Abstract
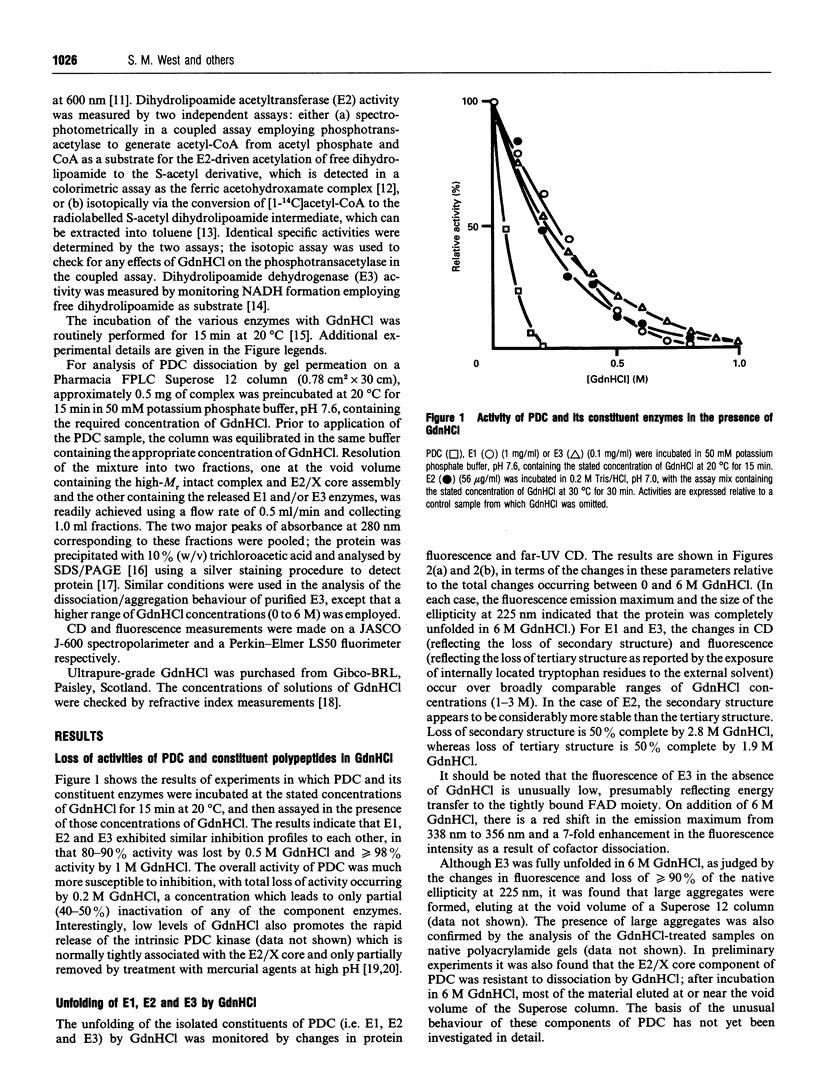
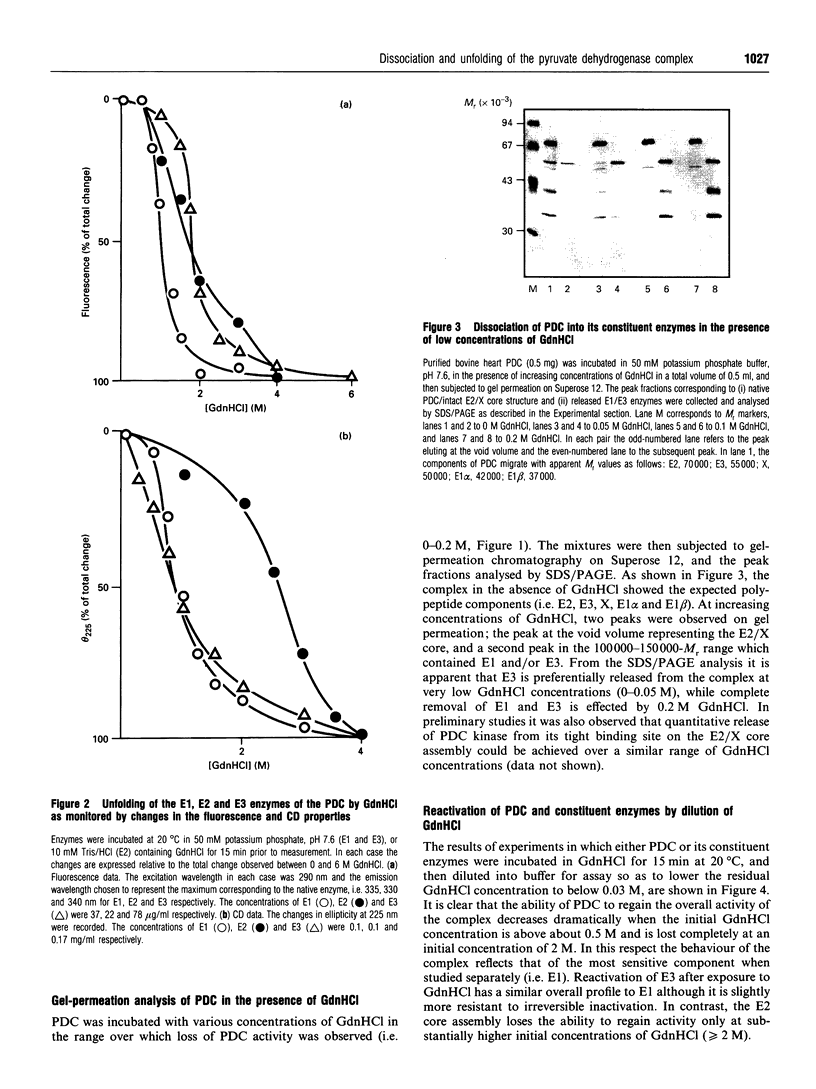
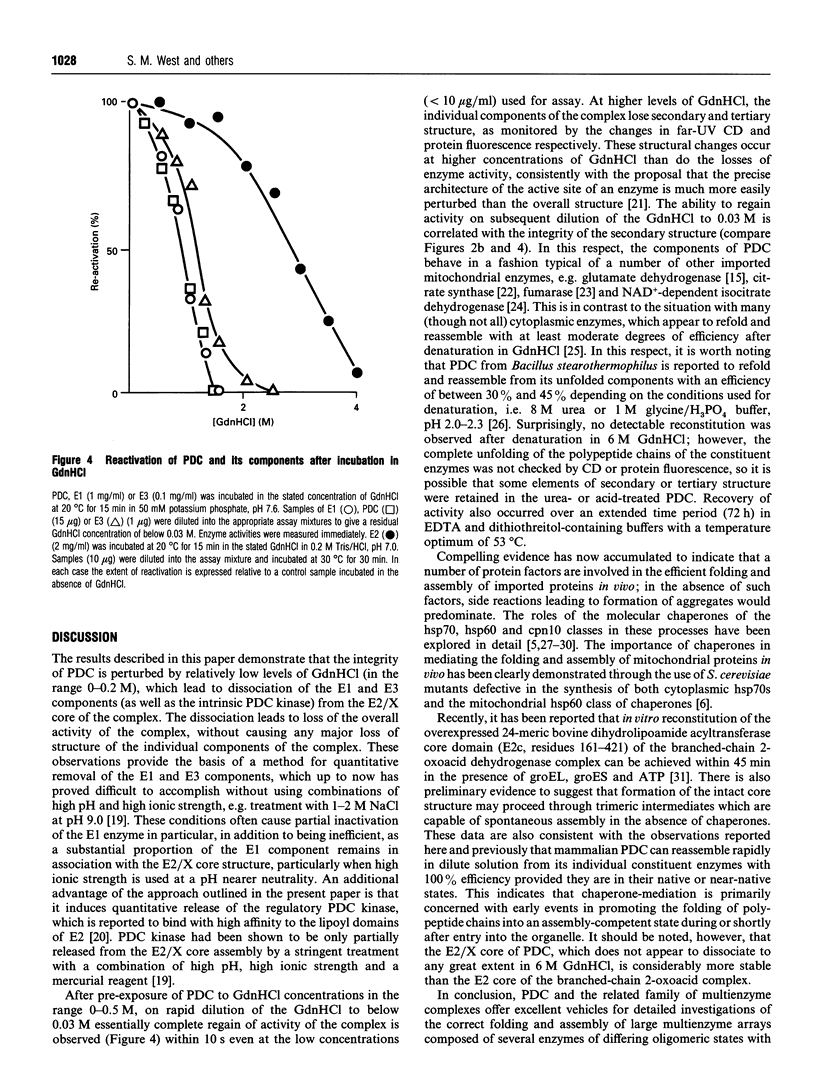
The effect of guanidinium chloride (GdnHCl) on the pyruvate dehydrogenase complex (PDC) from bovine heart and its constituent enzymes has been studied. The overall activity of the complex is lost reversibly at low levels of GdnHCl (0.2 M) which cause 40-50% inactivation but no loss of overall secondary or tertiary structures of the individual enzymes; the inactivation of the complex is shown to be caused by dissociation of the E1 and E3 components from the E2/X core assembly. This provides an improved procedure for controlled dissociation of the complex and efficient recovery of its component enzymes in their native states. Higher concentrations of GdnHCl (up to 4 M) lead to the unfolding and irreversible inactivation of the separate enzymes of the complex with the E2/X core proving the most resistant to GdnHCl-induced unfolding. Neither the 60-meric E2/X core assembly nor the dimeric E3 component are dissociated into monomers in the presence of 6 M GdnHCl; the latter enzyme forms higher-M(r) aggregates under these conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. P., Perham R. N. Selective inactivation of the transacylase components of the 2-oxo acid dehydrogenase multienzyme complexes of Escherichia coli. Biochem J. 1976 May 1;155(2):419–427. doi: 10.1042/bj1550419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth P. J., Tsai C. S., Eley M. H., Roche T. E., Reed L. J. A kinetic study of dihydrolipoyl transacetylase from bovine kidney. J Biol Chem. 1975 Mar 10;250(5):1921–1925. [PubMed] [Google Scholar]
- De Marcucci O., Lindsay J. G. Component X. An immunologically distinct polypeptide associated with mammalian pyruvate dehydrogenase multi-enzyme complex. Eur J Biochem. 1985 Jun 18;149(3):641–648. doi: 10.1111/j.1432-1033.1985.tb08972.x. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Glover L. A., Lindsay J. G. Targeting proteins to mitochondria: a current overview. Biochem J. 1992 Jun 15;284(Pt 3):609–620. doi: 10.1042/bj2840609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Jackman S. A., Hough D. W., Danson M. J., Stevenson K. J., Opperdoes F. R. Subcellular localisation of dihydrolipoamide dehydrogenase and detection of lipoic acid in bloodstream forms of Trypanosoma brucei. Eur J Biochem. 1990 Oct 5;193(1):91–95. doi: 10.1111/j.1432-1033.1990.tb19308.x. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Folding and association of proteins. Prog Biophys Mol Biol. 1987;49(2-3):117–237. doi: 10.1016/0079-6107(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Jaenicke R., Perham R. N. Reconstitution of the pyruvate dehydrogenase multienzyme complex from Bacillus stearothermophilus. Biochemistry. 1982 Jul 6;21(14):3378–3385. doi: 10.1021/bi00257a020. [DOI] [PubMed] [Google Scholar]
- Jilka J. M., Rahmatullah M., Kazemi M., Roche T. E. Properties of a newly characterized protein of the bovine kidney pyruvate dehydrogenase complex. J Biol Chem. 1986 Feb 5;261(4):1858–1867. [PubMed] [Google Scholar]
- Kelly S. M., Duncan D., Price N. C. Unfolding and refolding of the NAD(+)-dependent isocitrate dehydrogenase from yeast. Int J Biol Macromol. 1993 Apr;15(2):75–79. doi: 10.1016/0141-8130(93)90001-3. [DOI] [PubMed] [Google Scholar]
- Kelly S. M., Price N. C. The unfolding and refolding of pig heart fumarase. Biochem J. 1991 May 1;275(Pt 3):745–749. doi: 10.1042/bj2750745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khailova L. S., Bernkhardt R., Khiubner G. Izuchenie kineticheskogo mekhanizma piruvat-2,6-dikhlorfenolindofenolreduktaznoi aktivnosti myshechnoi piruvatdegidrogenazy. Biokhimiia. 1977 Jan;42(1):113–117. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langer T., Lu C., Echols H., Flanagan J., Hayer M. K., Hartl F. U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992 Apr 23;356(6371):683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Martin J., Mayhew M., Langer T., Hartl F. U. The reaction cycle of GroEL and GroES in chaperonin-assisted protein folding. Nature. 1993 Nov 18;366(6452):228–233. doi: 10.1038/366228a0. [DOI] [PubMed] [Google Scholar]
- Neagle J. C., Lindsay J. G. Selective proteolysis of the protein X subunit of the bovine heart pyruvate dehydrogenase complex. Effects on dihydrolipoamide dehydrogenase (E3) affinity and enzymic properties of the complex. Biochem J. 1991 Sep 1;278(Pt 2):423–427. doi: 10.1042/bj2780423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y. The preparation of guanidine hydrochloride. Methods Enzymol. 1972;26:43–50. doi: 10.1016/s0076-6879(72)26005-0. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Bassendine M. F., James O. F., Yeaman S. J. Human pyruvate dehydrogenase complex as an autoantigen in primary biliary cirrhosis. Clin Sci (Lond) 1993 Sep;85(3):289–293. doi: 10.1042/cs0850289. [DOI] [PubMed] [Google Scholar]
- Powers-Greenwood S. L., Rahmatullah M., Radke G. A., Roche T. E. Separation of protein X from the dihydrolipoyl transacetylase component of the mammalian pyruvate dehydrogenase complex and function of protein X. J Biol Chem. 1989 Mar 5;264(7):3655–3657. [PubMed] [Google Scholar]
- Reed L. J., Hackert M. L. Structure-function relationships in dihydrolipoamide acyltransferases. J Biol Chem. 1990 Jun 5;265(16):8971–8974. [PubMed] [Google Scholar]
- Stanley C. J., Perham R. N. Purification of 2-oxo acid dehydrogenase multienzyme complexes from ox heart by a new method. Biochem J. 1980 Oct 1;191(1):147–154. doi: 10.1042/bj1910147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp L. R., Pettit F. H., Yeaman S. J., Reed L. J. Purification and properties of pyruvate dehydrogenase kinase from bovine kidney. J Biol Chem. 1983 Aug 10;258(15):9454–9458. [PubMed] [Google Scholar]
- Stuart R. A., Cyr D. M., Craig E. A., Neupert W. Mitochondrial molecular chaperones: their role in protein translocation. Trends Biochem Sci. 1994 Feb;19(2):87–92. doi: 10.1016/0968-0004(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994 Jul 29;265(5172):659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- West S. M., Kelly S. M., Price N. C. The unfolding and attempted refolding of citrate synthase from pig heart. Biochim Biophys Acta. 1990 Mar 1;1037(3):332–336. doi: 10.1016/0167-4838(90)90034-d. [DOI] [PubMed] [Google Scholar]
- West S. M., Price N. C. The unfolding and refolding of glutamate dehydrogenases from bovine liver, baker's yeast and Clostridium symbosium. Biochem J. 1988 Apr 1;251(1):135–139. doi: 10.1042/bj2510135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Wynn R. M., Davie J. R., Zhi W., Cox R. P., Chuang D. T. In vitro reconstitution of the 24-meric E2 inner core of bovine mitochondrial branched-chain alpha-keto acid dehydrogenase complex: requirement for chaperonins GroEL and GroES. Biochemistry. 1994 Aug 2;33(30):8962–8968. doi: 10.1021/bi00196a014. [DOI] [PubMed] [Google Scholar]




