ABSTRACT
Background:
Respiratory symptoms may persist for several weeks following the initial coronavirus disease 2019 (COVID-19) infection. The aims and objectives were to assess the clinical symptoms, pulmonary functions, and radiological changes and to assess the cardio-vascular complications in post-COVID-19 patients.
Methods:
This observational study was conducted in the Department of Pulmonary Medicine in collaboration with the Department of Cardiology, SCBMCH, Cuttack, from March 2021 to August 2022 on 75 post-COVID-19 patients with respiratory symptoms from 4 weeks to 2 years after treatment for COVID-19 infection. Post-COVID patients having previous respiratory diseases were excluded from the study.
Results:
Among 75 patients, the most common age group was 18–30 years with a male-to-female ratio of 2.5:1. Based on O2 requirement, patients were divided into the mild symptomatic group and moderate to severe pneumonia group. The most common respiratory symptom was dyspnea, followed by cough with expectoration. Bilateral crepitations were found in 17% of cases. C-reactive protein (CRP) and D-dimer were increased in 38.6% and 32% of patients, respectively. 42.6% had abnormal chest X-ray, and the most common abnormal finding was reticular thickening. In spirometry, the restrictive pattern and mixed pattern were the predominant types documented in 49.3% and 13.3% of cases, respectively, which were significant in the moderate–severe group. Diffusion capacity of the lungs for carbon monoxide (DLCO) was performed in only 19 patients (mild group 13 and moderate–severe group 6). Twelve (63.2%) patients had abnormal DLCO. P- values were significant for RV (0.0482) and RV/TLC (0.0394). High-resolution computed tomography (HRCT) of the thorax was abnormal in 55.7% with the most common abnormalities as inter- and intra-lobular septal thickening. The left ventricular ejection fraction was preserved in all patients, with right atrium and right ventricle enlargement in 2.6% and pulmonary hypertension in 4.0% of participants.
Conclusion:
All post-COVID-19 patients having respiratory symptoms after recovery from acute COVID-19 may be referred by family care physicians to a dedicated post-COVID center for further evaluation, management, and early rehabilitation to decrease the morbidity in recovered patients. Persistent increased blood parameters like TLC, N/L ratio, RBS, CRP, and D-dimer seen in recovered post-COVID-19 patients. The long-term impact of CT findings on respiratory symptoms, pulmonary functions, and quality of life is unknown. Cardiovascular abnormalities in post-COVID-19 patients are infrequent.
Keywords: COVID-19 infection, DLCO, HRCT thorax, left ventricular ejection fraction, post-COVID-19, spirometry
Introduction
The first official report of a novel respiratory virus emerged from Wuhan city, Hubei province of China; on December 31, 2019, it was subsequently shown to be a coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-Cov2).[1] On March 11, 2020, World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) a pandemic.[2] Viral pneumonia is the most frequent serious clinical manifestation of COVID-19 prominently featuring fever, cough, dyspnea, hypoxemia, and bilateral opacities on chest X-ray. Severe hypoxemic respiratory failure/ARDS develops in a significant proportion of patients with COVID-19 pneumonia, which may require mechanical ventilation and have a high risk for death.[3] The disease course is uneventful, leading to recovery from the initial bout of infection in a majority of cases.[4] However, a proportion of patients can develop a constellation of signs and symptoms in the convalescent phase of the disease process often as a sequela to the initial infection can be termed as post-COVID-19 syndrome.[5] The respiratory system bears the maximum brunt of the direct viral damage that may persist for several weeks following the initial infection. These long-term effects of COVID-19 can be due to damage inflicted by the virus itself, by widespread damage due to cytokine storm, by the immune response of the body, due to underlying co-morbidities, as a consequence of the therapy used to treat the disease, or as a combination of all of them. The pulmonary abnormalities encountered after recovery from the acute COVID-19 illness include diffuse lung disease (inflammatory and/or fibrotic), respiratory muscle weakness, sequelae of pulmonary thromboembolism, and pulmonary infections (i.e., Klebsiella, Pseudomonas, Acinetobacter, Aspergillosis, Candida, Mucormycosis).[6] It is essential to detect alterations in pulmonary structure and function for the diagnosis and follow-up of patients with respiratory sequelae produced by COVID-19. So we decided to carry out an observational study aimed to determine the prevalence of obstructive patterns, restrictive patterns, and altered diffusion in patients treated for COVID-19 infection.[7] Different types of functional and radiological evaluations were carried out, that is, chest X-ray PA view, 6-minute walk test, spirometry, diffusion capacity of the lungs for carbon monoxide (DLCO), high-resolution computed tomography (HRCT) of the thorax, and two-dimensional echocardiography (2D ECHO), to determine the consequences of COVID-19 sequelae. Computed tomography pulmonary angiography (CT-PA) was carried out in participants having D-dimer ≥1500 μg/ml[8] and/or 2D ECHO having regional wall motion abnormality[9] to evaluate the pulmonary vasculature and associated abnormalities. The primary aims and objectives were to assess the clinical symptoms, pulmonary functions, and radiological changes and assess the risk factors associated with deranged pulmonary functions and to assess the cardio-vascular complications in post-COVID-19 patients. The secondary objective is attributable morbidity associated with symptomatic treated cases of COVID-19 infection.
Material and Methods
This observational study was conducted in the Department of Pulmonary Medicine in collaboration with the Department of Cardiology, SCBMCH, Cuttack, from March 2021 to August 2022. This observational study enrolled 75 adult post-COVID-19 patients who developed new respiratory symptoms after 4 weeks of COVID-19 infection irrespective of recovery from acute COVID-19. Post-COVID patients having previous respiratory diseases like post-tubercular obstructive airway disease (post-TB OAD), bronchial asthma, chronic obstructive pulmonary disease (COPD), and interstitial lung disease and refusal of consent were excluded from the study. The study was approved by the institutional ethical committee of SCBMC Cuttack, Odisha. After a detailed clinical history and physical examination, the patients were subjected to routine blood investigations, which include complete blood count (CBC), random blood sugar (RBS), liver function test (LFT), serum sodium and potassium, and inflammatory markers like C-reactive protein-quantitative (CRP-Q) and D-dimer. Then chest X-ray PA view followed by 6-minute walk test, spirometry, and DLCO were done. After that, patients were subjected to HRCT thorax and 2D ECHO. CT-PA was carried out in patients having D-dimer ≥1500 μg/ml and/or 2D ECHO having regional wall motion abnormality [Figure 1].
Figure 1.
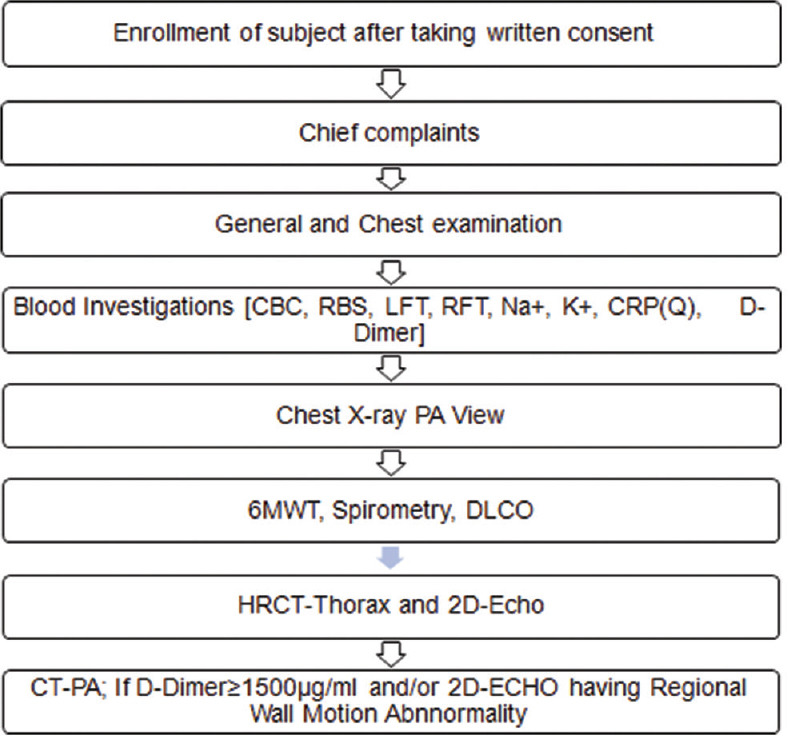
Flow chart of the study methodology
After compiling all the data, statistical analysis was performed using SPSS software. Descriptive frequencies were expressed using mean and standard deviation (SD). Differences between means of continuous variables are compared using the unpaired Student t-test, and qualitative variables are compared by using Chi-square test. The level of significance was expressed as probability values (P- values). P < 0.05 was considered statistically significant.
Results
According to the requirement of O2 at the time of COVID-19 infection, patients were divided into the mild symptomatic group (49, 65.33%) and moderate to severe pneumonia group (26, 34.66%). In the latter group, O2 therapy by mask/non-rebreather mask and non-invasive ventilation were used in 21 (27.6%) and 5 (6.6%), respectively.
Table 1 shows 53 (70.6%) cases were male and 22 (29.4%) cases were female with a male-to-female ratio of 2.5:1. The most common age group was 18–30 years (25.3%). Hypertension (10.6%) was the most common co-morbidity, followed by diabetes mellitus (8%). The most common addiction was alcoholism (21.3%), followed by smoking (14.6%) and chewing tobacco (13.3%). The most common post-COVID symptom was dyspnea in 56 (74.6%), followed by cough with expectoration in 28 (37.3%), dry cough in 20 (26.6%), and chest pain in 18 (24%). Dyspnea was predominant in the moderate–severe group (96.1%, 25) which was statistically significant (P = 0.001) when compared to the mild group. The most common auscultatory finding was normal vesicular breath sounds heard in 72% (54), followed by bilateral crepitation in 17.3% (13) and bilateral rhonchi in 5.3% (4). P- value was found significant for bilateral crepitations (0.0001) and normal breath sound (0.0003).
Table 1.
Baseline characteristics of post-COVID symptomatic patients
| Baseline Characteristics | Total n=75 | Mild Group n=49 (%) | Moderate-Severe Group n=26 (%) | P |
|---|---|---|---|---|
| Age (years), mean±SD | 42.9±15.1 | 39.77±14.93 | 48.9±13.7 | 0.01 |
| Males (%) | 53 (70.6) | 32 (59.25) | 21 (40.75) | 0.21 |
| Females (%) | 22 (29.4) | 17 (77.27) | 5 (22.72) | 0.003 |
| Comorbidities | ||||
| DM (%) | 6 (8) | 3 | 3 | 0.41 |
| Hypertension (%) | 8 (10.66) | 5 | 3 | 0.85 |
| Hypothyroidism (%) | 1 (1.33) | 0 | 1 | 0.17 |
| CLL (%) | 1 (1.33) | 0 | 1 | 0.17 |
| CKD (%) | 1 (1.33) | 0 | 1 | 0 |
| OA (%) | 2 (1.33) | 1 | 1 | 0.65 |
| Smoking | 11 (14.66) | 7 (9.33) | 4 (5.33) | 0.57 |
| Alcohol | 16 (21.33) | 8 (10.66) | 8 (10.66) | 0.14 |
| Tobacco | 10 (13.33) | 6 (8) | 4 (5.33) | 0.70 |
| Life style | ||||
| Light | 11 (14.66) | 6 (8) | 5 (6.66) | 0.41 |
| Moderate | 42 (72.66) | 42 (56) | 20 (26.66) | 0.49 |
| Rigorous | 2 (2.66) | 2 (1.33) | 1 (1.33) | 0.64 |
| Symptoms | ||||
| Chest pain (%) | 18 (24) | 14 (28.57) | 4 (15.38) | 0.203 |
| Chest tightness (%) | 3 (4) | 3 (6.12) | 0 (0) | 0.503 |
| Dry cough (%) | 20 (26.66) | 13 (26.53) | 7 (26.92) | 0.974 |
| Cough with exp. (%) | 28 (37.33) | 18 (36.73) | 10 (38.46) | 0.882 |
| Dyspnea (%) | 56 (74.66) | 31 (63.26) | 25 (96.15) | 0.001 |
| Hemoptysis (%) | 3 (4) | 3 (6.12) | 0 (0) | 0.503 |
| Auscultatory findings | ||||
| Normal (%) | 55 (73.33) | 42 (85.71) | 12 (46.15) | 0.0003 |
| ↓breath sounds (%) | 0 | 0 | 0 | |
| Left side absent breath sounds (%) | 2 (2.66) | 1 (2.04) | 1 (3.84) | 0.6472 |
| B/l rhonchi (%) | 4 (5.33) | 4 (8.16) | 0 | 0.1370 |
| B/l crepitation (%) | 13 (17.33) | 1 (2.04) | 12 (46.15) | 0.0001 |
| B/l crepitation + rhonchi (%) | 2 (2.66) | 1 (2.04) | 1 (3.84) | 0.6472 |
Table 2 summarizes baseline laboratory parameters of post-COVID symptomatic patients. P- value was found highly significant in TLC (0.012), neutrophil/lymphocyte ratio (N/L ratio) (0.0021), RBS (0.0006), and CRP (Q) (0.0060) and significant in D-dimer (0.032). 9.3% of patients had Hb below the normal range. 22.6%, 17.3%, and 33.3% had TLC, neutrophil, and N/L ratio above the normal range. The lymphocyte and total platelet count (TPC) are below the normal range in 16% and 10.6% of patients, respectively. RBS, serum bilirubin total (SB-T), SB-direct (SB-D), aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphatise (ALP) were above the normal range in 18.6%, 10.6%, 1.3%, 33.3%, 25.3%, and 5.3% of patients, respectively. 13.3% and 8% patients had decreased sodium and potassium below the normal range, respectively. Serum urea is increased in 10.6%, and creatinine decreased in 6.6% of patients. CRP (Q) and D-dimer were increased in 38.6% and 32% of patients, respectively.
Table 2.
Baseline laboratory parameters of post-COVID symptomatic patients
| Lab Parameter | Total n=75 | Mild Group n=49 (%) | Moderate-Severe Group n=26 (%) | P |
|---|---|---|---|---|
| HB (gm/dl) | 13.21±1.8 | 13.28±1.83 | 13.06±1.74 | 0.617 |
| TLC (10^3/uL) | 10.04±4.96 | 9.00±2.77 | 11.99±7.21 | 0.012 |
| Neutrophil (10^3/uL) | 65.36±12.83 | 64.04±8.47 | 68.33±18.31 | 0.103 |
| Lymphocyte (10^3/uL) | 36.6±10.72 | 28.62±7.52 | 25.91±14.93 | 0.379 |
| N/L ratio | 3.1±2.5 | 2.49±1.04 | 4.30±3.72 | 0.001 |
| TPC (10^3/uL) | 252.45±108.94 | 254.18±81.31 | 249.26±149.73 | 0.854 |
| RBS (mg/dl) | 130.23±63.26 | 112.53±35.65 | 163.58±87.39 | 0.001 |
| SB-D (mg/dl) | 0.25±0.15 | 0.24±0.13 | 0.27±0.16 | 0.500 |
| SB (T) SB-T (mg/dl) | 0.67±0.27 | 0.68±0.30 | 0.63±0.21 | 0.461 |
| AST (IU/L) | 38.91±19.91 | 35.89±15.49 | 44.59±25.72 | 0.072 |
| ALT (IU/L) | 37.80±21.03 | 34.81±14.29 | 43.44±29.43 | 0.091 |
| ALP (IU/L) | 226.37±78.76 | 226.70±76.69 | 225.73±84.07 | 0.959 |
| Na+ (mEq/L) | 139.25±3.91 | 139.49±3.49 | 138.81±4.63 | 0.481 |
| K+ (mEq/L) | 4.66±4.51 | 4.19±0.50 | 5.54±7.65 | 0.221 |
| Urea (mg/dl) | 27.12±9.99 | 26.43±9.22 | 28.42±11.38 | 0.414 |
| Creatinine (mg/dl) | 0.74±0.21 | 0.72±0.20 | 0.76±0.24 | 0.492 |
| CRP (Q) (mg/L) | 10.82±22.95 | 5.60±7.4 | 20.65±36.02 | 0.006 |
| D-Dimer (µg/ml) | 458.03±385.05 | 388.92±377.73 | 588.28±371.37 | 0.032 |
Table 3 shows restrictive disorder was found in 49.3% (37), followed by mixed obstructive and restrictive disorder in 13.3% (10). Spirometry was normal in 37.3% (28). P- value was significant for normal lung function (0.0190) and restrictive disorder (0.0442). Among 37 restrictive disorders, P- value was highly significant (P = 0.0095) for moderately severe restrictive patterns. The mean ± SD of pre-broncho-dilator values of FVC%, FEV1%, FEV1/FVC, FEF25-75, and PEFR% of 75 post-COVID patients were calculated. Also, the mean ± SD for all these parameters were calculated for the mild and moderate–severe groups and compared to calculate P- values. P- values were found highly significant for FVC% (0.007) and significant for FEV1% (0.049). 6MWT could not be performed by 8 out of 75 patients. The mean ± SD of 6-minute walk distance (MWD), 6MWD%, baseline SpO2, end exercise SpO2, baseline pulse rate, and end exercise pulse rate of 67 patients were recorded. P- value was found highly significant in 6MWD (0.001), baseline SpO2 (0.003), and end exercise SpO2 (0.001).
Table 3.
Spirometry (n=75) and 6MWT (n=67) in post-COVID patients
| Interpreted result in spirometry | Total n=75 n (%) | Mild group n=49; n (%) | Mod-severe group n=26; n (%) | P |
|---|---|---|---|---|
| Normal | 28 (37.33) | 23 (46.93) | 5 (19.23) | 0.0190 |
| Mixed obstructive and restrictive disorder | 10 (13.33) | 6 (12.24) | 4 (15.38) | 0.7053 |
| Restrictive disorder | 37 (49.33) | 20 (40.81) | 17 (65.38) | 0.0442 |
| Type of restriction | ||||
| Mild restriction | 13 (17.33) | 9 (18.36) | 4 (15.38) | 0.7472 |
| Moderate restriction | 12 (16) | 7 (14.28) | 5 (19.23) | 0.5804 |
| Moderately severe restriction | 6 (8) | 1 (2.04) | 5 (19.23) | 0.0095 |
| Severe restriction | 5 (6.66) | 2 (4.08) | 3 (11.53) | 0.2213 |
| Very severe restriction | 1 (1.33) | 1 (2.04) | 0 (0) | 0.4664 |
| Others | 38 (50.66) | 29 (59.18) | 9 (34.61) | 0.0442 |
|
| ||||
| Mean±SD | Mean±SD | Mean±SD | P | |
|
| ||||
| Spirometry parameters | ||||
| FVC% | 67.79±14.62 | 74.12±17.22 | 59.71±15.72 | 0.007 |
| FEV1% | 78.67±17.10 | 80.79±19.47 | 71.89±12.71 | 0.049 |
| FEV1/FVC | 91.94±7.92 | 90.09±8.17 | 93.01±6.48 | 0.586 |
| FEF25-75% | 85.34±15.78 | 83.10±19.13 | 90.71±16.23 | 0.279 |
| PEFR% | 64.48±13.45 | 67.71±18.78 | 62.26±16.32 | 0.547 |
|
| ||||
| n=67 | n=46 | n=21 | P | |
|
| ||||
| 6 Minute walk test (n=67) | ||||
| 6MWD (IN METRES) | 449.05±191.13 | 492.89±172.02 | 353.04±172.92 | 0.001 |
| 6MWD % | 82.75±86.45 | 92.24±102.94 | 61.96±29.44 | 0.190 |
| Base line spo2% | 97.71±30.38 | 97.95±23.74 | 97.19±39.07 | 0.003 |
| End exercise spo2% | 97.58±30.34 | 97.93±23.74 | 96.80±38.93 | 0.001 |
| Base line pulse rate | 91.58±31.41 | 91.41±25.52 | 91.95±39.74 | 0.886 |
| End exercise pulse rate | 102.88±35.60 | 101.58±29.03 | 105.71±45.46 | 0.349 |
Table 4 shows DLCO was performed in only 19 patients (mild group 13 and moderate–severe group 6). Twenty-one patients could not perform the test, 20 patients did not give their consent, and the remaining 15 patients were absent on their follow-up dates. Seven (36.8%) patients had normal DLCO, whereas 6 (31.5%), 5 (26.3%), and 1 (5.26%) had mild, moderate, and severely reduced DLCO, respectively. Mean ± SD were calculated for DLCO, DLCO/VA, TLC, RV, and RV/TLC for total cases, the mild group, and the moderate–severe group. P- values were significant for RV (0.0482) and RV/TLC (0.0394).
Table 4.
DLCO findings in post-COVID patients
| Interpreted result | Total (n=19) | Mild group (n=13) | Moderate-severe group (n=6) | P |
|---|---|---|---|---|
| Normal (%) | 7 (36.84) | 6 (46.15) | 1 (16.66) | 0.478 |
| Mild reduction (%) | 6 (31.57) | 4 (30.76) | 2 (33.33) | 0.912 |
| Moderate reduction (%) | 5 (26.31) | 3 (23.07) | 2 (33.33) | 0.636 |
| Severe reduction (%) | 1 (5.26) | 0 (0) | 1 (16.66) | 0.1411 |
| DLCO, mean±SD | 69.57±17.41 | 72.76±16.91 | 62.66±17.92 | 0.2508 |
| DLCO/VA, mean±SD | 95.68±28.72 | 95.23±31.04 | 96.66±25.60 | 0.9230 |
| TLC, mean±SD | 75.73±20.17 | 80±21.54 | 66.5±14.18 | 0.1821 |
| RV, mean±SD | 79.94±36.44 | 91±39.28 | 56±8.46 | 0.0482 |
| RV/TLC, mean±SD | 103.21±24.51 | 110.92±24.27 | 86.5±16.08 | 0.0394 |
Table 5 summarizes radiological findings of post-COVID patients. Chest X-ray was normal in 38 (77.5%) and 5 (19.2%) from the mild group (N = 49) and moderate–severe group (N = 26), respectively. The most common finding was reticulation, 41.33% (31), followed by ground glass opacity (GGO), 22.66% (17). P value was highly significant for normal chest X-ray (0.0001), GGO (P = 0.0001), and reticulation (P = 0.0001). HRCT thorax was normal in 30 (61.2%) of the mild group (N = 49) and 4 (15.38%) in the moderate–severe group (N = 26). The most common HRCT finding was inter- and intra-lobular septal thickening in 22 (29.33%), followed by fibrotic bands 19 (25.33%) and GGO in 18 (24%). P- values were highly significant for GGO (0.0001), inter- and intra-lobular septal thickening (0.0001), and fibrotic bands (0.0004) and significant in traction bronchiectasis (0.0262).
Table 5.
Radiological findings in post-COVID patients
| Type of lesion in chest x-ray PA view | Total n=75 (%) | Mild group n=49 (%) | Moderate-severe group n=26 (%) | P |
|---|---|---|---|---|
| GGO | 17 (22.66) | 4 (8.16) | 13 (50) | 0.001 |
| Consolidation | 2 (2.66) | 1 (2.04) | 1 (3.84) | 0.644 |
| Reticulation | 31 (41.33) | 11 (22.44) | 20 (76.92) | 0.001 |
| Collapse | 2 (2.66) | 1 (2.04) | 1 (3.84) | 0.644 |
| Effusion | 3 (4) | 1 (2.04) | 2 (7.69) | 0.234 |
| NormalCXR | 43 (57.33) | 38 (77.55) | 5 (19.23) | 0.0001 |
| Type of lesion in HRCT thorax | ||||
| GGO | 18 (24) | 5 (10.20) | 13 (50) | 0.001 |
| Consolidation | 2 (2.66) | 1 (2.04) | 1 (3.84) | 0.644 |
| Lymphadenopathy | 8 (10.66) | 3 (6.12) | 5 (19.23) | 0.08 |
| Inter- and intra-lobular septal thickening | 22 (29.33) | 7 (14.2) | 15 (57.69) | 0.001 |
| Fibrotic bands | 19 (25.33) | 6 (12.24) | 13 (50) | 0.001 |
| Traction bronchiectasis | 13 (17.33) | 5 (10.20) | 8 (30.76) | 0.025 |
| Honey-combing | 7 (9.33) | 3 (6.12) | 4 (15.38) | 0.189 |
| Atelectasis | 7 (9.33) | 4 (8.16) | 3 (11.53) | 0.189 |
| Effusion | 5 (6.66) | 3 (6.12) | 2 (7.69) | 0.795 |
| Pleural thickening | 1 (1.33) | 1 (2.04) | 0 | - |
| Emphysema | 5 (6.66) | 4 (8.16) | 1 (3.84) | 0.462 |
| Pneumothorax | 2 (2.66) | 1 (2.04) | 1 (3.84) | 0.644 |
| Normal HRCT | 34 (45.33) | 30 (61.22) | 4 (15.38) | 0.0002 |
Table 6 summarizes 2D ECHO findings in post-COVID patients. Out of 75 patients, left ventricular hypertrophy, left ventricular diastolic dysfunction, right ventricular systolic dysfunction, and right ventricular diastolic dysfunction were seen in 6 (8.0%), 12 (16.0%), 3 (4.0%), and 3 (4.0%), respectively. 18.6% had LVID-s (left ventricular internal diameter systole) below the normal range. IVSed (inter-ventricular septum end diastole), LVPWed (left ventricle posterior wall end diastole), right atrium size, right ventricle size, and TRPG (tricuspid regurgitation pressure gradient) were above the normal range in 9.3%, 9.3%, 2.6%, 2.6%, and 4.0% of patients, respectively.
Table 6.
2D ECHO findings in post-COVID patients
| Parameters | Total mean±SD | Mild group mean±SD | Moderate-severe group mean±SD | P |
|---|---|---|---|---|
| LVID-d | 40.76±4.79 | 40.73±4.88 | 40.80±4.72 | 0.950 |
| LVID-s | 26.70±4.34 | 26.73±4.37 | 26.65±4.37 | 0.940 |
| LVEF | 64.65±5.14 | 65.12±5.29 | 63.78±4.83 | 0.287 |
| LVFS | 34.54±4.23 | 34.93±4.34 | 33.79±3.99 | 0.268 |
| IVSed | 10.07±1.31 | 10.10±1.32 | 10.01±1.33 | 0.793 |
| LVPWed | 10.08±1.24 | 10.1±1.24 | 10.05±1.28 | 0.890 |
| Lt. atrium size | 30.88±3.77 | 30.65±3.99 | 31.30±3.33 | 0.478 |
| Rt. atrium size | 26.73±5.02 | 27.00±4.99 | 26.23±5.14 | 0.532 |
| Rt. ventricle size | 20.16±2.20 | 20.28±2.47 | 19.92±1.59 | 0.502 |
| TAPSE | 1.88±0.14 | 1.89±0.15 | 1.87±0.13 | 0.562 |
| TRPG/PH | 14.65±8.91 | 13.59±8.77 | 16.66±9.00 | 0.157 |
CT-PA was performed in one patient from the moderate–severe group, having D-dimer level >1500 μg/ml. The findings were main pulmonary trunk, 2.7 cm; right pulmonary trunk, 1.7 cm; and left pulmonary trunk, 1.7 cm, and there was no evidence of pulmonary embolism.
Discussion
In this single-center observational study, we evaluated 75 post-COVID-19 patients from 1 month to 2 years with a mean ± SD of 9.5 ± 7.1 months after treatment of COVID-19 infection. The most common age group was 18–30 years (25.3%) with a male-to-female ratio of 2.5:1, which is close to a study by Dararat Eksombatchai et al.[10] Hypertension (8 cases, 10.6%) was the most common co-morbidity, followed by diabetes mellitus (6 cases, 8%). The mean BMI was 25.5 ± 4.8, which is similar to Dararat Eksombatchai et al.[10] and Thyagaraj V et al.[11] Patients were classified into the mild group (49,65%) and moderate to severe group (26, 65%), which is similar to study designs by A.W. Wong et al.,[12] Fabio Anastasio et al.,[13] and Daniel Cruz Bretas et al.[14]
The most common persistent respiratory symptoms were dyspnea (74.6%), followed by cough with expectoration (37.3%), dry cough (20.6%), and chest pain (24.0%), which are supported by National Comprehensive Guidelines for management of post-COVID sequelae, MOHFW, Govt. of India.[6] The three most frequent symptoms were dyspnea, fatigue, and anosmia, listed as long-term symptoms by the National Institute of Health and Care Excellence and CDC.[15] Dyspnea in the mild group is unrelated to persistent damage in the lungs, which may be due to chronic fatigue syndrome, obesity, or previous co-morbid conditions, which is supported by Thyagaraj V et al.[11] and Klaus J Wirth et al.[16] In the moderate to severe group, dyspnea was significant (P- value = 0.002) compared with the mild symptomatic group, coinciding with the results by Desai SV et al.[17] On auscultation of chest, we found crepitations were significantly more in the moderate to severe group (46.15% vs 2.04% in the mild group, P- value < 0.0001), which was supported by Faverio P et al.[18]
In the current study, CBC showed decreased hemoglobin from the normal range in 9.3% of patients, which coincides with the study by Thomas Sonnweber et al.,[19] who found 9% had anemia categorized as anemia of inflammation. A meta-analysis of nine studies by Sulmaz Ghahramani et al.[20] showed a significant decrease in hemoglobin in severe groups compared to the non-severe group. Increased NLR was found in 33.3% of patients, which coincides with the study by Meryam Maamar et al.[21] There was a significant increase in NLR (P = 0.0021) in the moderate to severe group as compared to the mild group, which was supported by the study of George et al.,[22] who followed severe COVID-19 patients 3 to 6 months after recovery and found a neutrophil-associated inflammatory phenotype apparent in patients with persistent pulmonary symptoms. We found a decrease in lymphocyte count in 16.0% of patients, which is consistent with Mandal et al., Mannan et al., and Julian Varghese et al.[23,24,25] Thrombocytopenia was found in 10.6% of our patients, which is supported by the study done by Marco Lucijanic et al. and Chen et al.[26,27]
There were increased RBS levels in 18.6% patients with a significant increase (P = 0.006) in the moderate to severe group compared to the mild symptomatic group, which coincides with a meta-analysis of four cohort studies by Ali Abdelhamid et al.[28] Also, a study by Ayoubkhani D et al. and Huang C et al. reported 4.9% and 3.3% cases of new-onset diabetes, respectively.[29,30] There was an increase in SB-T, AST, ALT, and ALP in 1.3%, 33.3%, 25.3%, and 5.3% of patients, respectively, which is supported by the study of Xuejiao Liao et al. and Liu et al.[31,32] Also, a nearly significant increase was found in AST and ALT among the moderate to severe group compared to the mild symptomatic group. Our study found a decrease in serum sodium and potassium in 13.3% and 8% of patients, respectively, as reported by Giuseppe Lippi et al.[33] Hyponatremia was also seen in a meta-analysis by Sulmaz Ghahramani et al.[20] in the severe group compared with the non-severe group. Serum urea was increased in 10.6% with a decrease in serum creatinine level in 6.6% of patients, which was consistent with Xu-Wei et al.,[34] and Sulmaz Ghahramani et al.[20] did a meta-analysis and found an increase in blood urea and creatinine in the severe group compared to the non-severe group. There was an increase in CRP (Q) and D-dimer in 28.4% and 32% of patients, respectively, which are supported by Muhammad Ali Gameil et al.[35] and Swapna Mandal et al.[23] A multi-centric study in the UK by Mandal S et al.[23] showed 30.1% of patients showed an elevated D-dimer value after 60 days of discharge from the hospital. The present study demonstrated that 42.6% had abnormal chest X-ray findings and the most common finding was reticular thickening (41.3%), followed by ground glass opacities (22.6%), which was partly supported by Arzoo Gupta et al.[36] Consolidation was seen only in 2.6% due to a longer meantime of follow-up (9.5 ± 7.1 months) after acute COVID-19 infection, which might have progressed from consolidation to reticulation and is supported by Han X et al.[37] Comparison between the mild and moderate to severe pneumonia groups revealed normal CXRs (P = 0.0001) in the mild group and a significant number of reticular thickening (P < 0.0001) and GGO (P = 0.0001) in the moderate to severe pneumonia group, which was supported by M Fogante et al.[38]
Previous studies reported that SARS has long-term effects on lung functions and related physiological characteristics in part of survivors, even after 1 year of discharge.[35] The mean 6MWD in the moderate to severe pneumonia group (353.04 ± 172.92 meters) was shorter than that of the mild symptomatic group (492.8 ± 172), but this was not statistically significant. These results were agreed by a study conducted by AW Wong et al.[12] and Gupta A et al.[36] Also, the baseline and end-exercise SPO2 and pulse rate were within normal limits with mean 97.7 ± 30.3, 97.5 ± 30.3, 91.5 ± 31.4, and 110.8 ± 35.6, respectively, and no significant changes were found between the two groups, which is supported by the study of Dararat Eksombatchai et al.[10]
The mean ± SD of spirometry parameters like FVC (67.79 ± 14.62), FEV1 (78.67 ± 17.10), and PEFR (64.48 ± 13.45) were below the normal limit, and FEV1 and FVC were significantly reduced in the moderate to severe group than in the mild symptomatic group (P values 0.021 and 0.049, respectively), which coincides with the study by Guler SA et al.[39] Interpreting the spirometry result, we revealed that the restriction pattern and mixed pattern were the predominant types documented in 49.3% and 13.3% of cases, respectively, with a restrictive pattern more significant in the moderate to severe group (P = 0.0442), which was supported by You J et al.[40] Fabio Anastasio et al.[13] studied on 379 patients after 4 months of SARS-CoV-2 and concluded a significant decrease in RV, TLC, and FVC in the severe group as compared to the non-severe group. Also, Mo X. et al., Frija-Masson J. et al., and Li X. et al. reported the spirometry test in post-COVID cases and found a prevalent restrictive pattern in 59% and obstructive pattern in 16% of post-COVID pneumonia cases.[41,42,43]
In this study, we found abnormal DLCO in 36.8%, decreased RV in 42.85%, and decreased TLC in 57.14% out of 19 patients. Zhang et al.[44] reported a 32% reduction in DLCO after severe COVID-19 after 8 months. Also, Bretas DC et al. reported altered DLCO in 33% of patients at 12 months in non-ICU patients.[14] But we found abnormal DLCO in 36.8%, which could be explained mostly by a lower number of patients undergoing DLCO with a mean period followed up of 1.2 months. This early follow-up shows a high prevalence of altered diffusion capacity as evidenced by studies of Mo X et al., Frija-Masson J et al., and Huang Y et al., who found a high prevalence of altered diffusion capacity between 44% to 56% after 1 month of post-infection.[41,42,45]
Also, the values of RV and RV/TLC were significantly reduced in the moderate to severe group compared with the mild symptomatic group with P- values of 0.0482 and 0.0394, respectively, supported by Fabio Anastasio et al.[13] We also found that impaired diffusion is significant (P = 0.0251) in the moderate to severe group as compared with the mild symptomatic group. A study by Huang Y et al.[45] showed a reduction of DLCO, TLC, and 6 MWD in severe COVID-19 compared to non-severe COVID-19.
HRCT of the thorax revealed abnormal patterns in 55.7%, with the most common abnormalities as inter- and intra-lobular septal thickening in 29.3%, fibrotic bands in 25.3%, GGO in 24%, and traction bronchiectasis in 17.3%, which was consistent with Liu C et al.[46] Similarly, in a study by Tabatabaei et al.,[47] 42% showed residual abnormalities, most commonly GGO and subpleural parenchymal bands. GGO, inter-lobular and intra-lobular septal thickening, and fibrotic bands were found to increase significantly in the moderate to severe group as compared to the mild symptomatic group with P- values of 0.0001, 0.0001, and 0.0004, respectively. A study of 3-month scans in 48 survivors of severe COVID-19 who were mechanically ventilated found normal imaging at 4%, GGO at 89%, and signs of fibrosis at 67%.[48] Gulati A et al.[49] reported on 6-month scans in 12 patients that fibrotic abnormalities occur in areas of the original changes during the acute phase of infection. All these studies agreed with our results.
Our study found right atrium and right ventricle sizes were above the normal ranges in 2.6% and pulmonary hypertension in 4.0% of participants. Sonnweber et al.[50] described a 3% prevalence of LV dysfunction and 10% pulmonary hypertension in a cohort studied 60 days after COVID-19 diagnosis. As per our study, the left ventricular ejection fraction is preserved in all patients, which is supported by a study by Baruch et al.[51]
In this study, we had taken the value of D-dimer >1500 μg/ml, the criterion for CT- pulmonary angiography (CT-PA) as per Alexander A Tuck et al.[8] Remy-Jardin et al.[52] followed up 55 patients remaining symptomatic 3 months after hospitalization for SARS- CoV-2 infection. CT angiography revealed the presence of endoluminal filing defects in three patients (5.4%) of the study population. We did CT-PA in one patient from the moderate–severe group, having D-dimer level >1500 μg/ml, and there was no evidence of pulmonary embolism.
Conclusion
This study shows that a noticeable amount of COVID-19-recovered patients continue to report respiratory symptoms, the most common being dyspnea. Any post-COVID-19 patient having respiratory symptoms after recovery from acute COVID-19 may be referred by family care physicians to a dedicated post-COVID center for further evaluation, management, and rehabilitation to decrease the morbidity in recovered patients.
Persistent increased blood parameters like TLC, N/L ratio, RBS, CRP, and D-dimer seen in recovered patients suggest persistence of a low-grade inflammation (LGI) as a post-COVID-19 sequelae. Decreased 6MWD and pulmonary function abnormalities were found in patients with severe lung involvement during SARS-CoV-2. This study has shown that chest X-ray and HRCT-thorax abnormalities like GGO, inter–intra-lobular septal thickening, and fibrotic bands may persist well up to several months after discharge in severely ill COVID-19 patients depending on the severity of initial lung involvement. Cardiovascular abnormalities in post-COVID-19 patients are infrequent and usually mild. The long-term impact of CT findings on respiratory symptoms, pulmonary functions, and quality of life is unknown. A long-term multi-disciplinary rehabilitation program including physical and psychological aspects of rehabilitation is essential to decrease morbidity in recovered patients.
Limitations
A single center and a small sample size.
Limited proportion of participants performing DLCO.
Chest X-ray or HRCT chest of the subjects during the time of acute COVID-19 infection was not available to compare the changes in lung parenchyma with progression of time.
There was no histopathologic confirmation, and inferences were based on CT signs. Hence, a more extended follow-up is needed to better understand the possible long-term evolutions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. Available from:http://www.covid19.who.int .
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray&Nadels Textbook of Respiratory Medicine. (7th ed) Chapter 46a. 620. [Google Scholar]
- 4.Kunal S, Madan M, Tarke C, Gautam DK, Kinkar JS, Gupta K, et al. Emerging spectrum of post-COVID-19 syndrome. Postgrad Med J. 2022;98:633–43. doi: 10.1136/postgradmedj-2020-139585. [DOI] [PubMed] [Google Scholar]
- 5.Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: Post-viral syndrome post COVID-19. Med Hypotheses. 2020;144:110055. doi: 10.1016/j.mehy.2020.110055. doi:10.1016/j.mehy. 2020.110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive guidelines for management of post Covid sequelae, mohfw, Govt of India. :29. [Google Scholar]
- 7.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology. 2021;27:328–37. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuck AA, White HL, Abdalla BA, Cartwright GJ, Figg KR, Murphy EN, et al. To scan or not to scan-D-dimers and computed tomography pulmonary angiography in the era of COVID-19. Clin Med (Lond) 2021;21:e155–60. doi: 10.7861/clinmed.2020-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghidini S, Gasperetti A, Winterton D, Vicenzi M, Busana M, Pedrazzini G, et al. Echocardiographic assessment of the right ventricle in COVID-19: A systematic review. Int J Cardiovasc Imaging. 2021;37:3499–512. doi: 10.1007/s10554-021-02353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eksombatchai D, Wongsinin T, Phongnarudech T, Thammavaranucupt K, Amornputtisathaporn N, Sungkanuparph S. Pulmonary function and six-minute-walk test in patients after recovery from COVID-19: A prospective cohort study. PLoS One. 2021;16:e0257040. doi: 10.1371/journal.pone.0257040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thyagaraj V, Rao A, Kulkarni A, Shankar T, R N, Unnikrishnan H, et al. Clinical and laboratory profile of patients visiting the post-COVID-19 clinic at a tertiary care hospital: A cross-sectional study. Cureus. 2022;14:e22888. doi: 10.7759/cureus.22888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong AW, López-Romero S, Figueroa-Hurtado E, Vazquez-Lopez S, Milne KM, Ryerson CJ, et al. Predictors of reduced 6-minute walk distance after COVID-19: A cohort study in Mexico. Pulmonology. 2021;27:563–5. doi: 10.1016/j.pulmoe.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anastasio F, Barbuto S, Scarnecchia E, Cosma P, Fugagnoli A, Rossi G, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021;58:2004015. doi: 10.1183/13993003.04015-2020. doi:10.1183/13993003.04015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bretas DC, Leite AS, Mancuzo EV, Prata TA, Andrade BH, Oliveira JDGF, et al. Lung function six months after severe COVID-19: Does time, in fact, heal all wounds? Braz J Infect Dis. 2022;26:102352. doi: 10.1016/j.bjid.2022.102352. doi:10.1016/j.bjid. 2022.102352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. Healthcare Workers. Centers for Disease Control and Prevention. 2020. [Last accessed on 2021 Apr 18]. Available from:https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html .
- 16.Wirth KJ, Scheibenbogen C. Dyspnea in post-COVID Syndrome following mild acute COVID-19 infections: Potential causes and consequences for a therapeutic approach. Medicina (Kaunas) 2022;58:419. doi: 10.3390/medicina58030419. doi:10.3390/medicina58030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med. 2011;39:371–9. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 18.Faverio P, Luppi F, Rebora P. Six-month pulmonary impairment after severe COVID-19: A prospective, multicentre follow-up study. Respiration. 2021;100:1078–87. doi: 10.1159/000518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonnweber T, Boehm A, Sahanic S. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients'performance: A prospective observational cohort study. Respir Res. 2020;21:276. doi: 10.1186/s12931-020-01546-2. doi:10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghahramani S, Tabrizi R, Lankarani KB. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: A systematic review and meta-analysis. Eur J Med Res. 2020;25:30. doi: 10.1186/s40001-020-00432-3. doi:10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maamar M, Artime A, Pariente E. Post-COVID-19 syndrome, low-grade inflammation and inflammatory markers: A cross-sectional study. Curr Med Res Opin. 2022;38:901–9. doi: 10.1080/03007995.2022.2042991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George PM, Reed A, Desai SR. A persistent neutrophil-associated immune signature characterizes post-COVID-19 pulmonary sequelae. Sci Transl Med. 2022;14:eabo5795. doi: 10.1126/scitranslmed.abo5795. doi:10.1126/scitranslmed.abo5795. [DOI] [PubMed] [Google Scholar]
- 23.Mandal S, Barnett J, Brill SE, Brown JS, Denneny EK, Hare SS, et al. Long Covid: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thora×. 2021;76:396–8. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannan A, Mehedi HMH, Chy N, Qayum MO, Akter F, Rob MA, et al. A multi-centre, cross-sectional study on coronavirus disease 2019 in Bangladesh: Clinical epidemiology and short-term outcomes in recovered individuals. New Microbes New Infect. 2021;40:100838. doi: 10.1016/j.nmni.2021.100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varghese J, Sandmann S, Ochs K. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci Rep. 2021;11:12775. doi: 10.1038/s41598-021-91270-8. doi:10.1038/s41598-021-91270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucijanic M, Krecak I, Soric E. Thrombocytosis in COVID-19 patients without myeloproliferative neoplasms is associated with better prognosis but higher rate of venous thromboembolism. Blood Cancer J. 2021;11:189. doi: 10.1038/s41408-021-00585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Li Z, Yang B. Delayed-phase thrombocytopenia in patients with coronavirus disease 2019 (COVID-19) Br J Haematol. 2020;190:179–84. doi: 10.1111/bjh.16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali Abdelhamid Y, Kar P, Finnis ME. Stress hyperglycaemia in critically ill patients and the subsequent risk of diabetes: A systematic review and meta-analysis. Crit Care. 2016;20:301. doi: 10.1186/s13054-016-1471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Huang L, Wang Y. 6-Month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 2021;397:220–32. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayoubkhani D, Khunti K, Nafilyan V. Post-covid syndrome in individuals admitted to hospital with COVID-19: Retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao X, Li D, Ma Z. 12-month post-discharge liver function test abnormalities among patients with COVID-19: A single-center prospective cohort study. Front Cell Infect Microbiol. 2022;12:864933. doi: 10.3389/fcimb.2022.864933. doi:10.3389/fcimb. 2022.864933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Wu D, Yan W, Wang X, Zhang X, Ma K, et al. Twelve-month systemic consequences of COVID-19 in patients discharged from hospital: A prospective cohort study in Wuhan, China. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab703. doi:10.1093/cid/ciab703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57:262–5. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong X-W, Chi Z-p, Liu G-y. Characteristics of renal function in patients diagnosed with COVID-19: An observational study. Front Med. 2020;7:409. doi: 10.3389/fmed.2020.00409. doi:10.3389/fmed. 2020.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gameil MA, Marzouk RE, Elsebaie AH. Long-term clinical and biochemical residue after COVID-19 recovery. Egypt Liver J. 2021;11:74. doi: 10.1186/s43066-021-00144-1. doi:10.1186/s43066-021-00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta A, Garg I, Iqbal A. Long-term X-ray findings in patients with coronavirus disease-2019. Cureus. 2021;13:e15304. doi: 10.7759/cureus.15304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han X, Fan Y, Alwalid O. Six-month Follow-up chest CT Findings after severe COVID-19 pneumonia. Radiology. 2021;299:E177–86. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogante M, Cavagna E, Rinaldi G. COVID-19 follow-up: Chest X-ray findings with clinical and radiological relationship three months after recovery. Radiography (Lond) 2022;28:531–6. doi: 10.1016/j.radi.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2021;57:2003690. doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You J, Zhang L, Zhang J, Hu F, Chen L, Dong Y, et al. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J Infect. 2020;81:e150–2. doi: 10.1016/j.jinf.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mo X, Jian W, Su Z, Chen M, Peng H, Peng P. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frija-Masson J, Debray MP, Gilbert M, Lescure FX, Travert F, Borie R, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days postinfection. Eur Respir J. 2020;56:2001754. doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Wang C, Kou S, Luo P, Zhao M, Yu K. Lung ventilation function characteristics of survivors from severe COVID19: A prospective study. Crit Care. 2020;24:1–2. doi: 10.1186/s13054-020-02992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S, Bai W, Yue J, Qin L, Zhang C, Xu S, et al. Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci Rep. 2021;11:13854. doi: 10.1038/s41598-021-93191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y, Tan C, Wu J. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu C, Ye L, Xia R. Chest computed tomography and clinical follow-up of discharged patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann Am Thorac Soc. 2020;17:1231–7. doi: 10.1513/AnnalsATS.202004-324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tabatabaei SMH, Rajebi H, Moghaddas F, Ghasemiadl M, Talari H. Chest CT in COVID-19 pneumonia: What are the findings in mid-term follow-up? Emerg Radiol. 2020;27:711–9. doi: 10.1007/s10140-020-01869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Gassel RJJ, Bels JLM, Raafs A. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med. 2021;203:371–4. doi: 10.1164/rccm.202010-3823LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gulati A, Lakhani P. Interstitial lung abnormalities and pulmonary fibrosis in COVID-19 patients: A short-term follow-up case series. Clin Imaging. 2021;77:186. doi: 10.1016/j.clinimag.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonnweber T, Sahanic S, Pizzini A, Luger A, Schwabl C, Sonnweber B, et al. Cardiopulmonary recovery after COVID-19-An observational prospective multi-center trial. Eur Respir J. 2020;57:2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baruch G, Rothschild E, Sadon S, Szekely Y, Lichter Y, Kaplan A, et al. Evolution of right and left ventricle routine and speckle-tracking echocardiography in patients recovering from coronavirus diseasea longitudinal study. Eur Heart J Cardiovasc Imaging. 2019;23:1055–65. doi: 10.1093/ehjci/jeab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Remy-Jardin M, Duthoit L, Perez T. Assessment of pulmonary arterial circulation 3 months after hospitalization for SARS-CoV-2 pneumonia: Dual-energy CT (DECT) angiographic study in 55 patients. EClinicalMedicine. 2021;34:100778. doi: 10.1016/j.eclinm.2021.100778. doi:10.1016/j.eclinm. 2021.100778. [DOI] [PMC free article] [PubMed] [Google Scholar]


