 93% | 77 | 66 | ||
| Anthracycline → paclitaxel v paclitaxel/carboplatin | |||||
| TRAIN-2 | III | FEC × 3 → wTCHP × 6 | 3-year OS, 98% | 211 | 67 |
| wTCHP × 9 | 3 year OS, 98% | 206 | 68 | ||
| Anthracycline → paclitaxel v docetaxel | |||||
| BERENICE | II | ddAC → wTHP | 5-year OS, 96% | 199 | 62 |
| FEC → THP | 5-year OS, 94% | 201 | 61 | ||
| Single-agent paclitaxel or nab-paclitaxel | |||||
| GeparSepto | III | wT + HP → EC + HP | 4-year iDFS, 89% | 199 | 54 |
| NabTHP → EC + HP | 197 | 62 | |||
| ADAPT HER2+/HR– | II | HP | 5-year OS, 94% | 92 | 34 |
| THP | 5-year OS, 98% | 42 | 90 |
NOTE. This table shows the regimens given before surgery (primary end point was pCR). Postoperative treatments varied across trials. The taxane used is shown in bold.
Abbreviations: C, carboplatin; ddAC, dose-dense doxorubicin and cyclophosphamide; DFS, disease-free survival rate; FEC, fluorouracil, epirubicin, and cyclophosphamide; H, trastuzumab; HER2, human epidermal growth factor receptor 2; iDFS, invasive DFS rate; NabT, nab-paclitaxel; OS, overall survival rate; pCR, pathological complete response rate; P, pertuzumab; T, docetaxel; wT, paclitaxel.
Taxanes
The E1199 trial showed that weekly paclitaxel ×12 (wT) is as effective as docetaxel (T) every 3 weeks ×4 in patients with early-stage breast cancer. Although this study was not HER2 specific, it supports the efficacy of both taxanes in the adjuvant setting.39,40 While docetaxel was the taxane used in the NeoSphere and TRYPHAENA trials, several clinical trials have explored the safety and efficacy of weekly paclitaxel in the preoperative treatment of HER2-positive early breast cancer (Table 2). As it was shown with docetaxel, the addition of trastuzumab or HP to weekly paclitaxel improved the pCR rates significantly. The TRAIN-2 phase III trial evaluated the efficacy of weekly paclitaxel and carboplatin, with or without anthracyclines in combination with HP in the neoadjuvant setting. This study showed high pCR rates regardless of the chemotherapy backbone.41 Lopresti et al42 investigated the role of weekly paclitaxel, carboplatin, and HP (wTCHP). After 12 weeks, responding patients continued wTCHP for another 6 weeks, whereas nonresponders switched to AC. In this study, the pCR rate was 77% (95% CI, 58 to 90). Only two patients transitioned to AC for nonresponse, of which one achieved pCR. In the DAPHNe trial reported by Waks et al at the Dana Farber Cancer Institute, patients were treated with weekly paclitaxel and HP (wTHP) for 12 weeks. Over half of the patients (56.7%) achieved pCR, and no breast cancer recurrences were observed in the short follow-up period. Patients without pCR in the DAPHNe trial were considered for individualized adjuvant therapy, with a preference for adjuvant ado-trastuzumab emtansine (T-DM1). Larger ongoing de-escalation trials such as CompassHER2-pCR aim to find out if patients achieving a pCR after standard neoadjuvant treatment can safely omit chemotherapy after surgery. If the long-term effectiveness of this approach is confirmed, it could potentially spare many patients with stage II-III HER2-positive breast cancer from the harsh side effects of traditional chemotherapy regimens.
In summary, both docetaxel and paclitaxel are acceptable options for patients with HER2-positive early breast cancer in the neoadjuvant setting. The choice of docetaxel or paclitaxel does not seem to be as relevant in the setting of HP neoadjuvant therapy, and it should be based on physician's and patient's preference.39 Nab-paclitaxel is as effective as weekly paclitaxel on the basis of the GeparSepto trial,43 and it is usually reserved for patients who have experienced hypersensitivity reactions to paclitaxel or have contraindications to the steroids typically administered with docetaxel or paclitaxel to reduce the risk of hypersensitivity reactions.
Carboplatin
Pegram et al44 showed a synergistic interaction of carboplatin and trastuzumab in preclinical models. This finding, together with phase II studies showing that a combination of docetaxel, carboplatin, and trastuzumab (TCH) was safe in the metastatic setting,45 led to the BCIRG-006 trial to determine the efficacy and safety of TCH in the adjuvant setting.46 In this study, the DFS rates were similar for the group treated with doxorubicin/cyclophosphamide followed by docetaxel/trastuzumab (AC-TH) and the group treated with TCH. Cardiac toxicity was lower in patients treated with TCH. The BCIRG-006 trial was very influential in clinical practice, resulting in the widespread use of TCH in the adjuvant setting. However, a contemporary study (BCIRG-007) that randomly assigned patients with HER2-overexpressing metastatic breast cancer to TH or TCH failed to show an improvement in the response rate or PFS rate by the addition of carboplatin.47 Current research has not definitively demonstrated an advantage to incorporating carboplatin into taxane-based herceptin and pertuzumab (HP) regimens for neoadjuvant or adjuvant treatment. It should be noted that the pivotal FDA registration trial for pertuzumab in the neoadjuvant setting (ie, NeoSphere) did not include carboplatin. The experimental group on NeoSphere consisted of THP. It was on the TRYPHAENA phase II trial where a group of patients received TCHP (including carboplatin), resulting in a high pCR rate compared with historical data. However, TRYPHAENA was a small, phase II study designed to assess the cardiac safety of HP in combination with different chemotherapy regimens. The trial was not powered to evaluate the role of carboplatin in addition to THP therapy in the neoadjuvant setting.34 Therefore, in our opinion the role of carboplatin remains controversial.
The preferred regimen according to National Comprehensive Cancer Network (NCCN) guidelines is TCHP. However, we believe that both TCHP (using either docetaxel every 3 weeks or weekly paclitaxel) and THP (without carboplatin for selected patients) ought to be recognized as standard neoadjuvant treatment options in early-stage HER2-positive breast cancer (Fig 1).
FIG 1.
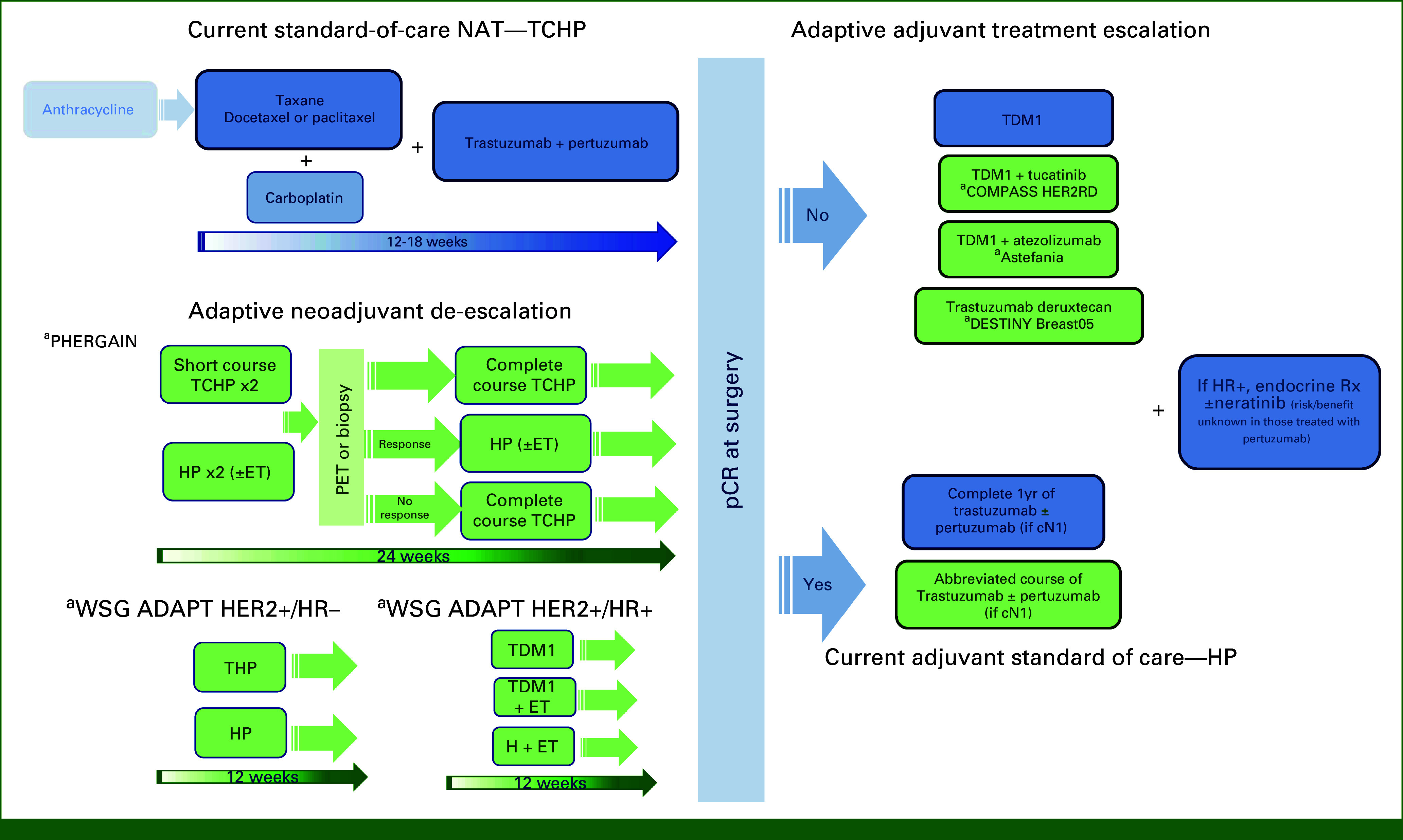
Evolving neoadjuvant treatment of HER2-positive breast cancer. Blue, standard of care; Green, investigational; aStudy name. C, carboplatin; ET, endocrine therapy; H, trastuzumab; HER2, human epidermal growth factor receptor 2; P, pertuzumab; T, docetaxel; TDM1, trastuzumab-DM1.
DE-ESCALATION STRATEGIES
In the NeoSphere trial, a group of patients treated with HP alone (without chemotherapy) achieved a pCR rate of 16%.33 This sparked interest in the development of chemotherapy-free regimens in the neoadjuvant setting (Table 3). The WSG ADAPT HER2+/HR– trial is exploring the feasibility of de-escalated neoadjuvant therapy in HER2-positive, HR-negative disease (Fig 1).48 Patients were randomly assigned to receive HP, with or without paclitaxel. Remarkably, the pCR rate was 90% in the de-escalated chemotherapy arm after 12 weeks of paclitaxel + dual HER2 blockade. Adjuvant therapy followed national guidelines. Patients who achieved pCR had the option to omit adjuvant chemotherapy, with 79% in the paclitaxel arm receiving no further chemotherapy. The 5-year invasive DFS (iDFS) rate was 98% for patients achieving pCR, regardless of whether they had received paclitaxel or not.
TABLE 3.
Neoadjuvant Clinical Trials With Omission of Chemotherapy in HER2-Positive Breast Cancer
| Trial | Phase | Treatment Arms | Survival | n | pCR, % |
|---|---|---|---|---|---|
| PHERGain | II | TCHP | NR | 71 | 58 |
| HP ± ET | 3-year iDFS, 95% | 285 | 38 | ||
| WSG ADAPT HER2+/HR– | II | HP | 5-year OS, 94% | 92 | 34 |
| wTHP | 5-year OS, 98% | 42 | 90 | ||
| ADAPT-TP HER2+/HR+ | II | T-DM1 | 5-year OS, 97% | 119 | 41 |
| T-DM1 + ET | 5-year OS, 96% | 127 | 41 | ||
| H + ET | 5-year OS, 96% | 129 | 15 |
Abbreviations: C, carboplatin; ET, endocrine therapy; H, trastuzumab; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NR, not reported; P, pertuzumab; pCR, pathological complete response; T, docetaxel; T-DM1, trastuzumab emtansine; wT, paclitaxel.
Overall, attempts to substitute T-DM1 for taxane and carboplatin therapy have not been successful. In the KRISTINE trial (n = 444) patients randomly assigned to docetaxel, carboplatin, and HP (n = 221) achieved a higher pCR rate than patients treated with T-DM1 + pertuzumab (n = 223).49 In the ADAPT-TP HER2+/HR+ trial, focusing on hormone receptor (HR) - positive disease, neoadjuvant treatment with T-DM1 alone or in combination with endocrine therapy resulted in a higher pCR rate compared with trastuzumab and endocrine therapy. Survival data from ADAPT-TP HER2+/HR+ indicated that patients achieving pCR had a similar 5-year DFS rate, regardless of adjuvant chemotherapy administration.50-52
The ongoing CompassHER2 trials are investigating chemotherapy de-escalation after surgery on the basis of pCR status. The CompassHER2-pCR trial is looking at recurrence-free survival in patients with HER2-positive stage II-IIIA breast cancer who achieve pCR after a 12-week neoadjuvant regimen of wTHP (paclitaxel × 12) followed by HP for a total of 1 year. Patients with residual invasive breast cancer receive standard T-DM1 treatment. Additional chemotherapy or hormone therapy may be administered as needed. Successful results from this trial could eliminate anthracycline and limit carboplatin use by treating patients with wTHP alone in the neoadjuvant setting.
The PHERGain study assessed the predictive value of 18F-labeled fluorodeoxyglucose-positron emission tomography (18F-FDG-PET) imaging in identifying patients who would benefit from HP (without chemotherapy). The trial involved two groups: one receiving a combination of chemotherapy and HER2-targeted therapies and the other undergoing dual HER2 blockade with HP. Patients in the latter group, on the basis of 18F-FDG-PET results, either continued dual HER2 blockade or switched to chemotherapy if they were nonresponders (Fig 1). The primary outcomes focused on pCR rates and 3-year iDFS in the dual HER2 blockade group.53 In the group using the HER2 blockade-alone approach, nearly 80% of patients had a positive response on PET scans, and the 3-year iDFS rate reached 95%. Notably, among PET responders, 37% achieved a pCR without undergoing chemotherapy, resulting in an impressive 98.8% 3-year iDFS rate.54 The ongoing ADAPT umbrella trial in Germany includes all major breast cancer subtypes, aiming to optimize treatment selection by combining prognostic and predictive markers (Table 3).51 These and other important ongoing trials (eg, CompassHER2-pCR, DESCRESCENDO) aim to personalize adjuvant therapy on the basis of pCR status after a de-escalated neoadjuvant course.
A novel de-escalation strategy involves the integration of trastuzumab deruxtecan (T-DXd), both in HER2-positive and HER2-low breast cancer. In the DESTINY-B04 trial, T-DXd prolonged progression-free survival when compared with physician's choice of single-agent cytotoxic chemotherapy in a pretreated patient population with metastatic HER2-low breast cancer, defined as 1+ or 2+ by immunohistochemistry and fluorescent in situ hybridization negative. On the basis of these results, clinical trials are exploring the safety and efficacy of T-DXd in the adjuvant and neoadjuvant settings both for HER2-positive and HER2-low early breast cancer (Table 4). For example, The DESTINY-B11 and the ADAPT-HER2-IV studies are evaluating the efficacy and safety of T-DXd in the neoadjuvant setting for patients with early HER2-positive breast cancer. The phase II TRIO-US B-12 TALENT trial showed preliminary evidence of clinical activity for neoadjuvant T-DXd in HER2-low, hormone receptor–positive early breast cancer. If confirmatory studies support the use of T-DXd in early-stage HER2-low breast cancer, this would have major implications on the treatment paradigms and patient classification in the future.
TABLE 4.
Neoadjuvant Trastuzumab-Deruxtecan Trials
| Trial | Inclusion Criteria | Treatment Arms | Primary Objective(s) | |
|---|---|---|---|---|
| ADAPTHER2-IV (NCT05704829) Phase II |
HER2-positive Cohort 1: low-intermediate risk for recurrence, cT1c-cT2 (1-3 cm, cN0, cT1a/b excluded) Cohort 2: intermediate-high risk recurrence cT2 (>3 cm-5 cm, cN0) Patients age 65 years and older could be assigned to any cohort |
T-DXd × 12 weeks in low-intermediate risk | pCR rate and dDFS rate | |
| T-DXd × 18 weeks in intermediate-high risk | ||||
| THP in low-intermediate risk × 12 weeks | pCR rate and dDFS | |||
| [T or wT] + C + HP × 18 weeks in intermediate-high risk | ||||
| DESTINY-Breast11 (NCT05113251) Phase III |
HER2-positive Clinical stage cT0-4 (including inflammatory BC) and N1-3; or >cT3/N0/M0 |
T-DXd | pCR rate | |
| T-DXd followed by wTHP | pCR rate | |||
| ddAC → wTHP | pCR rate | |||
| ARIADNE (NCT05900206) Phase II |
HER2-positive Clinical stage: cT2 with any cN or cN1-N3 with any cT |
T-DXd (cycles 1-3) | Cycle 4-6 on the basis of intrinsic molecular (PAM50) subtype Luminal A and ER+: Ribociclib, letrozole, and HP Luminal and ER– or basal-like: EC if no radiologic response after c1-3; if radiologic CR, then continue T-DXd or TCHP/wTCHP × 3 cycles HER2-enriched: T-DXd or TCHP/wTCHP × 3 more cycles |
pCR rate |
| TCHP or wTCHP (cycles 1-3) | ||||
| TRIO-US B12 TALENT (NCT04553770) Phase II |
HER2 low (1+ or 2+ by IHC and FISH negative) Clinical stage: cT2 or if cN1/N2 the tumor must be considered operable Hormone receptor–positive as per ASCO/CAP guideline |
T-DXd | pCR rate | |
| T-DXd + anastrazole | pCR rate | |||
Abbreviations: BC, breast cancer; C, carboplatin; CAP, College of American Pathologists; DFS, disease-free survival; ddAC, dose-dense doxorubicin and cyclophosphamide; dDFS, distant DFS; EC, epirubicin and cyclophosphamide; ER, estrogen receptor; H, trastuzumab; HER2, human epidermal growth factor receptor 2; P, pertuzumab; pCR, pathological complete response; T, docetaxel; T-Dxd, trastuzumab deruxtecan; wT, paclitaxel.
MANAGEMENT OF RESIDUAL DISEASE AFTER NEOADJUVANT HER2 THERAPY/ADAPTIVE TREATMENT ESCALATION
The management of residual disease after neoadjuvant HER2 therapy is crucial in determining the subsequent treatment approach. Patients who achieve a pCR are typically recommended to continue HER2-targeted therapy for a total duration of 1 year, encompassing both neoadjuvant and adjuvant settings.33,34 However, for patients who still have residual disease after neoadjuvant HER2-based chemotherapy, current guidelines advise the use of adjuvant treatment with T-DM1, as supported by findings from the KATHERINE trial.55 The CompassHER2-RD is assessing the potential benefit of combining T-DM1 with tucatinib, an oral potent HER2-specific TKI that has shown efficacy in metastatic HER2-positive breast cancer. The ASTEFANIA trial is studying the addition of atezolizumab (PD-L1 inhibitor) to T-DM1. The rationale for this combination is the synergy observed in preclinical models and early clinical trials for the combination of HER2-targeted therapies with cancer immunotherapy, which should be particularly relevant in the early-stage setting, where the immune system has not been weakened by heavy pretreatment.56 The DESTINY Breast05 is assessing the benefit of trastuzumab deruxtecan in this setting. These studies will further contribute to the understanding of optimal treatment strategies for this higher-risk patient population with residual disease after neoadjuvant therapy (Table 5).
TABLE 5.
Adaptive Adjuvant Treatment Escalation Trials
| Trial | Inclusion Criteria | Treatment Arms | Primary Objective: iDFS | n |
|---|---|---|---|---|
| Katherine Phase III |
cT1-4/N0-3/M0 (excluding T1a or T1bN0) HER2-positive with residual disease NACT: 9 weeks minimum of taxane and trastuzumab, 16 weeks or 6 cycles |
T-DM1 once every 3 weeks ×14 | 3-year iDFS, 88% | 743 |
| Trastuzumab once every 3 weeks ×14 | 3-year iDFS, 77% | 743 | ||
| COMPASS HER2-RD Phase III |
cT1-4/N0-3/M0 (excluding T1a/bN0) HER2-positive with residual disease Any ER, but if ER+ must be LN+ NACT: minimum of 16 weeks or 6 cycles of preoperative taxane and trastuzumab-based chemotherapy |
T-DM1/placebo once every 3 weeks ×14 | NA | |
| T-DM1/tucatinib once every 3 weeks ×14 | NA | |||
| Astefania Phase III |
cT4/anyN/M0, any cT/N2-3/M0, cT1-3/N0-1/M0 (excluding cT1mi/T1a/T1b/N0) HER2-positive with residual disease NACT: minimum 9 weeks of taxane and trastuzumab |
T-DM1/placebo once every 3 weeks ×14 | NA | |
| T-DM1 + atezolizumab once every 3 weeks ×14 | NA | |||
| DESTINY Breast05 Phase III |
cT4/N0-3/M0 or T1-3/N2-3/M0 or cT1-3/N0-1/M0 but with positive LN at surgery (ypN1-3) HER2-positive with residual disease (must have completed NACT) |
T-DM1 | NA | |
| T-DXd | NA |
NOTE. The experimental treatments are shown in bold.
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; iDFS, invasive disease-free survival; LN, lymph nodes; NA, not available; NACT, neoadjuvant chemotherapy; T-DM1, trastuzumab-DM1; T-DXd, trastuzumab deruxtecan.
In conclusion, neoadjuvant dual HER2-targeted therapy using HP is the standard of care in patients with high-risk HER2-positive early breast cancer. Patients with tumors larger than 2 cm should be considered for HP-based neoadjuvant therapy regardless of lymph node status. Although TCHP is considered the preferred regimen by NCCN guidelines, we believe weekly paclitaxel is an acceptable option on the basis of available safety and efficacy data. The role of carboplatin remains not well defined. De-escalation approaches including weekly paclitaxel in combination with HP is appropriate for patients with operable disease because of the high pCR rates attained, especially because of the possibility of using T-DM1 postoperatively (or additional AC chemotherapy if needed) in patients with residual disease. There is a need for better identification of the subset of patients for whom chemotherapy can be safely omitted (Fig 1). An expanding array of anti-HER2 agents is becoming rapidly available, including novel monoclonal antibodies, antibody-drug conjugates, tyrosine kinase inhibitors, and immune-based therapies. In the future, it will be crucial to determine the most suitable combination and sequence of these novel agents to optimize clinical outcomes for patients with HER2-positive and HER2-low breast cancer.
Francisco J. Esteva
Consulting or Advisory Role: Stemline Therapeutics, Genzyme, Novartis, AstraZeneca, Veracyte
Travel, Accommodations, Expenses: Genzyme
No other potential conflicts of interest were reported.
Footnotes
See accompanying Editorial, p. 1003
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Tailoring Neoadjuvant Therapy in Human Epidermal Growth Factor Receptor 2–Positive Early Breast Cancer: Recent Advances and Strategies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Francisco J. Esteva
Consulting or Advisory Role: Stemline Therapeutics, Genzyme, Novartis, AstraZeneca, Veracyte
Travel, Accommodations, Expenses: Genzyme
No other potential conflicts of interest were reported.
REFERENCES
- 1. Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2. Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: Association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Esteva FJ, Sahin AA, Cristofanilli M, et al. Molecular prognostic factors for breast cancer metastasis and survival. Semin Radiat Oncol. 2002;12:319–328. doi: 10.1053/srao.2002.35251. [DOI] [PubMed] [Google Scholar]
- 4. Nahta R, Esteva FJ. Trastuzumab: Triumphs and tribulations. Oncogene. 2007;26:3637–3643. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 5. Esteva FJ, Yu D, Hung MC, et al. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7:98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 6. Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: Results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- 7. Tolaney SM, Tarantino P, Graham N, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023;24:273–285. doi: 10.1016/S1470-2045(23)00051-7. [DOI] [PubMed] [Google Scholar]
- 8. Broglio KR, Quintana M, Foster M, et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: A meta-analysis. JAMA Oncol. 2016;2:751–760. doi: 10.1001/jamaoncol.2015.6113. [DOI] [PubMed] [Google Scholar]
- 9. Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): Follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- 10. Bianchini G, Kiermaier A, Bianchi GV, et al. Biomarker analysis of the NeoSphere study: Pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res. 2017;19:16. doi: 10.1186/s13058-017-0806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 12. I-SPY2 Trial Consortium. Yee D, DeMichele AM, et al. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: Three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6:1355–1362. doi: 10.1001/jamaoncol.2020.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous trastuzumab (herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26(4 suppl 12):78–83. [PubMed] [Google Scholar]
- 14. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 15. Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 16. Esteva FJ, Wang J, Lin F, et al. CD40 signaling predicts response to preoperative trastuzumab and concomitant paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide in HER-2-overexpressing breast cancer. Breast Cancer Res. 2007;9:R87. doi: 10.1186/bcr1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rastogi P, Tang G, Hassan S, et al. Long-term outcomes of dual vs single HER2-directed neoadjuvant therapy in NSABP B-41. Breast Cancer Res Treat. 2023;199:243–252. doi: 10.1007/s10549-023-06881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buzdar AU, Suman VJ, Meric-Bernstam F, et al. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): A randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1317–1325. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guarneri V, Griguolo G, Miglietta F, et al. Survival after neoadjuvant therapy with trastuzumab-lapatinib and chemotherapy in patients with HER2-positive early breast cancer: A meta-analysis of randomized trials. ESMO Open. 2022;7:100433. doi: 10.1016/j.esmoop.2022.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boulbes DR, Arold ST, Chauhan GB, et al. HER family kinase domain mutations promote tumor progression and can predict response to treatment in human breast cancer. Mol Oncol. 2015;9:586–600. doi: 10.1016/j.molonc.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu C, Stebbing J, Esteva FJ. Clinical development of CT-P6 in HER2 positive breast cancer. Expert Opin Biol Ther. 2019;19:987–992. doi: 10.1080/14712598.2019.1665019. [DOI] [PubMed] [Google Scholar]
- 22. Zinzani PL, Dreyling M, Gradishar W, et al. Are biosimilars the future of oncology and haematology? Drugs. 2019;79:1609–1624. doi: 10.1007/s40265-019-01193-y. [DOI] [PubMed] [Google Scholar]
- 23. Esteva FJ, Baranau YV, Baryash V, et al. Efficacy and safety of CT-P6 versus reference trastuzumab in HER2-positive early breast cancer: Updated results of a randomised phase 3 trial. Cancer Chemother Pharmacol. 2019;84:839–847. doi: 10.1007/s00280-019-03920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stebbing J, Baranau Y, Baryash V, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: A randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18:917–928. doi: 10.1016/S1470-2045(17)30434-5. [DOI] [PubMed] [Google Scholar]
- 25. Lemery SJ, Esteva FJ, Weise M. Biosimilars: Here and now. Am Soc Clin Oncol Educ Book. 2016;35:e151–e157. doi: 10.1200/EDBK_155954. [DOI] [PubMed] [Google Scholar]
- 26. Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 27. Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 28. Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 29. Gianni L, Llado A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:1131–1137. doi: 10.1200/JCO.2009.24.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 32. Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 33. Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 34. Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 35. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan AR, Im SA, Mattar A, et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): A randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021;22:85–97. doi: 10.1016/S1470-2045(20)30536-2. [DOI] [PubMed] [Google Scholar]
- 37. O'Shaughnessy J, Sousa S, Cruz J, et al. Preference for the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with HER2-positive early breast cancer (PHranceSCa): A randomised, open-label phase II study. Eur J Cancer. 2021;152:223–232. doi: 10.1016/j.ejca.2021.03.047. [DOI] [PubMed] [Google Scholar]
- 38. Swain SM, Ewer MS, Viale G, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29:646–653. doi: 10.1093/annonc/mdx773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sparano JA, Zhao F, Martino S, et al. Long-term follow-up of the E1199 phase III trial evaluating the role of taxane and schedule in operable breast cancer. J Clin Oncol. 2015;33:2353–2360. doi: 10.1200/JCO.2015.60.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Ramshorst MS, van der Voort A, van Werkhoven ED, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1630–1640. doi: 10.1016/S1470-2045(18)30570-9. [DOI] [PubMed] [Google Scholar]
- 42. Lopresti ML, Bian JJ, Sakr BJ, et al. Neoadjuvant weekly paclitaxel and carboplatin with trastuzumab and pertuzumab in HER2-positive breast cancer: A Brown University Oncology Research Group (BrUOG) study. Breast Cancer Res Treat. 2021;189:93–101. doi: 10.1007/s10549-021-06266-9. [DOI] [PubMed] [Google Scholar]
- 43. Gianni L, Mansutti M, Anton A, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women with ERBB2/HER2-negative breast cancer—The Evaluating Treatment with Neoadjuvant Abraxane (ETNA) trial: A randomized phase 3 clinical trial. JAMA Oncol. 2018;4:302–308. doi: 10.1001/jamaoncol.2017.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pegram MD, Konecny GE, O'Callaghan C, et al. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–749. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- 45. Pegram MD, Pienkowski T, Northfelt DW, et al. Results of two open-label, multicenter phase II studies of docetaxel, platinum salts, and trastuzumab in HER2-positive advanced breast cancer. J Natl Cancer Inst. 2004;96:759–769. doi: 10.1093/jnci/djh133. [DOI] [PubMed] [Google Scholar]
- 46. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Valero V, Forbes J, Pegram MD, et al. Multicenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): Two highly active therapeutic regimens. J Clin Oncol. 2011;29:149–156. doi: 10.1200/JCO.2010.28.6450. [DOI] [PubMed] [Google Scholar]
- 48. Harbeck N, Gluz O, Christgen M, et al. De-escalation strategies in human epidermal growth factor receptor 2 (HER2)–positive early breast cancer (BC): Final analysis of the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early BC HER2- and hormone receptor–positive phase II randomized trial—Efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with or without endocrine therapy (ET) versus trastuzumab plus ET. J Clin Oncol. 2017;35:3046–3054. doi: 10.1200/JCO.2016.71.9815. [DOI] [PubMed] [Google Scholar]
- 49. Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: Three-year outcomes from the phase III KRISTINE study. J Clin Oncol. 2019;37:2206–2216. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gluz O, Nitz UA, Christgen M, et al. Efficacy of endocrine therapy plus trastuzumab and pertuzumab vs de-escalated chemotherapy in patients with hormone receptor-positive/ERBB2-positive early breast cancer: The neoadjuvant WSG-TP-II randomized clinical trial. JAMA Oncol. 2023;9:946–954. doi: 10.1001/jamaoncol.2023.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harbeck N, Nitz UA, Christgen M, et al. De-escalated neoadjuvant trastuzumab-emtansine with or without endocrine therapy versus trastuzumab with endocrine therapy in HR+/HER2+ early breast cancer: 5-year survival in the WSG-ADAPT-TP trial. J Clin Oncol. 2023;41:3796–3804. doi: 10.1200/JCO.22.01816. [DOI] [PubMed] [Google Scholar]
- 52. Waks AG, Desai NV, Li T, et al. A prospective trial of treatment de-escalation following neoadjuvant paclitaxel/trastuzumab/pertuzumab in HER2-positive breast cancer. NPJ Breast Cancer. 2022;8:63. doi: 10.1038/s41523-022-00429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Perez-Garcia JM, Gebhart G, Ruiz Borrego M, et al. Chemotherapy de-escalation using an (18)F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22:858–871. doi: 10.1016/S1470-2045(21)00122-4. [DOI] [PubMed] [Google Scholar]
- 54. Cortes J, Perez-Garcia JM, Ruiz Borrego M, et al. 3-year invasive disease-free survival of the strategy-based, randomized phase II PHERGain trial evaluating chemotherapy de-escalation in human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol. 2023;41 suppl 17; abstr LBA506. [Google Scholar]
- 55. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 56. Emens LA, Esteva FJ, Beresford M, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21:1283–1295. doi: 10.1016/S1470-2045(20)30465-4. [DOI] [PubMed] [Google Scholar]


