Abstract
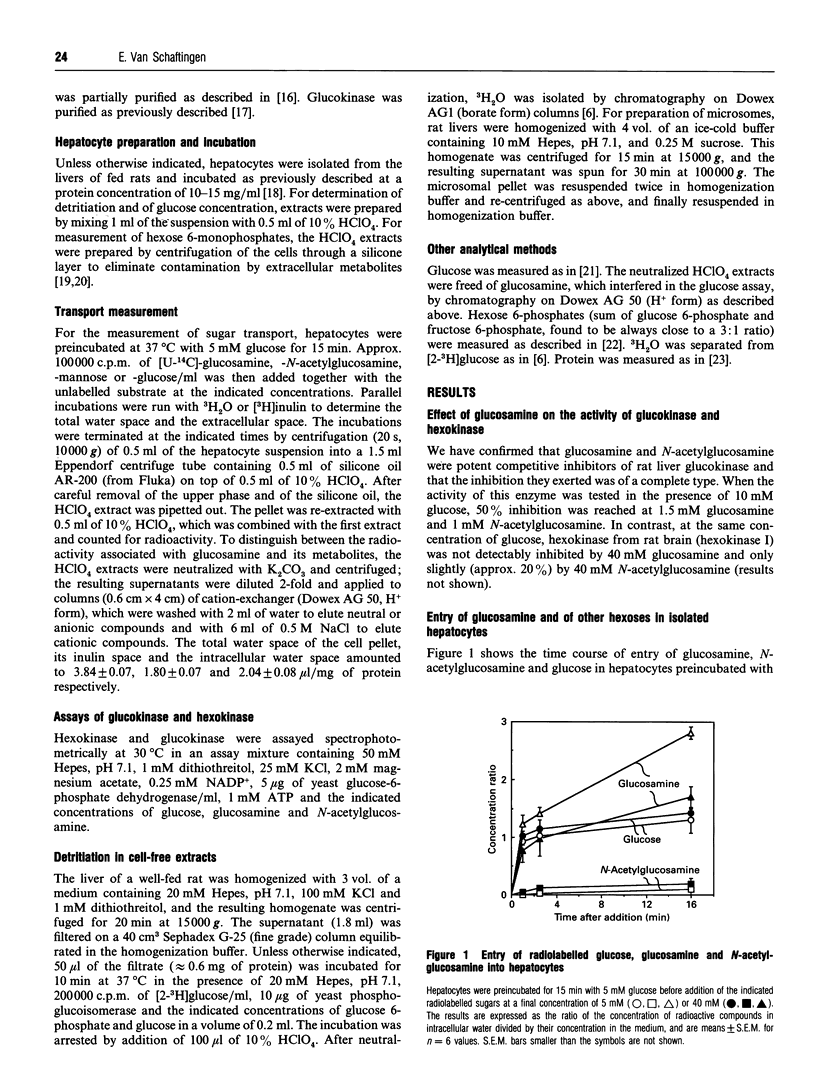
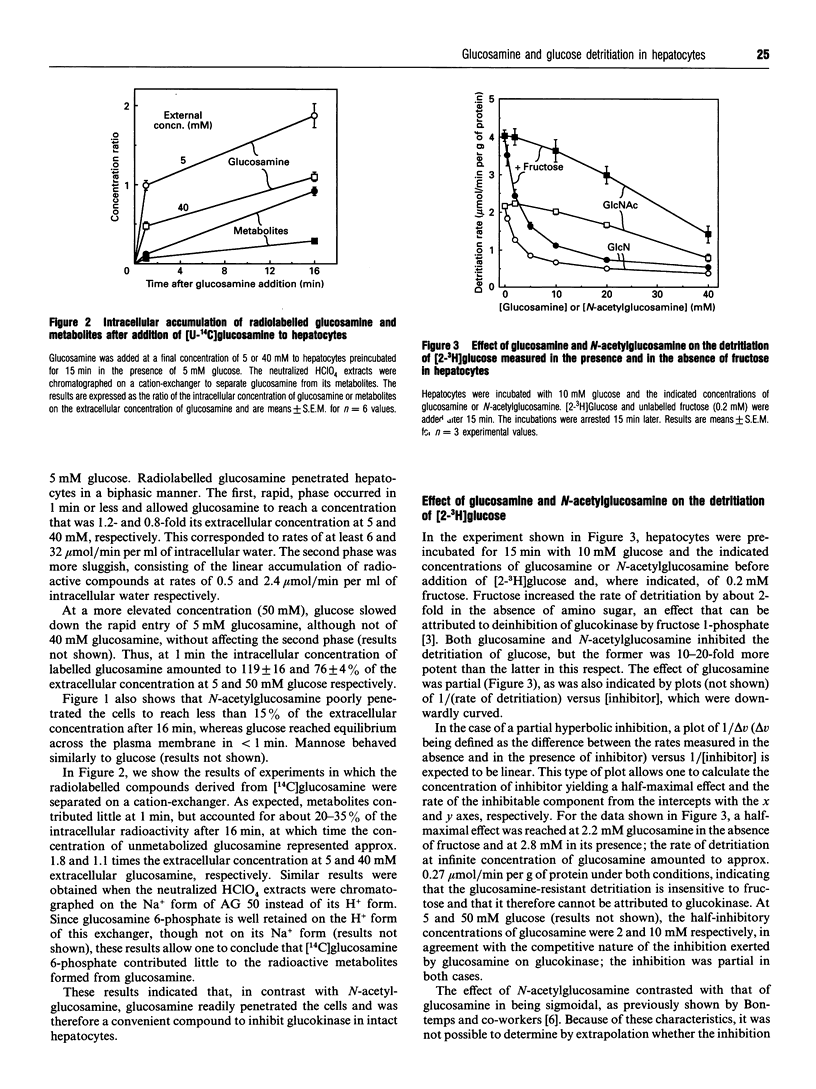
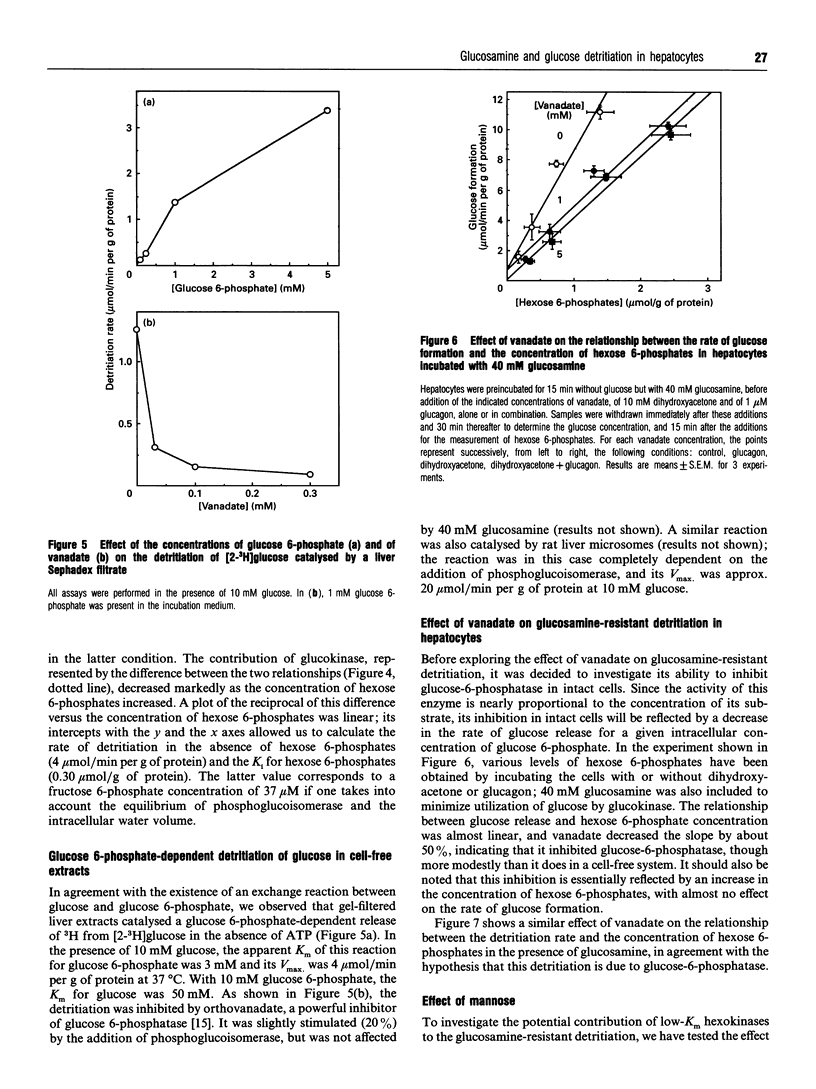
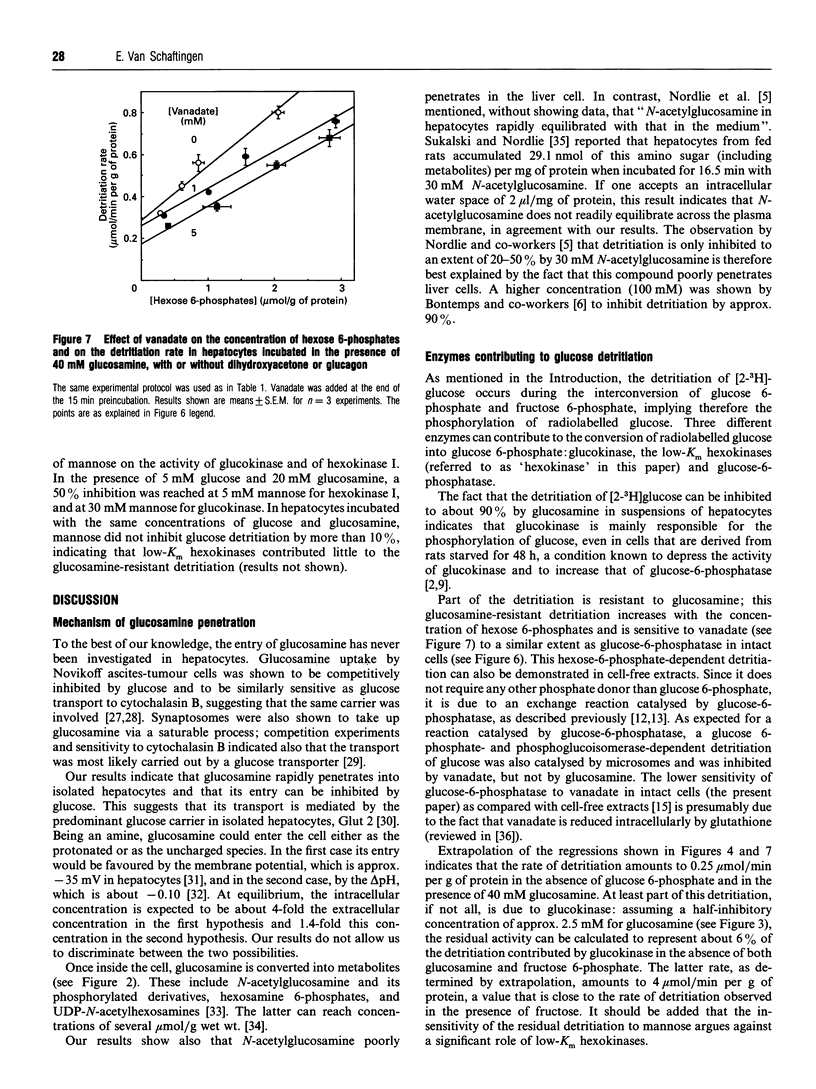
Glucosamine, a potent inhibitor of glucokinase (hexokinase IV or D), was used to estimate the contribution of this enzyme to glucose phosphorylation in freshly isolated rat hepatocytes and its sensitivity to fructose 6-phosphate in situ. Experiments with radiolabelled glucosamine indicated that this amino sugar, at concentrations of 5 or 40 mM, readily penetrated hepatocytes to reach in 1 min a total (i.e., glucosamine+metabolites) intracellular concentration equal to 0.8-1.2-fold its extracellular concentration. In marked contrast, N-acetylglucosamine barely penetrated the cells. The detritiation of [2-3H]glucose, used to estimate glucose phosphorylation in intact cells, was inhibited by glucosamine much more potently than by N-acetylglucosamine, half-maximal effects being reached at about 2.5 and 30 mM respectively. Extrapolation of the data indicated that about 12% of the detritiation was resistant to glucosamine. Dihydroxyacetone (10 mM), lactate (10 mM) + pyruvate (1 mM), and glucagon (1 microM) increased up to 8-fold the concentration of hexose 6-phosphates (glucose 6-phosphate+fructose 6-phosphate) and, against expectations, modestly decreased the detritiation rate measured in the absence of glucosamine. In the presence of 40 mM glucosamine, these agents increased the detritiation rate, which then positively correlated with the concentration of hexose 6-phosphates. This hexose 6-phosphates-dependent detritiation was sensitive to inhibition by vanadate, and was also catalysed by gel-filtered cell-free extracts, as well as by liver microsomes in the presence of phosphoglucoisomerase; it can be explained by an exchange reaction catalysed by glucose-6-phosphatase. When this exchange reaction is taken into account, it appears that the rate of glucose detritiation attributable to glucokinase decreases when the concentration of hexose 6-phosphates increases. This is in agreement with the known effect of fructose 6-phosphate to potentiate the inhibition of glucokinase by its regulatory protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou A. C., Wilson J. E. Purification and properties of rat brain hexokinase. Arch Biochem Biophys. 1972 Jul;151(1):48–55. doi: 10.1016/0003-9861(72)90471-7. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Detheux M., Van Schaftingen E. Fructose 1-phosphate and the regulation of glucokinase activity in isolated hepatocytes. Eur J Biochem. 1990 Sep 11;192(2):283–289. doi: 10.1111/j.1432-1033.1990.tb19225.x. [DOI] [PubMed] [Google Scholar]
- Detheux M., Vandercammen A., Van Schaftingen E. Effectors of the regulatory protein acting on liver glucokinase: a kinetic investigation. Eur J Biochem. 1991 Sep 1;200(2):553–561. doi: 10.1111/j.1432-1033.1991.tb16218.x. [DOI] [PubMed] [Google Scholar]
- Dipple I., Houslay M. D. The activity of glucagon-stimulated adenylate cyclase from rat liver plasma membranes is modulated by the fluidity of its lipid environment. Biochem J. 1978 Jul 15;174(1):179–190. doi: 10.1042/bj1740179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson D., Smith N. D., Boyer J. L. Bicarbonate-dependent and -independent intracellular pH regulatory mechanisms in rat hepatocytes. Evidence for Na+-HCO3- cotransport. J Clin Invest. 1989 Jul;84(1):312–321. doi: 10.1172/JCI114156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad P., Beck J. S., Boyer J. L., Graf J. Role of chloride ions in liver cell volume regulation. Am J Physiol. 1991 Aug;261(2 Pt 1):G340–G348. doi: 10.1152/ajpgi.1991.261.2.G340. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Yamanouchi K., Roach P. J., Yen T. T., Dominianni S. J., Stephens T. W. Stabilization of glycogen stores and stimulation of glycogen synthesis in hepatocytes by phenacyl imidazolium compounds. J Biol Chem. 1989 Sep 5;264(25):14674–14680. [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Hue L., Bontemps F., Hers H. The effects of glucose and of potassium ions on the interconversion of the two forms of glycogen phosphorylase and of glycogen synthetase in isolated rat liver preparations. Biochem J. 1975 Oct;152(1):105–114. doi: 10.1042/bj1520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Golden S., Rognstad R. Recycling of glucose by rat hepatocytes. Eur J Biochem. 1975 Dec 1;60(1):91–101. doi: 10.1111/j.1432-1033.1975.tb20979.x. [DOI] [PubMed] [Google Scholar]
- Keppler D. O., Rudigier J. F., Bischoff E., Decker K. F. The trapping of uridine phosphates by D-galactosamine. D-glucosamine, and 2-deoxy-D-galactose. A study on the mechanism of galactosamine hepatitis. Eur J Biochem. 1970 Dec;17(2):246–253. doi: 10.1111/j.1432-1033.1970.tb01160.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nordlie R. C. Metabolic regulation by multifunctional glucose-6-phosphatase. Curr Top Cell Regul. 1974;8(0):33–117. doi: 10.1016/b978-0-12-152808-9.50009-2. [DOI] [PubMed] [Google Scholar]
- Nordlie R. C., Singh J., Sukalski K. A. N-acetylglucosamine-insensitive glucose phosphorylation in isolated hepatocytes from rats fasted for 48 h. Biochim Biophys Acta. 1986 Nov 28;889(2):179–182. doi: 10.1016/0167-4889(86)90102-3. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Erbe J. Transport and metabolism of glucosamine by cultured Novikoff rat hepatoma cells and effects on nucleotide pools. Cancer Res. 1973 Mar;33(3):482–492. [PubMed] [Google Scholar]
- Reyes A., Cárdenas M. L. All hexokinase isoenzymes coexist in rat hepatocytes. Biochem J. 1984 Jul 15;221(2):303–309. doi: 10.1042/bj2210303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALAS J., SALAS M., VINUELA E., SOLS A. GLUCOKINASE OF RABBIT LIVER. J Biol Chem. 1965 Mar;240:1014–1018. [PubMed] [Google Scholar]
- Singh J., Nordlie R. C., Jorgenson R. A. Vanadate: a potent inhibitor of multifunctional glucose-6-phosphatase. Biochim Biophys Acta. 1981 Dec 18;678(3):477–482. doi: 10.1016/0304-4165(81)90129-x. [DOI] [PubMed] [Google Scholar]
- Stalmans W., Laloux M., Hers H. G. The interaction of liver phosphorylase a with glucose and AMP. Eur J Biochem. 1974 Nov 15;49(2):415–427. doi: 10.1111/j.1432-1033.1974.tb03847.x. [DOI] [PubMed] [Google Scholar]
- Sukalski K. A., Nordlie R. C. Implications of distinct inhibitory effects of N-acetylglucosamine on glucose uptake by an isolated perfusion system incorporating erythrocytes with livers from fed and 48-hour fasted rats. J Biol Chem. 1986 May 25;261(15):6860–6867. [PubMed] [Google Scholar]
- Tan C. H., Peterson N. A., Raghupathy E. D-Glucosamine uptake by rat brain synaptosomes. Biochim Biophys Acta. 1977 Jan 21;464(2):459–462. doi: 10.1016/0005-2736(77)90020-7. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Detheux M., Veiga da Cunha M. Short-term control of glucokinase activity: role of a regulatory protein. FASEB J. 1994 Apr 1;8(6):414–419. doi: 10.1096/fasebj.8.6.8168691. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Extracellular metabolites in suspensions of isolated hepatocytes. Biochem J. 1987 Dec 1;248(2):517–521. doi: 10.1042/bj2480517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Opperdoes F. R., Hers H. G. Effects of various metabolic conditions and of the trivalent arsenical melarsen oxide on the intracellular levels of fructose 2,6-bisphosphate and of glycolytic intermediates in Trypanosoma brucei. Eur J Biochem. 1987 Aug 3;166(3):653–661. doi: 10.1111/j.1432-1033.1987.tb13563.x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Vandercammen A. Stimulation of glucose phosphorylation by fructose in isolated rat hepatocytes. Eur J Biochem. 1989 Jan 15;179(1):173–177. doi: 10.1111/j.1432-1033.1989.tb14537.x. [DOI] [PubMed] [Google Scholar]
- Vandercammen A., Van Schaftingen E. The mechanism by which rat liver glucokinase is inhibited by the regulatory protein. Eur J Biochem. 1990 Jul 31;191(2):483–489. doi: 10.1111/j.1432-1033.1990.tb19147.x. [DOI] [PubMed] [Google Scholar]
- Weinhouse S. Regulation of glucokinase in liver. Curr Top Cell Regul. 1976;11:1–50. [PubMed] [Google Scholar]



