ABSTRACT
Background:
Hypertension (HTN) is a prevalent and impactful health concern associated with cardiovascular morbidity and mortality. This research delves into the intricate relationship between HTN and lower urinary tract symptoms (LUTS), acknowledging the multifaceted nature of these conditions and their potential impact on individuals’ quality of life.
Materials and Methods:
This cross-sectional study, conducted in Nablus, Palestine, employed convenient sampling to recruit hypertensive patients from the Ministry of Health clinics between September and December 2023. The inclusion criterion involved confirmed hypertensive patients, while the exclusion criterion included individuals with specific health conditions. The collected data included demographic, comorbidity, and lifestyle factor data. The study utilized the urogenital distress inventory 6 (UDI-6) and Incontinence Impact Questionnaire (IIQ)-7 short forms to assess urinary distress symptoms and the impact of urinary incontinence on quality of life. All the statistical analyses, including Pearson, Chi-square, Fisher’s exact, Kruskal-Wallis, and Mann-Whitney tests, were performed with various tests for significance, and the significance level was set at P < 0.05.
Results:
Among the 351 participants meeting the inclusion criteria, females (62.1%) and married individuals (83.5%) were the predominant participants. The majority were aged 58–67 (42.5%), had a high school education (39.0%), and had a body mass index (BMI) of 30–34.9 (30.2%). Significant correlations were found between UDI-6 scores and sex, age, marital status, education level, employment status, and comorbidities. The IIQ-7 score was significantly correlated with female sex, marital status, age, comorbidities, duration of HTN, employment status, and education level. Both the UDI-6 and IIQ-7 scores revealed considerable impacts on urinary symptoms and quality of life. A significant negative correlation (r = −0.579) between the IIQ-7 score and UDI-6 score emphasized the interconnectedness of urinary distress symptoms and their impact on quality of life (P < 0.001). These findings underscore the multifaceted nature of LUTS and their profound effects on hypertensive patients’ well-being.
Conclusion:
Our study revealed a significant association between HTN and LUTS, especially among females and specific demographic groups. This emphasizes the need for comprehensive management. The observed negative impact on quality of life emphasizes the significance of adopting holistic approaches to address both conditions.
Keywords: Hypertension, quality of life, severity of LUTS
Introduction
Hypertension (HTN) a persistent elevation in blood pressure, remains a major public health concern with profound implications for cardiovascular morbidity and mortality.[1] Adverse outcomes resulting from elevated blood pressure, including cardiovascular complications such as stroke, kidney damage, and cognitive effects, have been reported.[1,2] Millions of people are affected by HTN, and as a disease entity, it not only confirms health-related concerns but also results in a significant socioeconomic burden.[3]
The lower urinary tract symptom (LUTS) has been invariably studied and described in the literature. LUTSs were defined by the International Continence Society (ICS) and were categorized into storage symptoms, voiding symptoms, postmicturition symptoms, and others.[4] These symptoms are pervasive among both males and females, especially in the elderly population, and they notably diminish the quality of life (QoL) for affected individuals.[5]
Several factors, such as aging[6,7] and the presence of the metabolic syndrome (MetS),[6,8,9,10,11] have been implicated in affecting LUTS. MetS is considered a collection of several factors, including obesity, insulin resistance, dyslipidemia, and HTN, and several clinical and laboratory parameters are considered for its diagnosis.[12] Atherosclerosis and endothelial dysfunction have been hypothesized to contribute to both MetS and LUT.[13]
Studies have also explored the association between LUT and HTN,[6,13,14] with variable results, as one study showed no effect of HTN on the severity of LUTS,[6] while others showed an increase in LUTS either due to HTN itself or the medications used in its management.[13,15]
Various measurement tools, such as the International Prostate Symptoms Scale (IPSS),[6] the International Consultation on Inconsistency Questionnaire,[7] the Urogenital Distress Scale,[16] and the American Urological Association Symptoms Index,[15] have been used to assess the impact of different comorbidities on LUTS incidence.
In this study, we aimed to investigate the correlation between the severity of LUTS and the impact of LUTS on QoL among HTN patients.
Methods
Study design and setting
This cross-sectional study recruited HTN patients from the Ministry of Health clinics in Nablus, Palestine. This research utilized convenience sampling. From September to December 2023, data were collected.
Study population, sampling procedure, and sample size calculation
The study population included patients with HTN who visited the ministry of health clinics. The sample size was calculated using an online Raosoft sample size calculator,[17] which yielded a convenient sample size of 323. A confidence level of 95% error and a 5% margin were used according to the approximate number of HTN patients who visited the ministry of health clinics, which was approximately 2000.
Inclusion and exclusion criteria
Inclusion criteria
All patients were confirmed to have HTN, and patients who were also required to visit the ministry of health clinics due to HTN or its complications.
Exclusion criteria
Participants were excluded from the study if they had a history of previous urological disease, recent documented urinary tract infections (UTIs), or a psychiatric disease.
Data collection instruments
Participant demographics and characteristics
Age, sex, body mass index (BMI), smoking status, employment status, and marital status were included. Educational level, income level, duration of HTN, type of treatment, presence of other comorbidities, history of previous surgeries, and regular exercise were also collected.
The urogenital distress inventory 6 (UDI-6) short form
Like in the full version of the UDI-6, the intensity of urinary distress symptoms was based on the level of discomfort during the past month. It contains six multiple-choice questions organized into three categories: irritative symptoms (questions 1–2), stress symptoms (questions 3–4), and obstructive symptoms (questions 5–6). The participants rate each question on a scale ranging from zero to three. Therefore, the highest possible score is 18. The internal consistency of the short form of the UDI-6 was 0.71, as determined by the Cronbach’s alpha coefficient.[16] The UDI-6 scores were converted to a scale ranging from 0 to 100 to compare the measures with each other.[18]
Incontinence Impact Questionnaire-7 (IIQ-7) short form
A tool designed to assess the impact of urinary incontinence on QoL. The tool consists of seven items: household chores, physical recreation, entertainment activities, travel >30 min away from home, social activities, emotional health (nervousness, depression, etc.), and feeling frustrated. The severity of symptoms is rated on a scale from zero to three, where zero is the least severe and three is the most severe. The highest possible IIQ-7 score was 21. Scores for the IIQ-7 were converted to a scale of 0 to 100 to compare measures with each other.[19]
Statistical analysis
The data were entered and analyzed using the Social Sciences Statistical Package (SPSS) version 21. The data are expressed as the means and standard deviations for continuous variables and as frequencies and percentages for categorical variables. The Pearson test was used to assess the correlations and associations between IIQ-7 and UDI-6. Either the Chi-square test or Fisher’s exact test, as appropriate, was used to test the significance of differences between categorical variables. The Kruskal-Wallis test followed by Bonferroni-Dunn post hoc analysis or the Mann-Whitney test was used to test for differences in the means between categories. A P-value <0.05 indicated statistical significance.
Ethical considerations
All aspects of the study protocol, including access to and use of patient clinical information, were authorized by the Institutional Review Boards (IRBs) of An-Najah National University. The study was conducted according to the ethical standards of the Human Experimentation Responsible Committee (institutional and national) and the Helsinki Declaration. Informed consent was obtained from all patients.
Results
Participant demographics and characteristics
Three hundred and fifty-one participants met our inclusion criteria. There were 218 females (62.1%), and most of the participants were married (293; 83.5%), followed by 33 widows (9.4%). A total of 149 (42.5%) of the participants were between 58 and 67 years old, and 110 (31.3%) were between 48 and 57 years old. Most of the participants had a high school education level of 137 (39.0). Most of the sample had a BMI of 30–34.9 kg/m2, with 106 (30.2%) participants. Two hundred and forty three (69.2%) were not employed, 100 (28.5%) were smokers, and only two (0.6%) had a history of alcohol consumption. Sixty-eight (19.4%) of the participants exercised regularly. Regarding level of income, the majority of participants had a moderate income (n = 205; 58.4%). A total of 228 (65%) patients had more than five years of HTN, with 232 (66.1%) having other comorbidities. A total of 113 (32.2%) patients were receiving a combined treatment regimen, whereas 238 (67.8%) were receiving monotherapy for HTN [Tables 1 and 2].
Table 1.
Relationships between participant characteristics and UDI-6 scores
| Variables | Total frequency (%) n=351 | Mean rank | Median (Q1–Q3) | P* |
|---|---|---|---|---|
| Gender | ||||
| Male | 133 (37.9) | 151.76 | 12.5 (4.1–20.8) | <0.001a |
| Female | 218 (62.1) | 190.79 | 16.6 (8.3–25.0) | |
| Age | ||||
| <38 | 2 (0.6) | 170.00 | 18.7 (0.00-37.5) | 0.024b |
| 38–47 | 31 (8.8) | 177.45 | 16.6 (4.1–25.0) | |
| 48–57 | 110 (31.3) | 161.77 | 12.5 (4.1–25.0) | |
| 58–67 | 149 (42.5) | 170.96 | 12.5 (8.3–20.8) | |
| >68 | 59 (16.8) | 214.69 | 25 (8.3–33.3) | |
| Marital status | ||||
| Single | 21 (60) | 134.31 | 4.1 (0.0–27.0) | 0.002b |
| Married | 293 (83.5) | 172.69 | 12.5 (4.1–25.0) | |
| Divorced | 4 (1.1) | 161.88 | 12.5 (1.0–33.3) | |
| Widow | 33 (9.4) | 233.67 | 25 (12.5–41.6) | |
| Employed | ||||
| Yes | 108 (30.8) | 143.91 | 8.3 (4.1–20.8) | <0.001a |
| No | 243 (69.2) | 190.31 | 16.6 (8.3–25.0) | |
| Residency | ||||
| City | 173 (49.3) | 173.74 | 12.5 (4.1–25) | 0.489b |
| Village | 141 (40.2) | 182.54 | 16.6 (4.1–25) | |
| Camp | 37 (10.5) | 161.65 | 8.3 (4.1–25) | |
| Level of education | ||||
| Illiterate | 28 (8.0) | 227.18 | 25.0 (9.3–44.7) | 0.003b |
| Secondary school | 93 (26.5) | 196.72 | 16.6 (8.3–25.0) | |
| High school | 137 (39.0) | 164.22 | 12.5 (4.1–22.9) | |
| Diploma | 40 (11.4) | 152.38 | 12.5 (4.1–23.9) | |
| Bachelor’s | 53 (15.1) | 160.90 | 8.3 (0.0–25.0) | |
| Income level | ||||
| Low | 141 (40.2) | 176.67 | 12.5 (8.3–25.0) | 0.974b |
| Moderate | 205 (58.4) | 175.21 | 12.5 (4.1–25.0) | |
| High | 5 (1.4) | 189.50 | 12.5 (6.2–35.4) | |
| BMI | ||||
| 18.5–24.9 | 48 (13.7) | 156.26 | 10.4 (0.0–25.0) | 0.203b |
| 25–29.9 | 135 (38.5) | 169.45 | 12.5 (4.1–25.0) | |
| 30–34.9 | 106 (30.2) | 184.06 | 16.6 (8.3–25.0) | |
| >35 | 62 (17.7) | 191.77 | 16.6 (4.1–34.3) | |
| Smoking | ||||
| Yes | 100 (28.5) | 162.45 | 12.5 (4.1–25.0) | 0.112a |
| No | 251 (71.5) | 181.40 | 16.6 (4.1–25.0) | |
| Regular exercise | ||||
| Yes | 68 (19.4) | 191.29 | 16.6 (8.3–25.0) | 0.164a |
| No | 283 (80.6) | 172.33 | 12.5 (4.1–25.0) | |
| Alcohol drinking | 2 (0.6) | |||
| Yes | 349 (99.4) | 50.75 | 2.0 (0.0–4.1) | 0.078a |
| No | 176.72 | 12.5 (4.1–25.0) | ||
| Duration of HTN in years | ||||
| 1–3 | 76 (21.7) | 161.63 | 12.5 (0.0–23.9) | 0.169b |
| 4–5 | 47 (13.4) | 163.10 | 12.5 (4.1–25.0) | |
| >5 | 228 (65) | 183.45 | 16.6 (8.3–25.0) | |
| Treatment type | ||||
| Monotherapy | 238 (67.8) | 166.63 | 12.5 (4.1–25.0) | 0.011a |
| Combined | 113 (32.2) | 195.74 | 16.6 (8.3–27.0) | |
| Comorbidities | ||||
| Yes | 232 (66.1) | 190.19 | 16.6 (8.3–25.0) | <0.001a |
| No | 119 (33.9) | 148.34 | 12.5 (4.1–20.8) | |
| Previous surgeries | <0.001a | |||
| Yes | 139 (39.6) | 202.57 | 16.6 (8.3–29.1) | |
| No | 212 (60.4) | 158.58 | 12.5 (4.1–20.8) |
BMI: body mass index. *The bold values indicate P<0.05. aStatistically significant values were calculated using the Mann-Whitney U test. bStatistically significant values were calculated using the Kruskal-Wallis test
Table 2.
Relationships between participant characteristics and IIQ-7 scores
| Variables | Frequency (%) n=351 | Mean rank | Median (Q1–Q3) | P* |
|---|---|---|---|---|
| Gender | ||||
| Male | 133 (37.9) | 150.83 | 0.0 (0.0–12.5) | <0.001a |
| Female | 218 (62.1) | 191.36 | 8.3 (0.0–25.0) | |
| Age | ||||
| <38 | 2 (0.6) | 152.25 | 6.25 (0.00–12.5) | 0.004b |
| 38–47 | 31 (8.8) | 152.56 | 0.0 (0.0–12.5) | |
| 48–57 | 110 (31.3) | 167.86 | 4.1 (0.0–20.8) | |
| 58–67 | 149 (42.5) | 169.85 | 4.1 (0.0–20.8) | |
| >68 | 59 (16.8) | 219.83 | 16.6 (0.0–33.3) | |
| Marital status | ||||
| Single | 21 (60) | 135.45 | 0.0 (0.0–8.3) | <0.001b |
| Married | 293 (83.5) | 171.15 | 4.1 (0.0–20.8) | |
| Divorced | 4 (1.1) | 230.50 | 22.9 (5.2–28.1) | |
| Widow | 33 (9.4) | 238.24 | 16.6 (4.1–50.0) | |
| Employed | 0.001a | |||
| Yes | 108 (30.8) | 149.44 | 0.0 (0.0–12.5) | |
| No | 243 (69.2) | 187.80 | 8.3 (0.0–25.0) | |
| Residency | ||||
| City | 173 (49.3) | 179.70 | 4.1 (0.0–20.8) | 0.703b |
| Village | 141 (40.2) | 175.37 | 4.1 (0.0–20.8) | |
| Camp | 37 (10.5) | 164.82 | 4.1 (0.0–14.5) | |
| Level of Education | ||||
| Illiterate | 28 (8.0) | 220.57 | 14.0 (0.0–36.4) | <0.001b |
| Secondary School | 93 (26.5) | 202.26 | 12.5 (0.0–27.0) | |
| High School | 137 (39.0) | 168.05 | 4.1 (0.0–18.7) | |
| Diploma | 40 (11.4) | 145.66 | 0.0 (0.0–11.4) | |
| Bachelor’s | 53 (15.1) | 149.81 | 0.0 (0.0–10.4) | |
| Income level | ||||
| Low | 141 (40.2) | 184.98 | 8.0 (0.0–25.0) | 0.363b |
| Moderate | 205 (58.4) | 169.92 | 4.1 (0.0–16.6) | |
| High | 5 (1.4) | 171.80 | 0.0 (0.0–52.0) | |
| BMI | 0.227b | |||
| 18.5–24.9 | 48 (13.7) | 184.94 | 4.1 (0.0–29.1) | |
| 25–29.9 | 135 (38.5) | 162.59 | 0.0 (0.0–16.6) | |
| 30–34.9 | 106 (30.2) | 186.37 | 8.3 (0.0–20.8) | |
| >35 | 62 (17.7) | 180.54 | 4.1 (0.0–20.8) | |
| Smoking | 0.524a | |||
| Yes | 100 (28.5) | 170.78 | 4.1 (0.0–16.6) | |
| No | 251 (71.5) | 178.08 | 4.1 (0.0–20.8) | |
| Regular exercise | 0.920a | |||
| Yes | 68 (19.4) | 177.07 | 4. 1 (0.0–20.8) | |
| No | 283 (80.6) | 175.74 | 4.1 (0.0–20.8) | |
| Alcohol drinking | 2 (0.6) | 0.968a | ||
| Yes | 349 (99.4) | 178.75 | 12.5 (0.0–25.0) | |
| No | 175.98 | 4.1 (0.0–20.8) | ||
| Duration of HTN in years | 0.041b | |||
| 1–3 | 76 (21.7) | 152.04 | 0.0 (0.0–12.5) | |
| 4–5 | 47 (13.4) | 173.90 | 4.1 (0.0–20.8) | |
| >5 | 228 (65) | 184.42 | 4.1 (0.0–20.8) | |
| Treatment type | 0.381a | |||
| Monotherapy | 238 (67.8) | 172.88 | 4.1 (0.0–20.8) | |
| Combined | 113 (32.2) | 182.58 | 4.1 (0.0–20.8) | |
| Comorbidities | 0.004a | |||
| Yes | 232 (66.1) | 186.78 | 6.2 (0.0–20.8) | |
| No | 119 (33.9) | 154.99 | 0.0 (0.0–12.5) | |
| Previous surgeries | 0.004a | |||
| Yes | 139 (39.6) | 197.28 | 8.3 (0.0–25.0) | |
| No | 212 (60.4) | 164.01 | 2.0 (0.0–19.7) |
BMI: body mass index. *The bold values indicate P<0.05. aStatistically significant values were calculated using the Mann-Whitney U test. bStatistically significant values were calculated using the Kruskal-Wallis test
Relationships between participant characteristics and UDI-6 scores
Gender was significantly correlated with the UDI-6 score, with a higher mean rank for females (190.79; P < 0.001). A significant correlation was found between age and the UDI-6 score, with a median of 16.6 (4.1–25.0) for patients aged 38–47 years and a median of 18.7 (0.00–37.5) for patients aged less than 38 years (P = 0.024). A history of previous surgeries, the presence of other comorbidities, and unemployment were significantly correlated with medians of 16.6 (8.3–29.1), 16.6 (8.3–25.0), and 16.6 (8.3–25.0), respectively (P < 0.001). A mean rank of 233.67 was associated with widow participants, and a mean rank of 172.69 was significantly correlated with the UDI-6 score (P = 0.002). Participants with a mean rank of 227.18 were illiterate, and a mean rank of 196.72 was secondary school, which was correlated with the score (P = 0.003). The duration of HTN, smoking status, regular exercise status, and BMI were not significantly different among the groups. [Table 1]
Relationship between the participants’ characteristics and their IIQ-7 score
Female sex, with a mean rank of 191.36, was significantly correlated with the IIQ-7 score (P < 0.001). A median age of 22.9 years (5.2–28.1) was divided into 2 groups, and a median age of 16.6 years (4.1–50.0) was associated with the IIQ-7 score (P < 0.001). Age was significantly correlated with the IIQ-7 score; patients older than 68 years had a median age of 16.6 years (0.0–33.3), while patients aged between 48 and 57 years and between 58 and 67 years had a median age of 4.1 years (0.0–20.8) (P = 0.004). The presence of other comorbidities, with a median of 6.2 (0.0–20.8) and a history of previous surgeries of 8.3 (0.0–25.0), was significantly correlated with the IIQ-7 score (P = 0.004). Patients with more than five years of HTN had a higher IIQ-7 score, with a mean rank of 184.42. Unemployed status and education level were significantly correlated with the IIQ-7 score (P < 0.05). Treatment type, BMI, exercise, and income level were not significantly correlated [Table 2].
Distribution of responses to each question on the short UDI-6 and the short IIQ-7 [Graph 1 and Garph 2]
Graph 1.
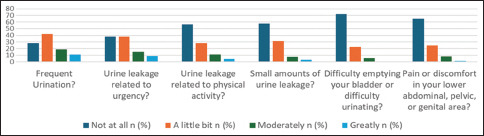
Distribution of responses to each question on the UDI-6
Graph 2.
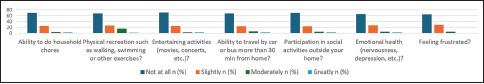
Distribution of responses to each item on the short-form IIQ-7
Graph 1 shows the median UDI-6 score was 4.0–25.0. A total of 148 (42.2%) participants had a little bit of frequent urination, 133 (37.9%) complained of urgency urinary incontinence just a little bit, and 31 (8.8%) had a great response to urgency incontinence. Most of the sample of 198 (56.4%) had never experienced an episode of incontinence with physical activity. Regarding difficulty emptying the bladder, the response options were as follows: 252 (71.8%), not at all; 78 (22.2%), a little bit; 20 (5.7%), moderately; and one (0.3%), greatly. Only six (1.7%) patients had pain in the lower abdomen or pelvis, while the majority did not have pain (228) (65.0%). A total of 202 (57.5%) patients reported that small amounts of urine leakage were mostly not present.
Graph 2 illustrates the median IIQ-7 score was 4.1 (0.0–20.8). A total of 110 (31.3%) mentioned that their urinary tract symptoms affected their ability to perform household chores. A total of 33.3% of the participants had limitations in physical recreation, such as walking, swimming, or other exercises, while 30.2% had an impact on entertainment activities. The scale also showed that 32.8% and 31.3% of the respondents had an effect on their ability to travel by car or bus more than 30 min from home and participate in social activities, respectively. Regarding the effect on emotional health, 65% of the participants reported having some kind of nervousness and depression. A total of 35.6% of the samples were frustrated to different degrees [Table 3].
Table 3.
Correlations between IIQ-7 and UDI-6
| n=351 | P | Pearson correlation |
|---|---|---|
| IIQ-7 and UDI-6 | <0.001 | 0.579** |
**Correlation is significant at the 0.01 level (two-tailed)
The correlation between IIQ-7 and UDI-6
There was a statistically significant correlation between the IIQ-7 score and the UDI-6 score (r = −0.579) (P < 0.001).
Discussion
The findings of our study provide insight into the risk factors for LUTS in a cohort of patients with HTN. Furthermore, our investigation examined the severity of LUTS within the same patient group.
A notable correlation was found between female sex and a higher UDI-6 score, indicating a more severe scale of LUTS among female participants. This finding contrasts with those of several prior studies that demonstrated comparable results between sexes, with voiding symptoms being more prevalent in males.[20]
Our study also revealed a significant increase in the severity of LUTS with advancing age, corroborating findings from other studies.[6,7,21] Participants who were widowed, married, or divorced exhibited a greater likelihood of having severe LUTS—a trend consistent with previous research linking marital status to the severity of LUTS.[21] Unemployment also showed a significant correlation with LUTS severity, aligning with findings in other studies where unemployment was identified as a risk factor for LUTS.[22]
A lower education level was found to be significantly correlated with LUTS, consistent with the findings of previous studies.[23,24,25] However, it is worth noting that some studies suggest an inverse relationship between LUTS and education level.
Our study identified several factors significantly correlated with LUTS, including combined treatment for HTN, the presence of other comorbidities, and prior surgical history. Medical therapy, depending on the type of medication used, was associated with increased LUTS in both sexes.[15] Interestingly, one study revealed that cotreatment of cardiovascular diseases and LUTS with angiotensin II receptor blockers reduced the severity of LUTS.[26] The combination of antihypertensive medications, such as amlodipine, and an alpha-blocker, such as terazosin, has been found to adequately control both HTN and LUTS.[27,28] The presence of comorbidities, such as DM[29] and MetS,[10] is also considered a risk factor for LUTS occurrence and severity.
A correlation between female sex and a higher IIQ-7 score implies a greater impact on QoL for female participants with HTN. Additionally, advancing age, being widowed, unemployment, a low educational level, duration of HTN, other comorbidities, and previous surgeries were also significantly correlated with IIQ-7 scores. These findings align with existing research confirming that LUTS negatively affect QoL and that underlying distress factors increase the severity of QoL impairment.[22,30]
Given these research findings, LUTS affects HTN patients. Several factors have been suggested to predispose and exacerbate LUTS. Early identification, a holistic assessment approach, and multidisciplinary team management might be needed for these patients for proper and adequate management of both conditions.[21]
Conclusions
LUTS emerge as a significant morbidity within hypertensive (HTN) patient populations. Various factors contribute to the severity of these symptoms among HTN patients, including advanced age, female sex, unemployment, lower educational attainment, employment of combined treatment modalities for HTN, and the presence of comorbidities. Furthermore, these factors collectively exert a notable influence on the QoL experienced by affected individuals.
Limitations of the study
Our study possesses several limitations. First, the primary constraint lies in its cross-sectional design, preventing the identification of causal relationships. Second, the study lacks consideration for HTN severity, necessitating a more precise measure to determine the adequacy of blood pressure control. Third, the initial limitation is associated with the sample’s nature, as a convenience sampling technique was employed.
Consent to participate
All subjects involved in the research were invited to participate voluntarily after the study’s purpose as well as the risks and the benefits of participation were explained. Informed consent was obtained from all individual participants is included in the study.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are grateful to the Palestinian Ministry of Health for assisting us in distributing and collecting questionnaires.
References
- 1.Deussen A, Kopaliani I. Targeting inflammation in hypertension. Curr Opin Nephrol Hypertens. 2023;32:111–7. doi: 10.1097/MNH.0000000000000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Makki A, DiPette D, Whelton PK, Murad MH, Mustafa RA, Acharya S, et al. Hypertension pharmacological treatment in adults: A world health organization guideline executive summary. Hypertension. 2022;79:293–301. doi: 10.1161/HYPERTENSIONAHA.121.18192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: A systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 5.Choi EPH, Wan EYF, Chin WY, Lam CLK. Lower urinary tract symptoms and health-related quality of life in Hong Kong primary care: A cross-sectional study. Qual Life Res. 2020;29:1311–21. doi: 10.1007/s11136-019-02402-7. [DOI] [PubMed] [Google Scholar]
- 6.Gondžetović N, Jatić Z, Omerbašić A. Assessment of lower urinary tract symptoms (LUTS) in hypertensive men. Hypertension. 2018;61:67.0. [Google Scholar]
- 7.Wang Y, Hu H, Xu K, Wang X, Na Y, Kang X. Prevalence, risk factors and the bother of lower urinary tract symptoms in China: A population-based survey. Int Urogynecol J. 2015;26:911–9. doi: 10.1007/s00192-015-2626-8. [DOI] [PubMed] [Google Scholar]
- 8.Tai HC, Yu HJ. Association between metabolic syndrome and lower urinary tract symptoms: Evidences from epidemiological studies. Low Urin Tract Symptoms. 2012;4(Suppl 1):8–10. doi: 10.1111/j.1757-5672.2011.00123.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoon H. Metabolic syndrome and lower urinary tract symptoms: Epidemiological study. Low Urin Tract Symptoms. 2012;4(Suppl 1):2–7. doi: 10.1111/j.1757-5672.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 10.Ineichen GB, Burkhard FC. Metabolic syndrome and male lower urinary tract symptoms. Panminerva Med. 2022;64:329–36. doi: 10.23736/S0031-0808.21.04496-7. [DOI] [PubMed] [Google Scholar]
- 11.Moul S, McVary KT. Lower urinary tract symptoms, obesity and the metabolic syndrome. Curr Opin Urol. 2010;20:7–12. doi: 10.1097/MOU.0b013e3283336f3f. [DOI] [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--A new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 13.Lim J, Bhoo-Pathy N, Sothilingam S, Malek R, Sundram M, Tan GH, et al. Cardiovascular risk factors and ethnicity are independent factors associated with lower urinary tract symptoms. PLoS One. 2015;10:e0130820. doi: 10.1371/journal.pone.0130820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugaya K, Kadekawa K, Ikehara A, Nakayama T, Gakiya M, Nashiro F, et al. Influence of hypertension on lower urinary tract symptoms in benign prostatic hyperplasia. Int J Urol. 2003;10:569–74. doi: 10.1046/j.1442-2042.2003.00707.x. discussion 75. [DOI] [PubMed] [Google Scholar]
- 15.Hall SA, Chiu GR, Kaufman DW, Wittert GA, Link CL, McKinlay JB. Commonly used antihypertensives and lower urinary tract symptoms: Results from the boston area community health (BACH) survey. BJU Int. 2012;109:1676–84. doi: 10.1111/j.1464-410X.2011.10593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altaweel W, Seyam R, Mokhtar A, Kumar P, Hanash K. Arabic validation of the short form of urogenital distress inventory (UDI-6) questionnaire. Neurourol Urodyn. 2009;28:330–4. doi: 10.1002/nau.20640. [DOI] [PubMed] [Google Scholar]
- 17.Raosoft 2004. Available from: http://www.raosoft.com/samplesize.html .
- 18.Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health-related quality of life measures for women with urinary incontinence: The incontinence impact questionnaire and the urogenital distress inventory. continence program in women (CPW) research group. Qual Life Res. 1994;3:291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 19.Skorupska K, Grzybowska ME, Kubik-Komar A, Rechberger T, Miotla P. Identification of the urogenital distress inventory-6 and the incontinence impact questionnaire-7 cutoff scores in urinary incontinent women. Health Qual Life Outcomes. 2021;19:87. doi: 10.1186/s12955-021-01721-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terai A, Matsui Y, Ichioka K, Ohara H, Terada N, Yoshimura K. Comparative analysis of lower urinary tract symptoms and bother in both sexes. Urology. 2004;63:487–91. doi: 10.1016/j.urology.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 21.Jeong JB, Lee JH, Choo MS, Ahn DW, Kim SH, Lee DS, et al. Association between life-style, metabolic syndrome and lower urinary tract symptoms and its impact on quality of life in men ≥ 40 years. Sci Rep. 2022;12:6859. doi: 10.1038/s41598-022-10904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TH, Han DH, Ryu DS, Lee KS. The impact of lower urinary tract symptoms on quality of life, work productivity, depressive symptoms, and sexuality in korean men aged 40 years and older: A population-based survey. Int Neurourol J. 2015;19:120–9. doi: 10.5213/inj.2015.19.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai UC, Wun YT, Luo TC, Pang SM. In a free healthcare system, why do men not consult for lower urinary tract symptoms (LUTS)? Asia Pac Fam Med. 2011;10:7. doi: 10.1186/1447-056X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badia X, Rodríguez F, Carballido J, García Losa M, Unda M, Dal-Ré R, et al. Influence of sociodemographic and health status variables on the American Urological Association symptom scores in patients with lower urinary tract symptoms. Urology. 2001;57:71–7. doi: 10.1016/s0090-4295(00)00894-3. [DOI] [PubMed] [Google Scholar]
- 25.Arslantas D, Gokler ME, Unsal A, Başeskioğlu B. Prevalence of lower urinary tract symptoms among individuals aged 50 years and over and its effect on the quality of life in a semi-rural area of western Turkey. Low Urin Tract Symptoms. 2017;9:5–9. doi: 10.1111/luts.12100. [DOI] [PubMed] [Google Scholar]
- 26.Semczuk-Kaczmarek K, Płatek AE, Szymański FM. Co-treatment of lower urinary tract symptoms and cardiovascular disease-Where do we stand? Cent European J Urol. 2020;73:42–5. doi: 10.5173/ceju.2020.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Liu P, Mao G, Chen G, Wang B, Qin X, et al. Efficacy of combined amlodipine/terazosin therapy in male hypertensive patients with lower urinary tract symptoms: A randomized, double-blind clinical trial. Urology. 2009;74:130–6. doi: 10.1016/j.urology.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 28.Mirzaei M, Daneshpajooh A, Anvari SO, Dozchizadeh S, Teimorian M. Evaluation of the clinical efficacy and complications of duloxetine in comparison to solifenacin in the treatment of overactive bladder disease in women: A randomized clinical trial. Urol J. 2021;18:543–8. doi: 10.22037/uj.v18i.6274. [DOI] [PubMed] [Google Scholar]
- 29.Qasrawi H, Tabouni M, Almansour SW, Ghannam M, Abdalhaq A, Abushamma F, et al. An evaluation of lower urinary tract symptoms in diabetic patients: A cross-sectional study. BMC Urol. 2022;22:178. doi: 10.1186/s12894-022-01133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao L, Chuang YC, Liu SP, Lee KS, Yoo TK, Chu R, et al. Effect of lower urinary tract symptoms on the quality of life and sexual function of males in China, Taiwan, and South Korea: Subgroup analysis of a cross-sectional, population-based study. Low Urin Tract Symptoms. 2019;11:O78–84. doi: 10.1111/luts.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.


