Abstract
Background and Objectives
Cognitive impairment (CI) in multiple sclerosis (MS) is frequent and determined by a complex interplay between inflammatory and neurodegenerative processes. We aimed to investigate whether CSF parvalbumin (PVALB), measured at the time of diagnosis, may have a prognostic role in patients with MS.
Methods
In this cohort study, CSF analysis of PVALB and Nf-L levels was performed on all patients at diagnosis (T0) and combined with physical, cognitive, and MRI assessment after an average of 4 years of follow-up (T4) from diagnosis. Cognitive performance was evaluated with a comprehensive neuropsychologic battery: both global (cognitively normal, CN, mildly CI, mCI, and severely CI, sCI) and domain cognitive status (normal/impaired in memory, attention/information processing speed, and executive functions) were considered. Cortical thickness and gray matter volume data were acquired using 3T MRI scanner.
Results
A total of 72 patients with MS were included. At diagnosis, PVALB levels were higher in those patients who showed a worsening physical disability after 4 years of follow-up (p = 0.011). CSF PVALB levels were higher in sCI patients than in CN (p = 0.033). Moreover, higher PVALB levels significantly correlated with worse global cognitive (p = 0.024) and memory functioning (p = 0.044). A preliminary clinical threshold for PVALB levels at diagnosis was proposed (2.57 ng/mL), which maximizes the risk of showing CI (in particular, sCI) at follow-up, with a sensitivity of 91% (specificity 30%). No significant results were found for these associations with Nf-L. In addition, patients with higher levels of PVALB at diagnosis showed higher cognitive (p = 0.024) and global fatigue (p = 0.043) at follow-up. Finally, higher PVALB levels also correlated significantly with more pronounced CTh/volume at T4 in the inferior frontal gyrus (p = 0.044), postcentral gyrus (p = 0.025), frontal pole (p = 0.042), transverse temporal gyrus (p = 0.008), and cerebellar cortex (p = 0.041) and higher atrophy (change T0-T4) in the right thalamus (p = 0.038), pericalcarine cortex (p = 0.009), lingual gyrus (p = 0.045), and medial frontal gyrus (p = 0.028).
Discussion
The significant association found between parvalbumin levels in the CSF at diagnosis and cognitive, clinical, and neuroradiologic worsening after 4 years of follow-up support the idea that parvalbumin, in addition to Nf-L, might represent a new potential prognostic biomarker, reflecting MS neurodegenerative processes occurring since early disease stages.
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated disorder of the CNS that affects both the white matter (WM) and the cortical/deep gray matter (GM) through inflammatory and neurodegenerative processes.1 While WM demyelinating lesions long have been thought of as a pathologic hallmark of MS, several studies have shown the relevance of focal (cortical lesions) and diffuse (atrophy) GM damage, which occurs from the earliest stages of the disease and worsens over time, explaining the accumulation of physical and cognitive disabilities.1 Furthermore, GM damage (especially cortical atrophy), reflecting the neuro-axonal loss and/or dysfunction, is not only strongly related to cognitive impairment (CI) but also considered one of the neuroradiologic parameters that best predict future cognitive decline (globally and in specific cognitive domains).2
Between 40% and 70% of patients with MS experience CI, depending on the population studied, the MS type, and the neuropsychologic tests performed.3 CI is acknowledged as a common and debilitating symptom that contributes significantly to patients' disability status.3 The most affected cognitive domains in MS are memory, information processing speed, and executive functions.3 Cognitive progression is characterized by significant interindividual variability, which can be explained by the different involvement patterns of WM and GM, occurring since the early stages of the disease without further neurologic deficits.3 Moreover, cognitive deficits are often present long before reaching the clinical threshold of detectability through neuropsychologic testing.4 CI in MS has progressively gained attention because it negatively affects the psychosocial status of patients, affecting their interpersonal interaction abilities, work life, and daily routine management.3 Considering the dramatic impact of CI on patients' quality of life, there is an urgent need to identify specific biomarkers capable of predicting patients that will be characterized by a more severe cognitive decline in the future.
Neurodegeneration plays a crucial role in the development of CI.5 The CSF contains different molecules recognized as biomarkers of neurodegeneration, including neurofilament light chain (Nf-L) and parvalbumin (PVALB). Nf-L is a biomarker of axonal damage, and it was the first serum biomarker in patients with MS shown to mirror acute disease activity in terms of relapses and lesion formation, to correlate with therapeutic response, and to foresee the progression of disability.6,7 However, its specificity as a biomarker is limited since its serum and CSF levels are also increased in several other acute and chronic neurodegenerative diseases and tend to follow the disease activity, increasing in association with flare-ups.8 However, Nf-L seems able to effectively track deficits of specific clinical and cognitive altered functions.9-13
On the other hand, PVALB is a protein responsible for calcium homeostasis expressed by specific groups of fast-spiking GABAergic interneurons,14 which represent a considerable part of the global cortical myelin, thus making them a preferential substrate for demyelinating diseases such as MS.15 These interneurons are also expressed in several subcortical regions, such as the hippocampus, where they are involved in neural plasticity processes related to memory and learning.16,17 We previously suggested that CSF levels in postmortem MS cases reflect cortical neuronal loss and that, in patients with MS at the time of diagnosis, it correlates at baseline with the cortical atrophy of specific brain areas known to be more affected by cortical pathology.18 In addition, PVALB levels were suggested able to reflect the different cognitive statuses of patients at diagnosis, potentially representing a new marker of patients with severe cognitive alterations. Instead, the correlations found for the Nf-L were lower or absent compared with those found for PVALB.18
In this study, we aimed to better investigate whether the CSF levels of PVALB analyzed at the time of diagnosis can predict the neuropsychologic performance and the neuroradiologic MS outcome after an average follow-up time of 4 years, evaluating a larger population than those examined in previous studies. Moreover, we also conducted the same analyses on Nf-L.
Methods
Study Population and Selection Criteria
Patients with MS from the MS Centre of Verona University Hospital (Verona, Italy) were enrolled in this prospective longitudinal study between January 2013 and October 2019.
At diagnosis (T0), each patient underwent a CSF withdrawal by lumbar puncture (LP), a neurologic examination including the measure of the Expanded Disability Status Scale (EDSS),19 a 3T MRI scan, and an extensive battery of neuropsychologic tests underwent close to the time of the LP, as part of the standard diagnostic process of the MS Centre. All patients underwent a multidisciplinary follow-up composed of the same evaluations listed above for baseline, except for the LP, executed only at diagnosis (neurologic examination every 6 months, MRI scan yearly, neuropsychologic assessment every 2 years).
The inclusion criteria were (1) all patients had to be part of our previous study,18 (2) they had to be followed over the years, and (3) the MRI and the neuropsychologic evaluations had to be paired in time. The exclusion criteria were (1) the presence of a concomitant neurologic condition (other than MS), (2) substance abuse, (3) hearing impairment, and (4) upper limb impairment that could interfere with neuropsychologic performance.
Standard Protocol Approvals, Registrations, and Patient Consents
The local Ethics Committee approved the study, and written informed consent was collected from all participants (MSBioB Biological Bank–A.O.U.I. Verona, protocol number 66418, 25/11/2019).
CSF Analysis
In accordance with the Consensus Guidelines for CSF and Blood Biobanking,20 CSF samples were collected at diagnosis as part of the diagnostic setup at least 1 month from the last relapse. The supernatant and cell pellet were separated after centrifugation and kept at −80°C. Two investigators (R.M. and V.M.), blinded to the clinical features, performed the CSF analysis. According to manufacturer instructions and previously optimized procedures,21 the levels of PVALB and Nf-L in the CSF were measured using a Parvalbumin ELISA kit (MBS2022353, MyBioSource) and Human Nf-L ELISA kit (MyBioSource, San Diego, CA), respectively. The samples were analyzed in random order and in duplicate and the intra-assay variability (coefficients of variation) of the samples was below 10%, as previously described.21
Neuropsychologic Assessment
A comprehensive battery of neuropsychologic tests was administered to all patients, with the same protocol of a previously published study.22 Patients underwent the Brief Repeatable Battery of Neuropsychological Tests and some executive functioning tests: the Stroop Test, the Phonemic Semantic Alternate Verbal Fluency, and the Modified Five Point Test.
Raw scores were adjusted for age, education, and sex: adjusted scores below the cutoff (5th percentile) of the Italian normative data of each test/battery were considered as failed. Patients were divided into 3 groups based on their cognitive performance on all neuropsychologic tests22: cognitively normal (CN, 0 failed subtests), mildly cognitive impaired (mCI, one or 2 failed subtests), and severely cognitive impaired (sCI, 3 or more failed subtests). A global cognitive functioning index and domain-specific cognitive functioning indices (memory−MEM, attention/information processing speed−ATT/IPS, and executive functions−EF) were estimated, averaging the z scores of all tests (global cognitive functioning) and of tests within a specific domain (domain cognitive functioning).22
To assess emotional state and fatigue, the Depression Anxiety Stress Scale (DASS-21) and the Modified Fatigue Impact Scale (MFIS) questionnaires were administered to all patients.
MRI Acquisition and Analysis
A Philips Achieva 3 T MRI scanner was used to perform the MRI evaluations. The following was the acquisition protocol:
A 3D T1-weighted turbo field echo (repeat time (TR)/echo time (TE) = 8.4/3.7 ms, voxel size of 1 × 1 × 1mm), total acquisition time of 5:51 minutes;
A 3D double inversion recovery (DIR) (TR/TE = 5,500/292 ms, inversion times (TI) TI1/TI2 = 525/2530 ms voxel size of 1 × 1 × 1 mm), turbo spin echo (TSE) readout, number of excitations 3, acquisition time of 10:49 minutes;
A 3D fluid-attenuated inversion recovery (FLAIR) sequence (TR/TE = 8,000/292 ms, TI = 2350 ms voxel size of 1 × 1 × 1 mm), same TSE readout as the DIR sequence, number of excitations 1, acquisition time of 4:50 minutes.
The acquired images were examined to assess the WM and GM lesion load. Through a semiautomatic lesion segmentation technique included in the MIPAV (Medical Image Processing and Visualisation; mipav.cit.nih.gov) software, WM lesions (WMLs) were found and segmented on the FLAIR images. Global and local cortical thicknesses were assessed on the 3D T1w scan using the online tool Freesurfer image analysis package.23,24 Each image was carefully examined for flaws or aberrations, and any error induced by tissue lesions was fixed using a semiautomated procedure, including lesion filling.
Statistical Analysis
Pearson correlation analysis was used to assess the association between PVALB and Nf-L levels and neuropsychologic and neuroradiologic outcomes, in which a p-value less than 0.05 was considered significant. Means±standard deviations (SD) were reported for continuous variables while medians [interquartile ranges, IQR] were reported for discrete variables. Considering the skewed distribution, we conducted analysis after log-transformation of the PVALB variable. We used the t test analysis, analysis of variance (ANOVA) with Bonferroni post hoc correction test, and Pearson correlation analysis to compare PVALB levels at diagnosis with clinical disability, neuropsychologic performance, and neuroradiologic outcomes over the follow-up, both in terms of absolute values (T4) and of longitudinal change (T0-T4).
The receiver operating characteristic (ROC) analysis was used to identify the PVALB cutoff, which maximizes the accuracy of identifying patients with cognitive alterations. Sensitivity, specificity, and area under curve (AUC) were reported.
In addition, we also performed the same analyses using Nf-L levels.
Data Availability
The corresponding author, who had complete access to all the study data, assumed responsibility for the data's integrity and the analyses' accuracy. The anonymized data set can be obtained from the corresponding author at a reasonable request.
Results
Study Population and Clinical Assessment
Seventy-two patients (50 female, 69.4%) were included (age at T4 = 42.1 ± 11.8 years, disease duration = 4.2 ± 1.8 years, education = 14.4 ± 3.3 years). The average follow-up time was 4 years (neuropsychologic follow-up time from LP: 4.2 ± 1.8 years, MRI follow-up time from LP: 4.3 ± 1.8 years).
MS diagnostic criteria25 identified all patients with a relapsing-remitting MS (RRMS) course. At T0, 27 patients (38%) received no treatment for MS, 33 patients (46%) were treated with low-efficacy disease-modifying treatments (DMTs), and 12 patients (17%) were treated with high-efficacy DMTs. At T4, 14 patients (19%) received no treatment for MS, 34 patients (47%) were treated with low-efficacy DMTs, and 24 patients (33%) were treated with high-efficacy DMTs.
The median EDSS at T0 was 2.0 [1.75] while at T4 was 1.0 [2.0]. However, when comparing PVALB levels among patients with an improved (n = 33, 45.8%), stable (n = 19, 26.4%), or worsened (n = 20, 27.8%) EDSS at T4 compared with T0 (in terms of absolute data), patients with an EDSS worsening at follow-up showed significantly higher levels of baseline PVALB compared with other 2 groups (worsened vs improved p = 0.021; worsened vs stable p = 0.019).
Association Between PVALB and Neuropsychologic Outcome
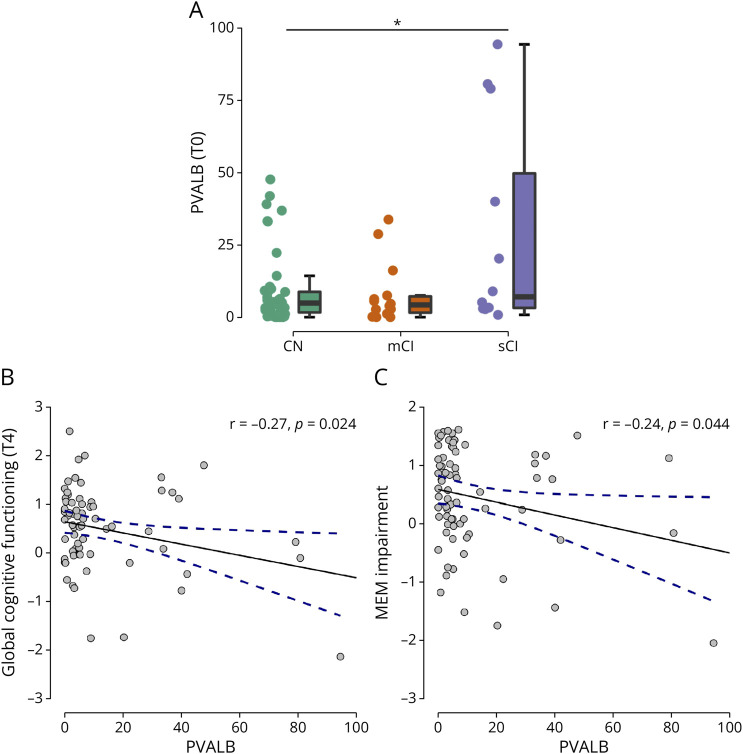
At T4, 46 patients were CN while 26 were CI (14 of whom were mCI and 12 were sCI). Patients with sCI at T4 had significantly higher PVALB levels at diagnosis (9.0 ± 12.5), compared with CN patients at T4 (28.5 ± 35.8) (p = 0.033) (Figure 1A). Higher PVALB levels at T0 was significantly associated with worse global cognitive functioning at T4 (r = −0.27, p = 0.024) (Figure 1B).
Figure 1. T0 PVALB and Global/Domain Cognitive Functioning/Status at T4.
Association between levels at MS diagnosis (T0) of PVALB with global cognitive status (A), global cognitive functioning (B), and memory functioning (C) at follow-up (T4). PVALB levels are expressed in ng/mL. * for p < 0.05. CN = cognitive normal; mCI = mild cognitive impairment; MEM = memory; sCI = severe cognitive impairment.
Noteworthy, patients with higher PVALB levels at diagnosis showed significantly worse memory functioning (r = −0.24, p = 0.044) at T4 (Figure 1C).
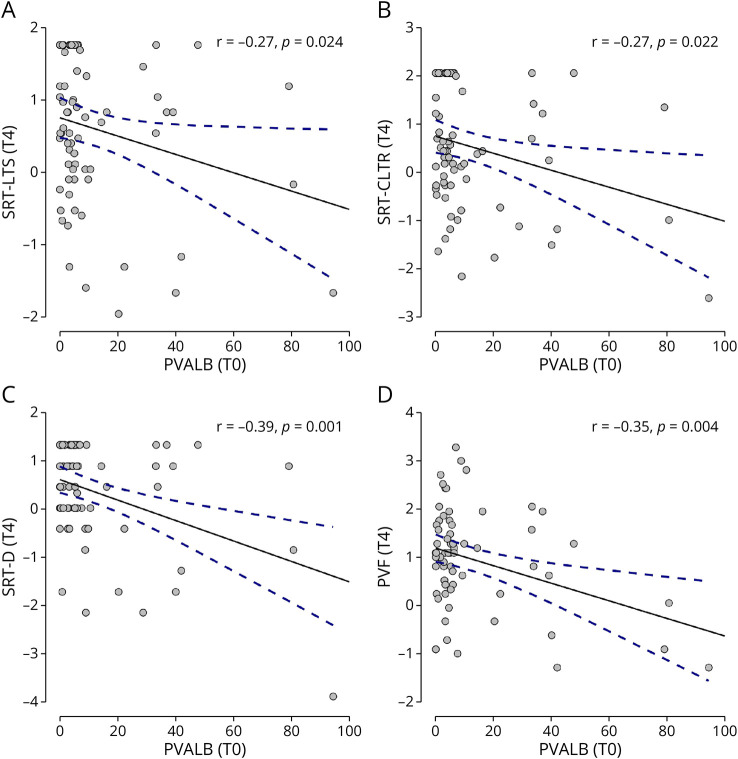
Higher PVALB levels at diagnosis were associated with a worse performance in single cognitive memory and executive tests at T4: SRT − LTS (r = −0.27, p = 0.024) (Figure 2A), SRT − CLTR (r = −0.27, p = 0.022) (Figure 2B), SRT−D (r = −0.39, p = 0.001) (Figure 2C), and PVF (r = −0.35, p = 0.004) (Figure 2D).
Figure 2. T0 PVALB and Specific Cognitive Tests at T4.
Significant association found between levels at MS diagnosis (T0) of PVALB and memory (A, B, C) and executive (D) tests at follow-up (T4). PVALB levels are expressed in ng/mL. PVF = phonemic verbal fluency; SRT-CLTR = selective reminding test consistent long-term retrieval; SRT-D = selective reminding test delayed; SRT-LTS = selective reminding test long-term storage.
We have to underline that the average follow-up period was of 4 years, which could be considered a limited time of time to accumulate cognitive disability, especially in patients with a very early MS that were evaluated close to the biological onset of the disease within their first years from diagnosis. CN and mCI patients represent most of our sample; however, they can be diagnosed with a mCI or sCI in the following years.
Fatigue was higher in patients with PVALB above median compared with others (MFIS cognitive: p = 0.024, MFIS tot: p = 0.043). No significant association was found for total/specific scores in the DASS-21 at T4 with both PVALB levels at diagnosis and cognitive status at T4.
Potential PVALB Threshold Identification for Longitudinal Cognitive Status
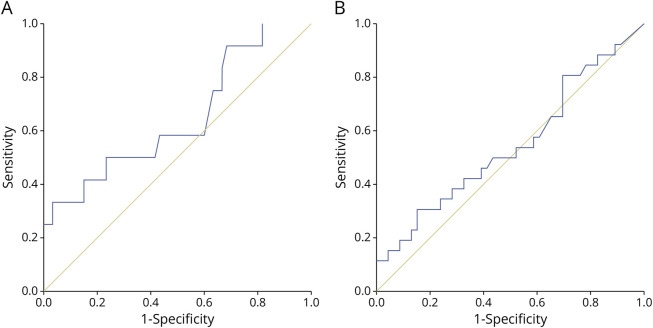
Moreover, ROC curve analysis was also performed, considering CSF PVALB levels at diagnosis with respect to future CI: results showed an area under the curve (AUC) of 0.55 and a clinical cutoff of 2.57 ng/mL with a high sensitivity of 81% (specificity 30%) (Figure 3A). We performed the same analysis considering the status of future severe CI as the outcome: results showed an AUC of 0.65, and the same clinical cutoff of 2.57 ng/mL showed an even higher sensitivity of 91% and the same specificity (Figure 3B). An additional ROC curve analysis was performed, combining CSF PVALB levels with baseline basic demographic/clinical characteristics (age, sex, and EDSS) both considering CI and sCI as future outcomes (eFigure 1).
Figure 3. ROC for T0 PVALB and Global CI at T4.
ROC curve analysis considering CSF PVALB levels at diagnosis with respect to the status of cognitive impairment (A) and severe cognitive impairment (B) at follow-up. ROC = receiver operating characteristics.
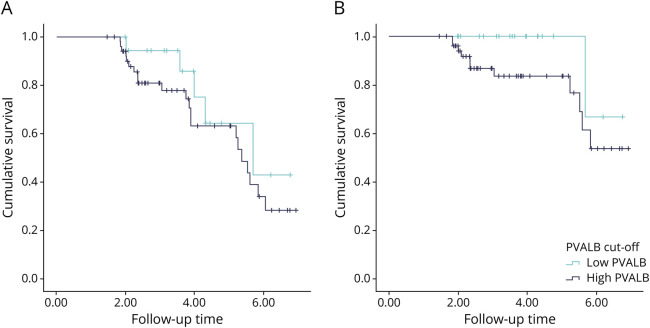
Kaplan-Meier curves were obtained for CI and specifically for sCI status outcomes by separating patients as having low or high levels of PVALB because of the cutoff proposed from the previous analysis (Figure 4).
Figure 4. KM for T0 PVALB and Global CI at T4.
Kaplan-Meier curves considering CSF PVALB levels at diagnosis with respect to the status of cognitive impairment (A) and severe cognitive impairment (B) at follow-up. KM = Kaplan-Meier.
Noteworthy, considering the importance of sensitivity in biomarkers, we can consider the cutoff of 2.57 ng/mL a valuable threshold for PVALB levels in the CSF for the identification of future cognitive impairment in MS: patients with CSF levels of PVALB higher than 2.57 ng/mL showed a significant increase of probability being classified as cognitively impaired (in particular, severely impaired) after 4 years of follow-up.
Association Between PVALB and Neuroradiologic Outcome
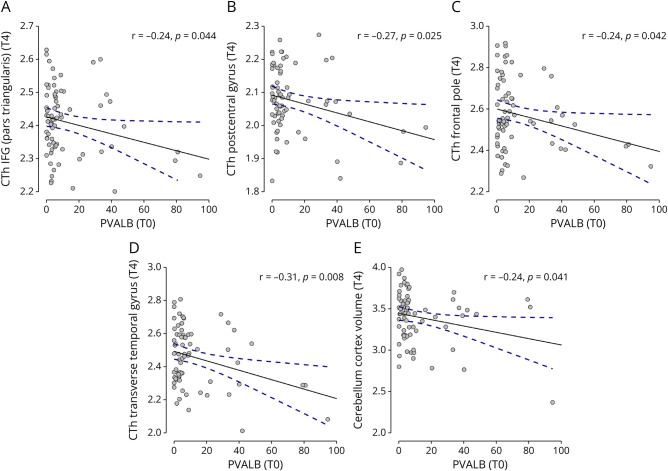
Higher PVALB levels at diagnosis also reflected cortical thickness (CTh) at T4 in the following areas: pars triangularis of the inferior frontal gyrus (r = −0.24, p = 0.044) (Figure 5A), postcentral gyrus (r = −0.27, p = 0.025) (Figure 5B), frontal pole (r = −0.24, p = 0.042) (Figure 5C), and transverse temporal gyrus (r = −0.31, p = 0.008) (Figure 5D). Moreover, a significant association was found between PVALB levels and cerebellum cortex volume (r = −0.24, p = 0.041) (Figure 5E). No significant correlation was found between PVALB levels at diagnosis and WMLs at T4 regarding lesion numbers and volumes.
Figure 5. T0 PVALB and MRI Outcomes at T4.
Significant association found between levels at MS diagnosis (T0) of PVALB and regional cortical thickness of inferior frontal gyrus (A), postcentral gyrus (B), frontal pole (C), transverse temporal gyrus (D), and volume of the cerebellum cortex (E) at follow-up (T4). PVALB levels are expressed in ng/mL. CTh = cortical thickness; IFG = inferior frontal gyrus.
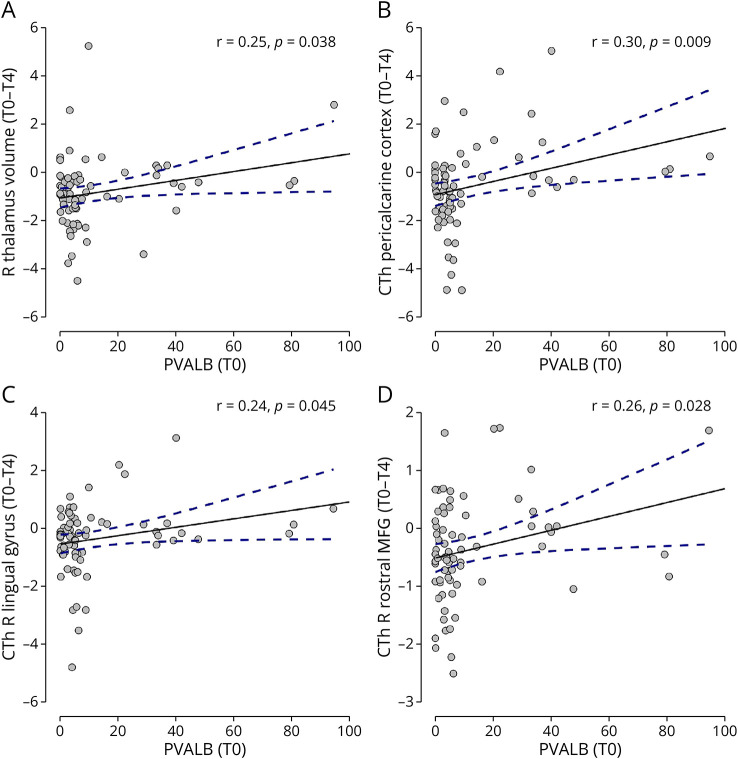
Furthermore, PVALB levels at the time of diagnosis were also able to reflect atrophy, in terms of decrease of CTh and volume between T0 and T4, in specific brain areas and regions. The main result can be observed for the thalamus: CSF PVALB levels at diagnosis were associated with the change in right thalamic volume (r = 0.25, p = 0.038) (Figure 6A). Baseline higher levels of PVALB also reflected greater atrophy in the following areas/regions: pericalcarine cortex (r = 0.30, p = 0.009) (Figure 6B), right lingual gyrus (r = 0.24, p = 0.045) (Figure 6C), and right rostral middle frontal gyrus (r = 0.26, p = 0.028) (Figure 6D).
Figure 6. T0 PVALB and MRI Outcomes Changes T0-T4.
Significant association found between levels at MS diagnosis (T0) of PVALB and change in volume of the thalamus (A) and in regional cortical thickness of pericalcarine cortex (B), right lingual gyrus (C), and right rostral middle frontal gyrus (D) over the follow-up period (change T0-T4). PVALB levels are expressed in ng/mL. CTh = cortical thickness; MFG = middle frontal gyrus; R = right.
Association Between Nf-L and Multidisciplinary Outcomes
Patients with MS with an EDSS worsening at follow-up showed at diagnosis no significant differences in terms of Nf-L levels compared with patients with improved and stable EDSS.
No significant results were obtained from the analysis of Nf-L at diagnosis and neuropsychologic data at T4 (global/domain cognitive functioning/status, specific cognitive test scores).
No significant association was also found for total/domain scores in the DASS-21 and in the MFIS at T4 with Nf-L levels at diagnosis.
Moreover, no significant results were obtained for the association between Nf-L levels at diagnosis and both regional CTh/volumes and WMLs numbers and volumes at T4.
Additional ROC curve analyses were performed on CSF Nf-L, alone and in combination with CSF PVALB levels, both considering CI and sCI as future outcomes (eFigures 2 and 3).
Discussion
Nowadays, prognostic biomarkers that would allow clinicians to identify, from the time of diagnosis, patients at higher risk of worse prognosis are critical unmet needs. In particular, it is crucial to identify as soon as possible those patients with elevated levels of neuro-axonal damage that could be affected by a more rapid and severe disability accumulation. The present preliminary prospective longitudinal study on patients with early MS aimed to identify the prognostic value of CSF PVALB, analyzed at the time of diagnosis, with respect to cognitive alterations, physical disability, fatigue, and radiologic GM damage over a mean follow-up time of 4 years.
First, PVALB levels in the CSF at diagnosis were associated with cognitive alterations, one of the most frequent hidden symptoms of MS that affect patients since the earliest phases of the disease. With respect to global cognitive functioning, we observed that patients with higher PVALB levels at baseline resulted in worse global cognitive functioning at follow-up and that sCI patients at T4 were the group with the highest PVALB levels at diagnosis. These results corroborate our previous study,18 in which this association was cross-sectionally detected at the time of diagnosis; moreover, this study supports the idea that the stratification of patients with MS according to PVALB CSF levels and the degree of CI starts since the beginning of the disease and is maintained over time, which makes PVALB a valid candidate as a prognostic biomarker. In addition to these results obtained with a 4-year observation period, we believe that extending the longitudinal follow-up would increase the strength of association between PVALB and cognitive functioning and worsening over time. Most of our patients were cognitively normal or with slight cognitive alterations, considering the earliest stages of the disease (very early, because their biological onset of MS could be overlapped to the diagnosis phase); although, a considerable number of them had at diagnosis discrete PVALB levels in their CSF, it would be helpful to continue to cognitively monitor these patients to evaluate potential worsening and, therefore, to increase statistical power and significance to present results.
We also performed ROC and Kaplan-Meier curve analyses to identify a clinical threshold for PVALB levels that can predict future prognosis: patients with PVALB levels higher than 2.57 ng/mL are at increased risk of cognitive impairment in the following 4 years.
By investigating the relation between CSF biomarkers and the functioning of single cognitive domains, we found that patients with higher PVALB levels at diagnosis had lower mnesic and executive functioning, as well as lower scores in tests of verbal learning and memory (SRT), visuospatial learning and memory (SPART), and verbal fluency (PVF) after 4 years.
All these results are in line with previous studies indicating that PVALB is a molecule involved in the modulation of neural plasticity related to learning and memory16,17 and associated with cortical atrophy of the hippocampus and other areas involved in memory circuits.18 The absence of significant correlations between PVALB and the SDMT and PASAT can be easily explained: both tests assess the information processing speed (IPS),26 a cognitive domain that relies on the functioning of multiple and integrated neural networks,27 and depends mainly on WM structural integrity28 which is poorly associated with PVALB levels.18 Moreover, our study population is composed of patients in the early stages of MS, characterized by slight cognitive alterations, whereas IPS impairment and worsening have been described in some studies as the disease progresses.29 Otherwise, the specific impairment in executive functioning has been demonstrated as one of the most typical of patients with early MS.29
Second, patients with MS with higher levels of PVALB at diagnosis were more physically and cognitively fatigued at follow-up than other patients. Fatigue is one of the most frequent symptoms reported by patients affected by multiple sclerosis; however, its pathophysiologic underpinnings remain to be elucidated. The results of our study are in line with several observations suggesting a relationship between neurodegeneration and the development of chronic fatigue in MS.30,31
The third exciting result from our study was the predictive value of PVALB at diagnosis with respect to neuroradiologic outcome. Our results confirm that PVALB appears as a specific marker of neuronal loss and/or dysfunction in the GM, as demonstrated by neuropathology and molecular studies.18 No significant correlations were found between PVALB levels and the number and the volume of WMLs, in line with our previous cross-sectional study at diagnosis,18 suggesting that PVALB cannot be considered adequate in reflecting focal WM demyelination and/or axonal damage.
On the contrary, we found a significant association between high PVALB levels at diagnosis and the future development of regional cortical atrophy in the inferior frontal gyrus, the postcentral gyrus, the frontal pole, the transverse temporal gyrus, and the cerebellar cortex, brain regions enriched in PVALB+ Gaba-ergic neurons.32,33 The frontotemporal cortex is characterized by high expression of PVALB32,33 and by severe cortical thinning, especially in patients with severe cognitive impairment.5 The somatosensory cortex presents more than twice PVALB+ cells compared with the motor cortex,34 proving that PVALB is preferentially associated with specific neuronal groups and terminals in the postcentral gyrus.35 Moreover, given that the cerebellar cortex is frequently subject to demyelination and atrophy in MS36 and that the Purkinje cells, the stellate, and basket cells all express large amounts of PVALB,37,38 these observations suggest the key role of PVALB as a possible prognostic biomarker of cerebellar neurodegeneration. This is in line with many studies,39,40 showing that the cerebellum is not only correlated with physical disability but plays an essential role in cognitive status in patients with MS.41 Damage to the cerebro-cerebellar circuitry can lead to cerebellar cognitive affective syndrome, characterized by deficits in executive function, language, attention, working memory, and visuospatial cognition.42 This evidence is also supported by a recent study from our group,43 which found a reduction in the percentage of the intracranial volume of cerebellar GM in cognitively impaired patients compared with those cognitively preserved.
Moreover, PVALB was able to predict EDSS increase over time: patients with higher levels of PVALB in the CSF at the time of diagnosis were also those who showed at follow-up a worsening in their physical disability, highlighting the importance of considering this molecule to know in advance the future evolution of MS in patients. We are aware that the strength of the correlation between PVALB and EDSS is weak; however, we have to remark that these patients are studied at clinical onset and that the follow-up is limited to 4 years. Considering the whole sample, we noticed a slight EDSS reduction between T0 and T4, probably due to the residual physical disability from the first clinical attack that was still present at the time of diagnosis.
Conversely, the analyses conducted for Nf-L showed no significant results. The fact that we did not detect the same results when examining the CSF levels of Nf-L in the same MS cohort may be related to the different pathologic sources of this marker compared with PVALB. CSF Nf-L are neuro-axonal cytoplasmic proteins highly expressed in large myelinated axons that could arise from acute neuro-axonal loss and damage related to MS activity, usually associated with WM and GM lesions,10 that may mainly reflect other functional systems than cognitive functioning.44 Instead, cognitive alterations seem to be more associated with diffuse GM neuronal and interneural loss that tend to accumulate as a result of a more smoldering activity process.18,45 High levels of Nf-L have been described in patients with early evolution to secondary progressive MS46; however, increased CSF levels of Nf-L were found in all neurodegenerative disorders (i.e., stroke, frontotemporal dementia, amyotrophic lateral sclerosis).47-49 It could be proposed that while Nf-L levels mainly reflect neuroinflammatory-related neuro-axonal damage, because it is highly associated with relapse occurrence and new gadolinium-enhancing lesions,50 PVALB levels could mirror the slow cortical neurodegeneration process that underlines cognitive impairment and cortical atrophy. In previous studies examining postmortem MS brains,18,51 PVALB gene expressions reflected the degree of meningeal inflammation and cortical neurodegeneration, whereas the expression reduction of the gene of Nf-L did not reflect these alterations, suggesting that CSF levels of PVALB protein provide more specific indication of cortical neurodegeneration compared with Nf-L in progressive MS. These results corroborate the hypothesis that PVALB may be more sensitive than Nf-L in detecting CI since the earliest phases of the disease, given its relationship with GM interneuron loss and/or dysfunction, and therefore, the combined analysis of PVALB and Nf-L could give a more comprehensive view of the MS neurodegeneration spectrum.
Our work has thus introduced new perspectives for PVALB as an additional useful MS neurodegeneration biomarker. As of now, few studies have investigated the correlation between PVALB and specific cognitive deficits16,17 while no study has evaluated its ability to predict clinical evolution in the long term. Otherwise, from this study, it seems to be a potential surrogate marker of the smoldering neurodegeneration processes characterizing MS and also a good prognostic biomarker of CI useful at the time of diagnosis because it is associated with global cognitive functioning and with the functioning of specific cognitive domains, such as memory and executive functions, and also with damage to brain areas directly involved in the same cognitive functions, especially those that most express PVALB.
We are aware that our study is not free from limitations. First of all, a more extended follow-up period could add relevance to the significant associations reported in this study because time is needed for physical and cognitive dysfunctions to emerge biologically. Furthermore, the number of patients should be increased, although our sample size is larger than that of other studies. Moreover, given that the CSF levels of inflammatory biomarkers also play a pivotal role in the evolution of MS and the development of neuropsychologic and neuroradiologic outcomes, it would be interesting to look into whether a complex profile of CSF inflammatory molecules can differentially reflect the longitudinal cognitive functioning of patients with MS. In addition, independent validation of our study on independent, different, and larger MS cohorts and other neurodegenerative diseases will be necessary to confirm our data and propose PVALB as an additional CSF marker of MS-specific neurodegeneration. We also did not consider different DMTs in our analyses; however, scarce evidence has been provided regarding a benefic effect of MS drugs on cognition. In fact, at the moment, no medication for treating CI has been approved.3 Finally, it has to be considered that PVALB is highly expressed not only in the cortex but also in other brain regions, such as the retina, thalamus, and cerebellum,52 explaining why one of the area that we found particularly associated with increased PVALB CSF levels was the cerebellum. Further studies are therefore needed to demonstrate that PVALB could be a unique MS marker of neurodegeneration occurring in a specific brain region. However, PVALB is also expressed in the kidney and parathyroid gland53; therefore, it remains still to be validated whether it could be also a potential good blood biomarker. This would help in the clinical practice, given the fact that CSF is less workable and repeatable than serum.
Parvalbumin levels in the CSF measured at the time of diagnosis could represent a potential prognostic neurodegeneration biomarker of GM damage and clinical status in terms of cognitive impairment, physical disability, and fatigue. Further studies will be necessary to verify the extent of the predictive value of parvalbumin and to validate it as a potential biomarker to be introduced in the clinical management routine of patients with MS. Thus, given the complexity of identifying valid prognostic biomarkers of worse prognosis in patients with MS and considering the impact that clinical conditions have on the quality of life of patients, our study contributed to identifying a potential additional specific neurodegeneration biomarker capable of predicting in advance which patients will be characterized by a more severe prognosis at year 4. This, in turn, would contribute to optimizing patient treatment at an early stage, identifying those who need more intensive treatment protocols, neuroprotective treatments, more frequent monitoring, and structured rehabilitation pathways.
Acknowledgment
The authors thank the LURM (Laboratorio Universitario di Ricerca Medica) Research Center, University of Verona.
Glossary
- AUC
area under curve
- CI
cognitive impairment
- DASS
Depression Anxiety Stress Scale
- DMT
disease-modifying treatment
- EDSS
Expanded Disability Status Scale
- FLAIR
fluid-attenuated inversion recovery
- GM
gray matter
- IPS
information processing speed
- LP
lumbar puncture
- MFIS
Modified Fatigue Impact Scale
- MS
multiple sclerosis
- ROC
receiver operating characteristic
- RRMS
relapsing-remitting MS
- WM
white matter
- WML
WM lesion
Appendix. Authors
| Name | Location | Contribution |
| Stefano Ziccardi, PhD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Agnese Tamanti, PhD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Major role in the acquisition of data |
| Claudia Ruggieri, MD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Drafting/revision of the manuscript for content, including medical writing for content |
| Maddalena Guandalini, MSc | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Major role in the acquisition of data |
| Damiano Marastoni, MD, PhD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Valentina Camera, MD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Major role in the acquisition of data |
| Luigi Montibeller, PhD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Major role in the acquisition of data |
| Valentina Mazziotti, PhD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Major role in the acquisition of data |
| Stefania Rossi, PhD | Department of Neurosciences, Biomedicine and Movement, University of Verona; Department of Oncology and Molecular Medicine, Istituto Superiore di Sanità, Rome, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Milena Calderone, MD | Radiology Unit, Cmsr Veneto Medica s.r.l., Altavilla Vicentina, Vicenza, Italy | Major role in the acquisition of data |
| Francesca Benedetta Pizzini, MD | Institute of Radiology, University of Verona, Italy | Major role in the acquisition of data |
| Stefania Montemezzi, MD | Institute of Radiology, University of Verona, Italy | Major role in the acquisition of data |
| Roberta Magliozzi, PhD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Massimiliano Calabrese, MD | Department of Neurosciences, Biomedicine and Movement, University of Verona, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
Work supported by #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006) - A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Disclosure
S. Ziccardi: nothing to disclose; A. Tamanti: nothing to disclose; C. Ruggieri: nothing to disclose; M Guandalini: nothing to disclose; D. Marastoni: received research support and/or honoraria for speaking and funds for travel from Roche, Sanofi-Genzyme, Merck-Serono, Biogen Idec, and Novartis and receives research support from Italian Minister of Health; V. Camera: received grant from European Charcot Foundation, received support for scientific meetings from Janssen, Novartis, BMS, Roche and speaking honoraria from Novartis; V. Mazziotti: nothing to disclose; L. Montibeller: nothing to disclose; S. Rossi: nothing to disclose; M. Calderone: nothing to disclose; F.B. Pizzini: nothing to disclose; S. Montemezzi: nothing to disclose; R. Magliozzi: received research support from Italian MS Foundation grant (2023/R-Single/038); M. Calabrese: received speaker honoraria from Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, and Roche and receives research support from the Progressive MS Alliance and Italian Minister of Health. Go to Neurology.org/NN for full disclosures.
References
- 1.Calabrese M, Magliozzi R, Ciccarelli O, Geurts JJ, Reynolds R, Martin R. Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci. 2015;16(3):147-158. doi: 10.1038/nrn3900 [DOI] [PubMed] [Google Scholar]
- 2.Eijlers AJC, van Geest Q, Dekker I, et al. Predicting cognitive decline in multiple sclerosis: a 5-year follow-up study. Brain. 2018;141(9):2605-2618. doi: 10.1093/brain/awy202 [DOI] [PubMed] [Google Scholar]
- 3.Benedict RHB, Amato MP, DeLuca J, Geurts JJG. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19(10):860-871. doi: 10.1016/S1474-4422(20)30277-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitteri M, Ziccardi S, Dapor C, Guandalini M, Calabrese M. Lost in Classification: lower cognitive functioning in apparently cognitive normal newly diagnosed RRMS patients. Brain Sci. 2019;9(11):321. doi: 10.3390/brainsci9110321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrese M, Rinaldi F, Mattisi I, et al. Widespread cortical thinning characterizes patients with MS with mild cognitive impairment. Neurology. 2010;74(4):321-328. doi: 10.1212/WNL.0b013e3181cbcd03 [DOI] [PubMed] [Google Scholar]
- 6.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 7.Comabella M, Sastre-Garriga J, Carbonell-Mirabent P, et al. Serum neurofilament light chain levels predict long-term disability progression in patients with progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93(7):732-740. doi: 10.1136/jnnp-2022-329020 [DOI] [PubMed] [Google Scholar]
- 8.Kuhle J, Leppert D, Petzold A, et al. Neurofilament heavy chain in CSF correlates with relapses and disability in multiple sclerosis. Neurology. 2011;76(14):1206-1213. doi: 10.1212/WNL.0b013e31821432ff [DOI] [PubMed] [Google Scholar]
- 9.Quintana E, Coll C, Salavedra-Pont J, et al. Cognitive impairment in early stages of multiple sclerosis is associated with high cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain. Eur J Neurol. 2018;25(9):1189-1191. doi: 10.1111/ene.13687 [DOI] [PubMed] [Google Scholar]
- 10.Gaetani L, Salvadori N, Lisetti V, et al. Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J Neurol. 2019;266(9):2157-2163. doi: 10.1007/s00415-019-09398-7 [DOI] [PubMed] [Google Scholar]
- 11.Kuhle J, Kropshofer H, Barro C, et al. The predictive value of neurofilament light chain levels in blood for cognitive impairment in patients with secondary progressive multiple sclerosis (S12.009). Neurology. 2019;92(15_supplement):666. doi: 10.1212/WNL.92.15_supplement.S12.009 [DOI] [Google Scholar]
- 12.Jakimovski D, Zivadinov R, Ramanthan M, et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: a longitudinal retrospective 5-year study. Mult Scler. 2020;26(13):1670-1681. doi: 10.1177/1352458519881428 [DOI] [PubMed] [Google Scholar]
- 13.Nourbakhsh BS, Gnanapavan S, Giovannoni G, et al. Association of serum neurofilament light chain with cognitive and disability measures in patients with very early MS: a longitudinal study. Neurologt. 2016;82:396. doi: 10.1212/WNL.86.16_supplement.P1.396 [DOI] [Google Scholar]
- 14.Hu H, Gan J, Jonas P. Interneurons. Fast-spiking, parvalbumin⁺ GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345(6196):1255263. doi: 10.1126/science.1255263 [DOI] [PubMed] [Google Scholar]
- 15.Micheva KD, Wolman D, Mensh BD, et al. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife. 2016;5:e15784. doi: 10.7554/eLife.15784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Lee K. Parvalbumin-expressing GABAergic interneurons and perineuronal nets in the prelimbic and orbitofrontal cortices in association with basal anxiety-like behaviors in adult mice. Behav Brain Res. 2021;398:112915. doi: 10.1016/j.bbr.2020.112915 [DOI] [PubMed] [Google Scholar]
- 17.Murray AJ, Sauer JF, Riedel G, et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat Neurosci. 2011;14(3):297-299. doi: 10.1038/nn.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magliozzi R, Pitteri M, Ziccardi S, et al. CSF parvalbumin levels reflect interneuron loss linked with cortical pathology in multiple sclerosis. Ann Clin Transl Neurol. 2021;8(3):534-547. doi: 10.1002/acn3.51298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 20.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914-1922. doi: 10.1212/WNL.0b013e3181c47cc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magliozzi R, Fadda G, Brown RA, et al. “Ependymal-in” Gradient of thalamic damage in progressive multiple sclerosis. Ann Neurol. 2022;92(4):670-685. doi: 10.1002/ana.26448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziccardi S, Pisani AI, Schiavi GM, et al. Cortical lesions at diagnosis predict long-term cognitive impairment in multiple sclerosis: a 20-year study. Eur J Neurol. 2023;30(5):1378-1388. doi: 10.1111/ene.15697 [DOI] [PubMed] [Google Scholar]
- 23.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050-11055. doi: 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.surfer.nmr.mgh.harvard.edu/
- 25.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 26.Benedict RH, DeLuca J, Phillips G, et al. Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler. 2017;23(5):721-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lengenfelder J, Bryant D, Diamond BJ, et al. Processing speed interacts with working memory efficiency in multiple sclerosis. Arch Clin Neuropsychol. 2006;21(3):229-238. doi: 10.1016/j.acn.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 28.Govindarajan ST, Liu Y, Parra CorralMA, et al. White matter correlates of slowed information processing speed in unimpaired multiple sclerosis patients with young age onset. Brain Imaging Behav. 2021;15(3):1460-1468. doi: 10.1007/s11682-020-00345-z [DOI] [PubMed] [Google Scholar]
- 29.Thrue C, Riemenschneider M, Hvid LG, Stenager E, Dalgas U. Time matters: early-phase multiple sclerosis is accompanied by considerable impairments across multiple domains. Mult Scler. 2021;27(10):1477-1485. doi: 10.1177/1352458520936231 [DOI] [PubMed] [Google Scholar]
- 30.Calabrese M, Rinaldi F, Grossi P, et al. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult Scler. 2010;16(10):1220-1228. doi: 10.1177/1352458510376405 [DOI] [PubMed] [Google Scholar]
- 31.Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev. 2016;15(3):210-220. doi: 10.1016/j.autrev.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 32.Kim Y, Yang GR, Pradhan K, et al. Brain-wide maps reveal stereotyped cell-type-based cortical architecture and subcortical sexual dimorphism. Cell. 2017;171(2):456-469.e22. doi: 10.1016/j.cell.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjerke IE, Yates SC, Laja A, et al. Densities and numbers of calbindin and parvalbumin positive neurons across the rat and mouse brain. iScience. 2020;24(1):101906. doi: 10.1016/j.isci.2020.101906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Brederode JF, Helliesen MK, Hendrickson AE. Distribution of the calcium-binding proteins parvalbumin and calbindin-D28k in the sensorimotor cortex of the rat. Neuroscience. 1991;44(1):157-171. doi: 10.1016/0306-4522(91)90258-p [DOI] [PubMed] [Google Scholar]
- 35.Gelfand JM. Multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation. Handb Clin Neurol. 2014;122:269-290. doi: 10.1016/B978-0-444-52001-2.00011-X [DOI] [PubMed] [Google Scholar]
- 36.Kutzelnigg A, Faber-Rod JC, Bauer J, et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17(1):38-44. doi: 10.1111/j.1750-3639.2006.00041.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosaka T, Kosaka K, Nakayama T, Hunziker W, Heizmann CW. Axons and axon terminals of cerebellar Purkinje cells and basket cells have higher levels of parvalbumin immunoreactivity than somata and dendrites: quantitative analysis by immunogold labeling. Exp Brain Res. 1993;93(3):483-491. doi: 10.1007/BF00229363 [DOI] [PubMed] [Google Scholar]
- 38.Schwaller B, Meyer M, Schiffmann S. 'New' functions for 'old' proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1(4):241-258. doi: 10.1080/147342202320883551 [DOI] [PubMed] [Google Scholar]
- 39.Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, Leggio MG. The cerebellar cognitive profile. Brain. 2011;134:3672-3686. doi: 10.1093/brain/awr266 [DOI] [PubMed] [Google Scholar]
- 40.Schoonheim MM, Douw L, Broeders TA, Eijlers AJ, Meijer KA, Geurts JJ. The cerebellum and its network: disrupted static and dynamic functional connectivity patterns and cognitive impairment in multiple sclerosis. Mult Scler. 2021;27(13):2031-2039. doi: 10.1177/1352458521999274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weier K, Penner IK, Magon S, et al. Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS One. 2014;9(1):e86916. doi: 10.1371/journal.pone.0086916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobyne SM, Ochoa WB, Bireley JD, et al. Cognitive impairment and the regional distribution of cerebellar lesions in multiple sclerosis. Mult Scler. 2018;24(13):1687-1695. doi: 10.1177/1352458517730132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ziccardi S, Pizzini FB, Guandalini M, Tamanti A, Cristofori C, Calabrese M. Making visible the invisible: automatically measured global and regional brain volume is associated with cognitive impairment and fatigue in multiple sclerosis. Bioengineering. 2022;10(1):41. doi: 10.3390/bioengineering10010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eshaghi A, Prados F, Brownlee WJ, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83(2):210-222. doi: 10.1002/ana.25145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovannoni G, Popescu V, Wuerfel J, et al. Smouldering multiple sclerosis: the 'real MS'. Ther Adv Neurol Disord. 2022;15:17562864211066751. doi: 10.1177/17562864211066751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salzer J, Svenningsson A, Sundström P. Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler. 2010;16(3):287-292. doi: 10.1177/1352458509359725 [DOI] [PubMed] [Google Scholar]
- 47.Tiedt S, Duering M, Barro C, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018;91(14):e1338-e1347. doi: 10.1212/WNL.0000000000006282 [DOI] [PubMed] [Google Scholar]
- 48.Meeter LH, Dopper EG, Jiskoot LC, et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann Clin Transl Neurol. 2016;3(8):623-636. doi: 10.1002/acn3.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feneberg E, Oeckl P, Steinacker P, et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology. 2018;90(1):e22-e30. doi: 10.1212/WNL.0000000000004761 [DOI] [PubMed] [Google Scholar]
- 50.Chitnis T, Qureshi F, Gehman VM, et al. Inflammatory and neurodegenerative serum protein biomarkers increase sensitivity to detect clinical and radiographic disease activity in multiple sclerosis. Nat Commun. 2024;15(1):4297. doi: 10.1038/s41467-024-48602-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magliozzi R, Hametner S, Facchiano F, et al. Iron homeostasis, complement, and coagulation cascade as CSF signature of cortical lesions in early multiple sclerosis. Ann Clin Transl Neurol. 2019;6(11):2150-2163. doi: 10.1002/acn3.50893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.proteinatlas.org/ENSG00000100362-PVALB/brain
- 53.proteinatlas.org/ENSG00000100362-PVALB/tissue
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author, who had complete access to all the study data, assumed responsibility for the data's integrity and the analyses' accuracy. The anonymized data set can be obtained from the corresponding author at a reasonable request.