ABSTRACT
Background:
COVID-19 an infectious disease caused by the SARS-CoV-2 virus, started in late 2019 and became a pandemic within a short period. To respond to the pandemic vaccines like Covishield, Covaxin, Sputnik V, Covovax, etc., were developed rapidly. However, there were raising concerns about the development of immunity as well as adverse events following vaccination.
Objectives:
To compare anti-SARS-CoV-2 IgG antibody titres at different time-points post-vaccination between baseline seropositive and seronegative groups and to assess the adverse events following the 1st dose of Covishield vaccine among adult beneficiaries attending vaccination centre in a tertiary care hospital of Upper Assam.
Materials and Methods:
Prospective Cohort study was conducted from July 2021 to June 2022 among adult beneficiaries receiving the Covishield vaccine. The oral questionnaire was used incorporating socio-demographic variables, and clinical profiles including co-morbidities and adverse events following vaccination. Data analysis was done by Microsoft Excel and SPSS
Results:
Out of a total of 146 study participants, IgG estimation showed 61% as seropositive and the rest as seronegative. A total of 55.40% had minor adverse events, majority of them were females (53.08%) and 88.80% belonged to 18–59 years compared to 11.11% above 60 years of age. The majority (71.60%) did not have any co-morbidities and the major AEFI was NIL among the study participants. The study group had 61% seropositive previously infected.
Conclusion:
Covishield vaccination induces an immune response and 90% seroconversion is achieved after 1st dose (booster dose). Antibody titres of the seropositive group by natural infection of SARS-CoV-2 were higher than seronegative cohort seroconverted by vaccination. The AEFI observed were minor and can be commented as safer.
Keywords: AEFI, co-morbidities, Covishield, SARS-CoV-2, serosurveillance
Introduction
The pandemic of the novel coronavirus (SARS-CoV-2), epi-centred in Wuhan, Hubei Province of the People’s Republic of China, has risen to a global health crisis causing widespread morbidity and mortality during the period 2020–2022. According to Worldometer, the rate of death due to COVID-19 was high in the year 2020–2021 but significantly declined in the year 2022. The global number of deaths in 2021 was 35,98,075 with the overall cumulative total number of death at the end of 2021 of 54,98,075. In the year 2022, the global number of deaths was 12,22,846 with the cumulative total number of deaths as of January 1, 2023 of 66,49,477.[1] Mass prophylactic vaccination was the only possible cost-effective way that curbed the pandemic. Several vaccines were approved for clinical trials and emergency use by various countries. India had also completed Phase III trials for the Covishield vaccine (ChAdOx1nCov 19 recombinant vaccines, M/S Serum Institute of India Private Limited)[2] which was approved by the Drugs Controller General of India (DGCI) for emergency use.[3] As a result of the pandemic, COVID-19 vaccines were rapidly developed with simultaneous Phase III trials and approved for emergency use. This however resulted in knowledge gaps highlighted by the WHO ad hoc committee statement on COVID-19 vaccines that was published on January 15, 2021.[4] The key highlights of the committee were: (i) real-world effectiveness of first-generation vaccines; (ii) duration of protection provided; (iii) effectiveness of first-generation vaccines among special populations like children, pregnant women and immune-compromised; (iv) impact of these vaccines on viral shedding and transmission; and (v) their long term safety.
This study was conducted against a backdrop of post-vaccine surveillance scenarios for the COVID-19 pandemic in a tertiary care hospital in Assam, a North-Eastern state of India. Covishield, a first-generation vaccine administered among the population was part of the vaccination drive. It was a non-replicating chimpanzee adenovirus vaccine developed by Oxford University and Astra Zeneca that conveys DNA encoding instructions for the spike protein of the SARS-CoV-2 virus to the human cells.[5] The messenger RNA (mRNA) is created when the DNA is released into the cytoplasm and subsequently transported to the nucleus. The ribosome eventually translates the mRNA into spike protein. This spike protein is incapable of infecting human cells but prepares the immune system to combat the virus.[6] Our hypothesis was that in the seropositive population, the 1st dose of vaccination acts as a booster dose and there is a robust immune response even after a single dose of vaccination. The eventual outcome is to serve sources in terms of vaccine doses. We also conducted post-vaccination surveillance for adverse events following immunization (AEFI). At the beginning of the study, there were very limited data on AEFI but now most of the studies published show minor AEFI except in very rare reports of major AEFI that are available.[2,5,6] An AEFI is an untoward medical incidence following immunization that may not have a causal relationship with the usage of vaccines.[7]
Objectives
To compare anti-SARS Cov2 IgG antibody titres after 28 days of 1st and 2nd dose of Covishield vaccine among baseline seropositive and seronegative groups of adult beneficiaries attending vaccine centres of Dibrugarh district of Upper Assam region.
To estimate the incidence of AEFI after Covishield vaccination among adult beneficiaries attending various vaccination centres of Upper Assam.
Subjects and Methods
Study design
This was a prospected Cohort study conducted at Dibrugarh district in the state of Assam. The period of study was from July 2021 to June 2022. Vaccine centres located at various places in Dibrugarh district including the vaccine centre of the tertiary care hospital were included in the study. The Multidisciplinary Research Unit of the tertiary centre was used for anti-SARS-CoV-2 IgG antibody estimation.
Inclusion and exclusion criteria
Inclusion criteria:
-
(1)
Vaccine beneficiaries.
-
(2)
Age: 18.0 years and above.
-
(3)
Gender: Male and Female.
-
(4)
Resident of the study area.
Exclusion criteria:
-
(1)
Known cases of Primary or secondary immune deficiency.
-
(2)
On chemotherapy/radiotherapy/immunomodulatory drugs.
-
(3)
Pregnancy and age less than 18 years.
Enrolment
Participants were enrolled based on inclusion and exclusion criteria. Pre-enrolment counselling included commentary on the risks and benefits of the study to the participants and its contribution to the general population.
Sample size
A total of 146 adult individuals between the age group 18–90 years participated in the study. A purposive sampling technique was used for the determination of sample size.
Data collection and follow up
Data were collected from various vaccination centres located in the study area. Data were collected through telephonic personal interviews with an oral questionnaire method. The questionnaire included demographic details of age, sex, pre-existing medical conditions (co-morbidities) and possible adverse events following 7 days and 28 days of 1st and 2nd dose of Covishield vaccination. All the participants were followed up during the time period.
Sample collection and storage for serosurveillance
Blood samples were collected from all the participants at baseline but subsequent samples were collected from those who were available during follow-up. Three blood samples were collected from the participants at different time points: pre-vaccination (baseline = 1st sample), after 28 days of 1st dose (2nd sample) and after 28 days of 2nd dose (3rd sample). The samples were collected in a gel tube with a clot activator, serum was separated and stored in − 70°C up to completion of the study.
Antibody test for anti-SARS-CoV-2 IgG
Antibody detection was carried out from the participants where all three samples were available. The quantitative estimation of anti-SARS-CoV2 spike protein IgG titre level was carried out using SCoV-2 Detect™ IgG ELISA kit (Make: InBios) as per kit protocol. Briefly, 50 μl of controls and samples were added to the SCoV-2 Antigen Coated Microtiter Strip plate and incubated at 37°C for 1 h. After incubation, the wells were washed six times with 300 μl wash buffer. To the washed wells added 50 μl per well of Conjugate Solution and further incubated at 37°C for 30 min. The wells were again washed six times with wash buffer as previously described. Added 75 μl of Liquid TMB substrate into each well and incubated at room temperature in the dark for 20 min. Added 50 μl of Stop Solution into each well uniformly. The optical density was recorded at 450 nm in an ELISA reader (MultiSkan Go, Make: Thermo Scientific). Instrument calibration was done as per the manufacturer’s instruction. Also, a comparative analysis was done of the 57 serosurveillance cases against their respective AEFI.
Surveillance for adverse events following immunization
-
(a)
Surveillance for adverse events was carried out from a tertiary care centre of the Dibrugarh district in the Upper Assam region.
-
(b)
Adverse events were recorded through telephonic interviews on day 7 and day 28 post-first and second doses of vaccination.
-
(c)
Participants were also provided with a helpline number to report adverse events throughout the period of study.
-
(d)
Adverse events recorded were categorized as minor and serious.[8] Minor adverse events were common body discomforts and self-limiting reactions. Serious adverse events were disabling, rarely life-threatening and did not lead to long-term problems.
-
(e)
Serious events were further investigated and a facility for hospitalization was provided wherever required.
-
(f)
Causality assessment was done as per WHO guidelines.[9]
Ethical approval
The study was approved by the Institutional Ethical Committee for Human of the tertiary care hospital. Informed consent was taken from the study participants.
Statistical analysis
Statistical analysis was done on Microsoft Excel and SPSS software. Quantitative data are presented in frequencies and percentages. The antibody titre calculated at different time points was interpreted as Immunological Status Ratio (ISR) with reference to the raw Optical Density values and cut-off value as mentioned in the kit protocol. Multivariate analysis for a test of significance and positive predictive value test was done.
Results
A total of 146 participants who received the Covishield vaccine were followed up after 7 days and 28 days of 1st dose and 2nd dose of immunization. Clinico-social characteristics of the participants are detailed in Table 1. The frequency of male and female recipients is shown in Figure 1. The average age of 146 recipients was 40.58 years with the lowest and highest recipient ages being 18 years and 87 years, respectively. The frequency of age distribution is shown in Figure 2. Among the participants, 30.13% of cases had more than one pre-existing medical condition (co-morbidities) with less than 1% were in immunocompromised state. The co-morbidities include hypertension, diabetes, hypothyroidism, cardiac, renal and respiratory problems. These are shown in Table 2.
Table 1.
Clinico-social characteristics of study participants
| Parameters | n=146 | |
|---|---|---|
| Gender Distribution % | ||
| Male | 63 | 43.15 |
| Female | 83 | 56.84 |
| Age Distribution % | ||
| 18–35 | 60 | 41.09 |
| 36–53 | 62 | 42.46 |
| 54–71 | 17 | 11.64 |
| 72–90 | 7 | 4.79 |
| Distribution of immunocompromised state % | ||
| Present | 1 | 0.69% |
| Absent | 145 | 99.31% |
| Distribution Co-morbidities % | ||
| Non co-morbid | 89 | 69.80% |
| Co-morbid | 44 | 30.13% |
| Distribution of AEFI % | ||
| Present | 81 | 55.40% |
| Absent | 51 | 34.90% |
| No response | 14 | 9.58% |
Figure 1.
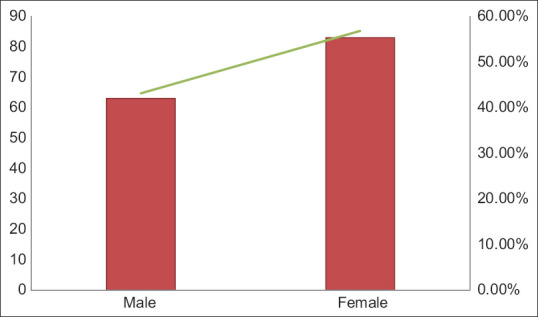
Distribution of gender among 146 participants enrolled for the study
Figure 2.
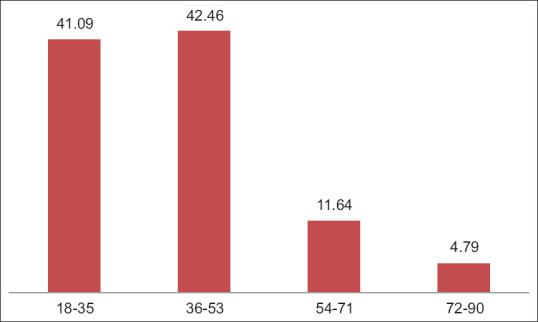
Age distribution among the study participants
Table 2.
Morbidity profile of study participants (n=44)
| Co-morbidities | No. of cases | Percentage |
|---|---|---|
| HTN | 24 | 54.55 |
| Diabetes | 10 | 22.73 |
| Hypothyroidism | 13 | 29.55 |
| Cardiac | 5 | 11.36 |
| Renal | 1 | 2.27 |
| Respiratory | 1 | 2.27 |
IgG antibody detection in human serum samples
Baseline characteristics of participants showed 39% were naive, and not exposed to SARS-CoV-2; whereas, 61% were already immunized by exposure to SARS-CoV-2 virus. The immune status of the 146 participants at baseline is shown in Table 3. Among the seropositive at baseline, after 1st dose of vaccination, 97% remained seropositive and 3% turned seronegative. After 2nd dose, a different participant remained seronegative [Table 3].
Table 3.
Comparison of post vaccine immune status between seropositive and seronegative participants
| Immune Status | Base line | After 1st Dose | After 2nd Dose | ||
|---|---|---|---|---|---|
|
|
|
||||
| Positive | Negative | Positive | Negative | ||
| Seropositive | 61% | 97% | 3% | 97% | 3% |
| Seronegative | 39% | 82% | 18% | 91% | 9% |
The mean ISR value at baseline for seropositive samples was found to be 3.4 in comparison to 0.233 for seronegative samples. The mean ISR values after 1st and 2nd dose for the seropositive group were 4.03 and 4.12, respectively [Table 4]. Through multivariate analysis, the P value between the two groups in contrast with baseline ISR and after 1st dose is less than 0.05, hence it is statistically significant [Table 5]. The positive predictive value of the seropositive group after 1st and 2nd doses of Covishield vaccination was 63.4% and 61.1%, respectively.
Table 4.
Comparison of post vaccine ISR values between sero-positive and sero-negative participants
| ISR values | Baseline | After 1st dose | After 2nd dose | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Lowest | Highest | Mean | Lowest | Highest | Mean | Lowest | Highest | Mean | |
| Seropositive | 1.09 | 6.14 | 3.4 | 1.175 | 6.13 | 4.03 | 1.23 | 6.14 | 4.12 |
| Seronegative | 0.055 | 0.774 | 0.233 | 0.138 | 0.36 | 0.209 | 0.09 | 0.54 | 0.246 |
Table 5.
Association of sero-surveillance with ISR values
| Groups | N | Baseline | After 1st Dose | After 2nd Dose | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean | P | Mean | P | Mean | P | ||
| Seropositive | 33 | 3.4 | 0.000 | 4.03 | 0.010 | 4.12 | 0.465 |
| Seronegative | 24 | 0.233 | 0.209 | 0.246 | |||
Adverse events following immunisation
In the study, AEFI was defined as any untoward medical consequence following immunization that does not necessarily have a causal relationship with the usage of vaccines. For the purpose of reporting, AEFI was classified as minor and serious through active and passive surveillance.[6,7] A total of 81 recipients (55.4%) who received the 1st dose of the Covishield vaccine showed adverse events during the first 7 days of administration. A total of 34.9% and 9.58% had no adverse events and no response to follow-up, respectively. The adverse event after 28 days of vaccine administration was negligible.
Among the study participants, only a few (4.79%) showed adverse events during 7 days of 2nd dose administration. Among them, mild fever was the most common. The male-female ratio of the participants was 63:83. Among the 81 AEFI cases, some showed more than one adverse event. The most common AEFI was fever (56.79%). Local AEFI reported pain in the injection site (2.46%). No other local AEFI was reported. Figure 3 shows the distribution of AEFI among 81 recipients. Among different age groups, AEFI was 37.3% in the 18–35 years age group followed by 23.5% in 36–53 years age group, while most AEFI positive in the age group 36–53 years. The distribution of AEFI among these 57 participants tested for SARS-CoV-2 antibody in terms of age and gender is shown in Figures 4 and 5.
Figure 3.
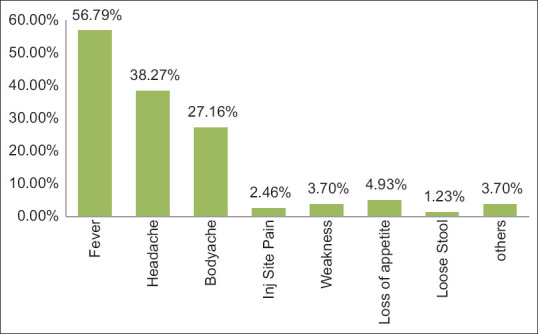
Distribution of adverse events among 81 AEFI cases
Figure 4.
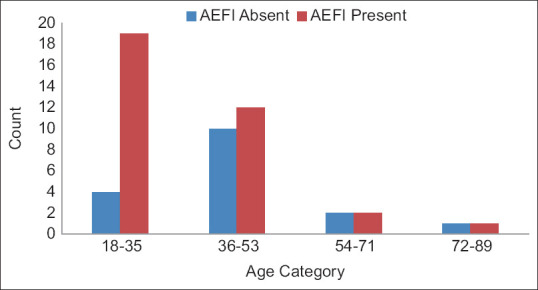
Distribution of age among 57 serosurveillance participants with respect to AEFI
Figure 5.
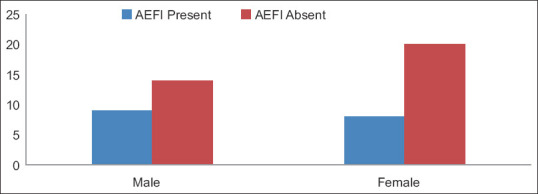
Distribution of gender among 57 serosurveillance participants with respect to AEFI
Discussion
The study was conducted in a period during July 2021 and enrolment continued for 6 months when Covishield vaccination used to be given in various vaccination centres. Sero-surveillance involves monitoring the presence of antibody, IgG in a population to assess the level of immunity against a particular pathogen, in this case, the SARS-CoV-2 virus. A comparison of anti-SARS-Cov-2 IgG antibody titres at different time-points post vaccination between baseline seropositive and seronegative groups was done. Among the 147 participants, 61% showed seropositivity against the SARS-CoV-2 virus in the baseline. The naïve seronegative group was 39% [Table 3]. Among the seronegative group at baseline after the 1st dose of vaccination, 82% became seropositive with high antibody titre (ISR 6.13) due to seroconversion. This finding is similar to the study conducted by Varghese et al. (2022). They found the 1st dose of vaccine-induced seroconversion in 91.7% of beneficiaries. Nearly one-third (30.2%) of them had high antibody titres, and it showed a significant association with the female gender (9.6 ± 5.5 vs. 7.6 ± 5.6) and younger age (P = 0.008). In addition, those with previous COVID-19 infection showed a more robust immune response when compared to others (P = 0.001).[10] Another immunogenicity study on the Covishield vaccine among 1638 healthcare workers in the NCR region revealed a seropositive rate of 48.2% at baseline which is lower than our study (61.4%).[11] The baseline mean IgG titre in the seropositive group was 3.4 ISR followed by an IgG titre of 4.03 ISR after 1st dose and 4.12 ISR after 2nd dose. Among the baseline seronegative group where seroconversion was due to Covishield vaccination, where seroconversion was 82% after 1st dose of vaccination and 91% after 2nd dose. The mean titre in the seronegative group was 2.74 ISR after 1st dose and 3.53 ISR after 2nd dose of vaccination [Table 4]. So, the evidence from our study can be inferred that natural infection by the SARS-CoV-2 virus generates a stronger immune response with a higher antibody titre and continues to maintain a higher antibody titre after vaccination. This finding is similar to another study which found high IgG titre among previously seropositive subjects with a single dose of vaccine.[12] This study also reflected the immune response after Covishield vaccination in naïve seronegative that was lower even after two doses of vaccination. Thus, the null hypothesis is rejected as there is a statistically significant association between the two groups. In the seropositive population, the 1st dose of vaccination acted as a booster dose leading to a rise in ISR levels. This finding had public health implications as a single dose of vaccine had a robust immune response.[13] In our study, after 1st dose of vaccination, all remained seropositive both by previous infection and by Covishield vaccination except one. Follow-up testing after 2nd dose of Covishield vaccination again 93% remained positive, and the mean IgG titre was slightly higher after 2nd dose. In our study, two participants remained seronegative during the entire study period and the reason behind this requires deeper investigation and understanding. Both were young healthy and with no co-morbidity.
We found in our study majority of adverse events reported after the Covishield administration were minor (non-serious). A total of 146 participants, 55.40% had reported AEFI and all were non-serious or minor adverse events. The male-female ratio of the participants was 63:83. AEFI was observed highest in the age group of 18–35 years followed by 36–53 years and lowest in 71–90 years age. So AEFI were inversely proportional to the age. We also correlated AEFI with co-morbidities. Among our study participants, 30.1% had co-morbidities like hypertension, hyperthyroidism, diabetes, etc., We observed that participants with co-morbidity least experienced any adverse events following immunization. The common adverse event in our study was fever (56.7%), followed by headache (38.2%) and bodyache (27.16%). A study done in the South-Eastern part of India by Kamal et. al. (2021), found 96.1% of participants with non-serious AEFI which is higher than our study (56.7%). They found a lower percentage of fever (12.5%) and headache (17.4%) with feeling unwell (20.6%) in south-eastern India during that period.[6] In another similar study done on AEFI following Covishield vaccination, 85% showed minor adverse events with 37% suffering from fever and 26% suffering from headache.[14] In our study, the majority of AEFI was reported during 7 days of 1st dose of Covishield vaccination. No AEFI was reported after 28 days of 1st dose of vaccination. After the 2nd dose, very few participants (6.8%) showed minor AEFI. There was no report of major (serious) adverse events during the entire study period. However, the incidence of AEFI varies based on age, co-morbidities and other factors. Primary care physicians can effectively manage the adverse events following Covishield vaccination as almost all were minor in nature with simple measures like prescribing paracetamol and counselling to alley fear and informing benefit of immunity generation by taking vaccination in the community.[15]
Limitation of the study: The main limitation of the study was the loss of follow-up of participants due to travelling back to their workplaces and receiving the 2nd dose in other locations. However, they continued to participate in AEFI.
Conclusion
Covishield vaccination induces an immune response and 90% seroconversion is achieved after the booster dose. Antibody titres of the seropositive group by natural infection of SARS-CoV-2 were higher than naïve seronegative cohort seroconverted by vaccination. The AEFI observed were minor and Covishield vaccines can be commented as safer.
List of abbreviations
| Abbreviation | Definition |
|---|---|
| AEFI | Adverse Events Following Immunization |
| IgG | Immunoglobulin G |
| ISR | Immunological Status Ratio |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome-Corona virus-2 |
Financial support and sponsorship
MRU-ICMR funding.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Worldometer COVID-19 Coronavirus Pandemic. 2021, 2022a. 2023. [[Last accessed on 2024 Apr 14]]. Available from: https://www.worldometers. info/coronavirus/
- 2.Kulkarni PS, Padmapriyadarsini C, Vekemans J, Bavdekar A, Gupta M, Kulkarni P, et al. A phase 2/3, participant-blind, observer-blind, randomised, controlled study to assess the safety and immunogenicity of SII-ChAdOx1 nCoV-19 (COVID-19 vaccine) in adults in India. EClinicalMedicine. 2021;42:10218. doi: 10.1016/j.eclinm.2021.101218. doi: 10.1016/j.eclinm.2021.101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drugs Controller General of India. Press Statement by the Drugs Controller General of India (DCGI) on Restricted Emergency approval of COVID-19 virus vaccine. 2021. (https://www.icmr.gov.in/pdf/press_realease_files/HFW_DCGI_energency_use_authorisation_03012021_2.pdf)
- 4.World Health Organization. COVID-19 vaccines: Knowledge gaps and research priorities WHO ad hoc consultation. 2021. (https://www.who.int/publications/m/item/covid-19-vaccines-knowledge-gaps-and-research-priorities---who-ad-hoc-consultation)
- 5.Marfoh K, Samba A, Okyere E, Acheampong F, Owusu E, Darko DN, et al. Adverse events following immunisation (AEFI) of COVISHIELD vaccination among healthcare workers in Ghana. BMJ Open. 2023;13:e061643. doi: 10.1136/bmjopen-2022-061643. doi: 10.1136/bmjopen-2022-061643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamal D, Thakur V, Nath N, Malhotra T, Gupta A, Batlish R. Adverse events following ChAdOx1 nCoV-19 Vaccine (COVISHIELD) amongst health care workers: A prospective observational study. Med J Armed Forces India. 2021;77:S283–8. doi: 10.1016/j.mjafi.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global Manual on Surveillance of Adverse Events Following Immunization. 2014. [[Last updated on 2016 Mar;Last accessed on 2021 Jan 10]]. Available from: https://www.who.int/vaccine_safety/publications/Global_Manual_on_Surveillance_of_AEFI.pdf .
- 8.Ministry of Health and Family Welfare. Adverse Events Following Immunization- Operational Guidelines. Covid 19 vaccine. 2021. (https://www.mohfw.gov.in/pdf/COVID19VaccineOG111Chapter16.pdf. )
- 9.World Health Organization. Causality Assessment of an Adverse Event Following Immunization (AEFI), User Manual for the Revised WHO Classification. 2nd ed. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 10.Varghese SM, Sachu A, Jacob L, George G, Chandy GM, Samuel Johnson AK, et al. COVID-19 vaccines portray the bright side of human creativity, but it means nothing until they prove their worth: A study on seroconversion after the first dose of Covishield vaccine in central Kerala. Indian J Community Med. 2022;47:213–7. doi: 10.4103/ijcm.ijcm_671_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kataria S, Sharma P, Deswal V, Kumar K, Singh M, Alam S, et al. A real world evaluation of the safety and immunogenicity of the Covishield vaccine, ChAdOx1 nCoV- 19 corona virus vaccine (recombinant) in health care workers (HCW) in National Capital Region (NCR) of India: A preliminary report. medRxiv. 2021 doi: 10.1101/2021.04.14.21255452. [Google Scholar]
- 12.Borkakoty B, Sarmah MD, Bhattacharjee CK, Bali N, Gogoi G. Antibody response after a single dose of ChAdOx1-nCOV (Covishield™®) vaccine in subjects with prior SARS-CoV2 infection: Is a single dose sufficient? medRxiv. 2021 doi: 10.1101/2021.06.15.21258346. [Google Scholar]
- 13.Bradley T, Grundberg E, Selvarangan R, LeMaster C, Fraley E, Banerjee D, et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. NEJM. 2021;384:1959–61. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha S, Devbhandari RP, Shrestha A, Aryal S, Rajbhandari P, Shakya B, et al. Adverse events following the first dose of ChAdOx1 nCoV-19 (COVISHIELD) vaccine in the first phase of vaccine roll out in Nepal. JPAHS. 2021;8:9–17. [Google Scholar]
- 15.Kumar R, Singh J, Singh N, Bhandari V. A study of COVID-19 vaccine (COVISHIELD) pharmacovigilance in primary healthcare workers in Punjab, India. AMEI's CTDT. 2021;5:6–11. [Google Scholar]


