Abstract
Introduction
We aim to report the anatomical and functional outcomes of ruthenium-106 brachytherapy in the management of circumscribed choroidal hemangiomas (CCH).
Methods
This is a single-center, retrospective case series including patients with unilateral symptomatic CCH treated with ruthenium-106 brachytherapy at the Cairo University Ocular Oncology Service. Patient records were analyzed for patients’ demographics, best corrected visual acuity (BCVA), tumor dimensions (thickness and largest base diameter), foveal subretinal fluid, radiation-related complications, and recurrence.
Results
Seven patients were included in the study (including 6 males) with a mean age of 39.3 ± 15.4 years; ruthenium-106 plaque was used to deliver 50 Gray to the tumor apex. After a mean follow-up duration of 12.5 months, all patients had significant improvement in BCVA after treatment, mean tumor height decreased significantly from 4.76 ± 1.76 mm to 1.70 ± 1.2 mm (p value 0.01). The largest tumor base diameter also decreased significantly from 9.13 ± 2.68 mm to 4.65 ± 3.75 mm (p value 0.05). Subretinal fluid and exudative retinal detachment resolved in all patients, and no significant radiation-related complications were observed in any patient. None of the patients needed any further treatment or experienced recurrence within the follow-up period.
Conclusion
Ruthenium-106 brachytherapy is an effective tool in the management of symptomatic CCH with a good visual prognosis and safety profile.
Keywords: Circumscribed choroidal hemangioma, Brachytherapy, Ruthenium-106, Treatment outcome
Introduction
Circumscribed choroidal hemangiomas (CCH) are orange-red vascular hamartomatous lesions that mostly affect the posterior pole. Some cases may remain asymptomatic, while others may present with visual impairment due to exudative retinal detachment, cystoid macular edema, photoreceptor changes, and subretinal fibrosis. Despite being a benign lesion, if left untreated, up to 40% of patients with CCH may end up with progressive retinal detachment and phthisis [1–3].
Numerous interventional modalities have been attempted for the management of CCHs with varying degrees of success. Photocoagulation, transpupillary thermotherapy (TTT), and photodynamic therapy (PDT) were considered the first lines of treatment but are not suitable for cases with extensive subretinal fluid or exudative retinal detachment. External beam radiotherapy (EBR), proton beam radiation, and gamma knife radiotherapy have all shown promising results in such cases. However, the collateral damage caused by these modalities, coupled with the asymmetrical delivery of the radiation dose to the tumor, has prompted the search for more localized methods of treatment [1, 2].
In order to mitigate these hazards, episcleral brachytherapy has been attempted in the management of CCHs with rather conflicting results. In this article, we present our experience with a case series of patients with symptomatic CCHs treated with ruthenium-106 plaque brachytherapy together with a brief review of the literature.
Methods
This was a single-center retrospective case series of patients with unilateral symptomatic CCH who were treated with ruthenium-106 plaque brachytherapy at the Ocular Oncology Service, Ophthalmology Department, Faculty of Medicine, Cairo University in the period between June 2019 and June 2023. The study adhered to the tenets of the Declaration of Helsinki and was approved by the research ethics committee of the Faculty of medicine, Cairo University (N-6-2024). Informed consent was obtained from all patients prior to brachytherapy.
Diagnosis of CCH was based on a combination of the findings of clinical examination by binocular indirect ophthalmoscopy and the characteristic ultrasonographic features (dome-shaped choroidal mass with high internal reflectivity) (shown in online suppl. Fig. 1, 2; for all online suppl. material, see https://doi.org/10.1159/000539384).
Only patients with symptomatic CCH associated with extensive subretinal fluid and/or retinal detachment involving the macula causing diminution of vision were included. Patients were excluded if they were previously treated for CCH or having other ocular conditions including glaucoma, diabetic retinopathy, macular hole, and rhegmatogenous retinal detachment.
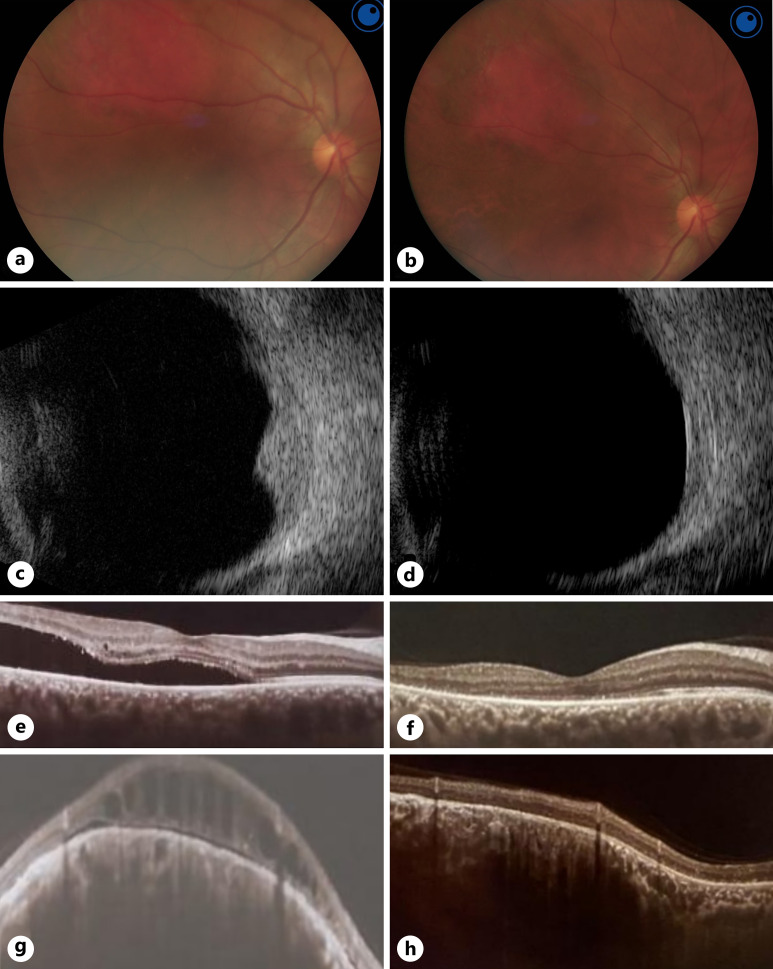
Demographic data of the patients were obtained, best corrected visual acuity (BCVA) was assessed for all patients, intraocular pressure was measured using a Goldmann applanation tonometer, and anterior segment examination by slit lamp and detailed fundus examination by indirect ophthalmoscopy were done for each patient at the initial visit and all subsequent follow-up visits. All patients had preoperative and postoperative colored fundus photography, A and B scan ultrasonography (to assess the dimensions of the tumor), and optical coherence tomography (OCT) scans on the macular area (to assess for the presence of and quantify the subretinal fluid) (shown in Fig. 1).
Fig. 1.
Treatment outcome of ruthenium-106 brachytherapy in case 7 with right CCH. a Preoperative colored fundus photograph of the right eye showing a supero-temporal CCH with associated exudative retinal detachment. b Postoperative colored fundus photograph of the right eye showing flattening and scarring of the CCH with resolution of the exudative detachment after brachytherapy. c Preoperative B scan ultrasonography showing a dome-shaped choroidal lesion with high internal reflectivity. d Postoperative B scan ultrasonography showing reduction in lesion height and base diameter after brachytherapy. e Preoperative OCT line scan passing through the fovea showing significant subfoveal fluid. f Postoperative OCT line scan passing through the fovea showing resolution of the subretinal fluid after brachytherapy. g Preoperative OCT line scan passing through the CCH showing dome-shaped choroidal lesion with overlying cystoid spaces and subretinal fluid. h Postoperative OCT line scan passing through the CCH showing flattening of the lesion with resolution of the associated cystoid edema and subretinal fluid after brachytherapy.
Ruthenium-106 ophthalmic applicator (BEBIG Isotopen und Medizintechnik GmbH, Berlin, Germany) was applied under general anesthesia. The tumor location was identified using indirect binocular ophthalmoscopy and indentation with marking of the anterior border of the tumor on the sclera using a marker. Intraoperative ultrasonography was deployed to confirm proper plaque placement and positioning. The plaque was removed under general anesthesia after delivering a predetermined target radiation dose of 50 Gray (Gy) to the CCH apex.
The primary efficacy outcomes included the changes in CCH height and BCVA at the last follow-up visit compared to baseline. Treatment is considered successful if there is improvement of BCVA by at least two lines, resolution of subretinal fluid and exudative retinal detachment in OCT, and reduction in tumor dimensions by ultrasound. Safety outcomes included the occurrence of complications such as radiation retinopathy, radiation papillopathy, cataract formation, iris neovascularization, secondary glaucoma, and subretinal fibrosis.
Statistical analysis was performed using Microsoft Excel. Paired t test was used to compare changes in CCH dimensions on ultrasound between the baseline and last follow-up visit. A two-sided p value of less than 0.05 was considered to be statistically significant.
Results
Seven patients with unilateral symptomatic CCH were included for analysis in this case series. Six patients were males and only one female with a mean age of 39.3 ± 15.4 (range 16–56) years. The presenting symptom in all patients was gradual progressive diminution of vision with a mean duration of 2.43 (range 1–4) months between the onset of symptoms and applying brachytherapy. The left eye was involved in 5 patients (71.4%), and the macula was involved by the CCH in 3 patients (42.9%). Two patients (28.57%) had an ipsilateral facial hemangioma and were diagnosed to have Sturge-Weber syndrome. The mean baseline tumor height was 4.76 ± 1.76 mm, and the mean baseline largest tumor diameter was 9.13 ± 2.68 mm. All patients had subretinal fluid at the macula, and 6 patients had associated exudative retinal detachment. Individual patients’ characteristics are listed in Table 1.
Table 1.
Demographic data and clinical features of study subjects
| Age | Gender | Laterality | Facial hemangioma | Involved quadrant | Macular/extramacular | RD | SRF | Duration of symptoms, months | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | Male | Left | No | Nasal | Extramacular | Yes | Yes | 4 |
| 2 | 35 | Male | Left | No | Temporal | Extramacular | Yes | Yes | 3 |
| 3 | 56 | Male | Left | No | Temporal | Macular | No | Yes | 2 |
| 4 | 22 | Male | Right | Yes | Temporal | Macular | Yes | Yes | 2 |
| 5 | 16 | Female | Left | Yes | Temporal | Macular | Yes | Yes | 1 |
| 6 | 46 | Male | Left | No | Superior | Extramacular | Yes | Yes | 2 |
| 7 | 49 | Male | Right | No | Temporal | Extramacular | Yes | Yes | 3 |
RD, retinal detachment; SRF, subretinal fluid.
An apex dose of 50 Gy was delivered in all patients using the ruthenium-106 applicator, COB was used in 5 patients (71.4%), and CCB was used in the remaining 2 patients (28.6%). The mean treatment duration was 83.7 ± 43.6 (range 34–144) hours.
After a mean follow-up duration of 12.5 months, all patients had significant improvement in their BCVA after treatment, mean tumor height decreased significantly from 4.76 ± 1.76 mm preoperatively to 1.70 ± 1.2 mm at the last follow-up (p value 0.01). The largest tumor base diameter also decreased significantly from 9.13 ± 2.68 mm at baseline to 4.65 ± 3.75 mm at the last follow-up (p value 0.05). Subretinal fluid and exudative retinal detachment resolved in all patients, and no significant radiation-related complications were observed in any patient (shown in Fig. 1 and online suppl. Fig. 1–3). None of the patients required further treatment or experienced recurrence within the follow-up period. Treatment outcomes for individual patients are listed in Table 2.
Table 2.
Treatment details and outcomes
| Plaque type | Apex dose, Gy | Treatment duration, h | BCVA | Height, mm | Diameter, mm | Complications | Reccurence | Follow-up duration, months | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre | post | pre | post | pre | post | |||||||
| 1 | COB | 50 | 72 | 0.05 | 0.9 | 3.35 | 2.77 | 6.6 | 4.9 | No | No | 6 |
| 2 | CCB | 50 | 72 | CF50 | 0.8 | 4.02 | Flat | 10.5 | Flat | No | No | 16 |
| 3 | COB | 50 | 72 | HM | 0.3 | 3.71 | 2.44 | 8.08 | 7.47 | No | No | 10 |
| 4 | COB | 50 | 144 | HM | 0.2 | 7.57 | Flat | 12.59 | Flat | No | No | 9 |
| 5 | COB | 50 | 144 | HM | 0.1 | 7.03 | 2.40 | 12.11 | 10.29 | No | No | 24 |
| 6 | COB | 50 | 48 | 0.1 | 0.8 | 3.53 | 2.57 | 5.58 | 4.07 | No | No | 15 |
| 7 | CCB | 50 | 34 | 0.05 | 0.4 | 4.09 | 1.78 | 8.43 | 5.58 | No | No | 8 |
BCVA, best corrected visual acuity; Gy, Gray.
Discussion
CCHs are rather uncommon congenital vascular tumors that are more frequently encountered in Caucasians and show a slight male predilection. Unlike their diffuse counterparts, CCHs are usually not correlated with systemic disease. However, 2% of CCHs are associated with ipsilateral facial hemangiomas and other features of Sturge-Weber syndrome [1, 2].
CCHs are usually present in the posterior pole as an orange-red mass that ranges in size from 3 to 19 mm in diameter and 1–8 mm in thickness. Their clinical presentation may range from incidental discovery on routine examination to metamorphopsia, blurring of vision and visual field loss related to subretinal fluid accumulation, lipid exudation, and exudative retinal detachment. Other associated findings may include neovascularization of the iris, RPE hyperplasia, and retinoschisis [1, 2].
Several posterior pole lesions may mimic CCHs, including amelanotic choroidal melanomas, choroidal metastasis, and other causes of exudative retinal detachment. These include central serous chorioretinopathy, choroidal melanoma, choroidal metastasis, and posterior nodular scleritis. The most concerning of these are choroidal melanomas and choroidal metastases because of their risk of ocular and systemic morbidity and mortality [1, 2].
In addition to their unique clinical appearance, a battery of investigations may be deployed to rule out mimicking conditions, including ultrasonography, fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA), and optical coherence tomography angiography. On ultrasonography, CCHs tend to exhibit high internal reflectivity owing to their vascular elements, which is considered an important differentiating factor from choroidal melanomas that tend to demonstrate characteristic acoustic hollowness and low to medium internal reflectivity [1, 4]. On the other hand, FFA in these lesions presents with an early lacy pattern that increases in the late stages, corresponding to the subretinal leakage frequently seen in these cases. ICGA gives a similar picture to FFA with a more pronounced picture of the choroidal vasculature. The choroidal filling in FFA and IGCA is slower and less intense in choroidal melanoma and metastasis [1, 5].
OCT may be helpful in demonstrating and quantifying macular edema and lipid exudation as well as monitoring foveal involvement. In EDI-OCT, CCH shows a smooth, sloping anterior surface with expansion of medium and large choroidal vessels without choriocapillaris compression, a feature which helps in differentiation from choroidal melanoma [6, 7]. Optical coherence tomography angiography can show the irregular vascular network within the CCH and can be used to monitor response to treatment [8]. Despite the above, the results of ancillary testing may remain inconclusive in some cases in which a biopsy may be needed to confirm the diagnosis.
Various treatment strategies have been proposed for the management of CCHs. Asymptomatic cases with no exudation involving the macula may be regularly followed up for signs of progression. In more advanced cases with affection of the central vision, the primary strategy is induction of tumor regression, resorption of macular edema and exudation as well as prevention against the development of neovascular glaucoma [1].
Historically, the first management option for these cases was xenon arc photocoagulation, which was later switched to argon photocoagulation. The premise behind photocoagulation was halting of the vascular permeability of the tumor, thus inducing fluid regression. Despite limited success, many of these cases suffered significant chorioretinal scarring and poor visual outcome which limited the use of photocoagulation to more peripheral lesions in which central vision may be spared [1, 9–11].
With advances in laser technology, the diode laser emerged as a more precise option capable of penetrating deeper tissues, inducing a thermal occlusion of blood vessels with minimal affection of the surrounding healthy tissue, a process known as TTT. TTT has been explored as a primary option for small lesions measuring less than 10 mm in diameter and 4 mm in thickness, or as an adjunct treatment modality in larger tumors. Despite resolution of subretinal fluid in most patients and regression of tumor thickness, the visual outcomes were not impressive, and most patients required multiple TTT sessions, which is more likely to be associated with pigmentary epithelial affection and chorioretinal atrophy with visual field and acuity loss. Furthermore, TTT may result in the development of other complications, such as retinal tears, preretinal fibrosis, and vascular occlusions [2, 12, 13].
In recent years, PDT has been explored in multiple retinochoroidal pathologies as a selective method to occlude vascular lesions. This is achieved by injecting a photochemical known as Verteporfin, followed by the application of a laser beam that reacts with the chemical and induces the destruction of the tissue. Owing to the selective application of these burns, PDT has been utilized in subfoveal CHHs with sparing of the overlying retinal tissue [12–16]. Eighty-five percent of the patients had an improvement in visual acuity (mean 3.3 lines), and the mean tumor height declined from 3.0 mm to 0.4 mm after an average of two PDT treatments [17].
For decades, larger tumors and those with extensive subretinal fluid have posed a more difficult conundrum. Photocoagulation and TTT have not been beneficial in providing adequate regression of the tumor activity and have thus gone largely unmanaged. With the introduction of radiation therapy for the management of benign and malignant growths, numerous attempts have been made at exploring its role in larger CCHs [18–21].
External beam irradiation has been deployed to larger, more diffuse lesions allowing treatment of the entire choroid homogenously and has demonstrated favorable results in induction of tumor regression [22]. However, EBR is often associated with slow absorption of subretinal fluid hindering the resultant visual outcome. In addition, the use of radiation therapy is not without its possible list of complications. Cataracts, radiation retinopathy, optic neuropathies, and dry eye have all been reported [17, 18, 23].
To minimize collateral damage brought on by the irradiation, proton beam radiotherapy and gamma knife radiosurgery may be useful, more precise alternative treatments to external beam therapy especially in larger and more diffuse hemangiomas. In a series of 71 patients treated using proton beam therapy with a dose of 20 Gy, tumor regression and retinal reattachment were achieved in all patients and visual acuity improved in 52% of patients [19]. Kong et al. [20] reported comparable results with gamma knife radiosurgery. These treatments, however, are limited by their unavailability in most centers and the relatively expensive cost.
Brachytherapy involves the introduction of a radioactive plaque to the episclera until the required dosage of radiation has been delivered to the tumor. It was first described in management of CCH by MacLean and Maumenee [24] in 1960 when they used Radon seeds [24]. A variety of isotopes have been used as cobalt-60 [25], palladium-103 [17], iodine-125 [3, 23], and ruthenium-106 [8, 23, 26–28]. Ruthenium-106 plaques, emitting beta particles, have a lower penetration rate than iodine-125 offering the advantage of delivering a limited range of radiation to a very restricted volume [29]. Brachytherapy was successful in improving visual acuity and inducing tumor regression with resolution of subretinal fluid in most cases. It offers the advantage of targeted delivery of radiation to the tumor while sparing healthy tissues (Table 3).
Table 3.
Treatment outcome of brachytherapy in circumscribed choroidal hemangiomas in different studies
| Present study | Li et al. [26] | Madreperla et al. [23] | Naseripour et al. [27] | Joshi et al. [28] | Cennamo et al. [8] | Aizman et al. [17] | Lopez-Caballero et al. [3] | Zografos et al. [25] | |
|---|---|---|---|---|---|---|---|---|---|
| Patients, n | 7 | 25 | 8 | 21 | 8 | 7 | 5 | 8 | 41 |
| Isotope | Ru | Ru | Ru and I | Ru | Ru | Ru | Pal | I | Co |
| Mean dose, Gy | 50 | 84.4 | 50 | 38.5 | 32.5 | 100 | 29 | 46.9 | 50 |
| Improved VA, % | 100 | 56 | 63 | 57 | NA | NA | 60 | 0 | NA |
| Mean tumor height, mm | |||||||||
| Baseline | 4.76 | 3.99 | 4.8 | 3.87 | 5.0 | 4.46 | 3.2 | 4.4 | 2.9 |
| Final visit | 1.70 | 0.84 | 2.1 | 0.7 | NA | 2.29 | 1.6 | 1.19 | NA |
| Mean largest diameter, mm | |||||||||
| Baseline | 9.13 | 9.36 | 10.6 | 10.0 | 12.7 | 9.27 | 8.32 | 11.3 | 8.1 |
| Final visit | 4.65 | 7.40 | NA | 8.33 | NA | 4.76 | NA | NA | NA |
| Radiation-related complications, n (%) | |||||||||
| Retinopathy | 0 | 2 (8) | 0 | 5 (23.8) | 0 | NA | 1 (20) | 3 (37.5) | 3 (7.3) |
| Papillopathy | 0 | 0 | 0 | 1 (4.8) | 0 | NA | 0 | 0 | 0 |
| Mean FU, months | 12.5 | 28 | 25 | 38.6 | NA | 12 | 18.6 | 83 | 24–120 |
Co, cobalt-60; I, iodine-125; Pal, palladium-103; Ru, ruthenium-106; VA, visual acuity; FU, follow-up.
In the current series, brachytherapy was used as the first line of management of symptomatic CCHs. Although PDT might be considered the treatment of choice in many centers over the past years, however, the unavailability of the dye in our country, especially after the era of anti-VEGFs, has limited its role and urged the need for an alternative management option as brachytherapy. Ruthenium-106 plaque was used in all cases, since it was the only available isotope at our facility; all cases showed a significant reduction in tumor size with a resolution of subretinal fluid and exudative retinal detachment, consistent with findings in other studies.
We, however, noticed an improvement in visual acuity in 100% of cases compared to about 60% in most other reported series (Table 3). While Li et al. [26] used a higher radiation dose than our study, the other 3 reports with lower visual improvements used similar and even lower radiation dose. The first explanation could be the relatively short duration between the onset of symptoms and the application of plaque in our series (2.43 months) compared to 9.7 months in the series by Naseripour et al. [27]. The longer the duration of the subretinal fluid accumulation and exudative retinal detachment, the more the photoreceptor degenerative changes with subsequent reduced visual prognosis, especially in patients with macular involvement [1]. This highlights the importance of prompt treatment of symptomatic CCHs.
A second explanation could be the shorter follow-up duration in our study (12.5 months) compared to other studies with longer follow-up duration allowing for the appearance of radiation-related complications. Radiation retinopathy, papillopathy, and other radiation-induced complications were not detected in any of the patients in the current series. In contrast, radiation retinopathy and papillopathy were detected in Naseripour’s series in 23.8% and 4.8% of patients, respectively, despite the lower mean apex dose (38.5 Gy), probably due to the relatively longer follow-up duration of 38.6 months in that series [27]. Long-term follow-up is mandatory for early detection and prompt management of radiation-induced complications. In addition to the relatively short follow-up duration, the current series is also limited by its retrospective nature and small sample size.
In conclusion, CCHs are uncommon benign choroidal tumors that result in loss of central vision predominantly through macular edema and exudative retinal detachment. Ultrasonography, FFA, ICGA, and OCT all play a pivotal role in evaluating the damage caused by these lesions and excluding mimicking conditions. Observation may be warranted in small peripheral lesions with dry maculae, while larger tumors affecting the macula or causing leakage affecting the central vision may be managed with TTT, PDT, external beam, and proton pump radiotherapy as well as brachytherapy depending on the tumor height, width, and location. Early management is crucial for a better visual prognosis. Large comparative studies are needed to determine the superiority of any of these treatment modalities over the other.
Statement of Ethics
All procedures performed were in accordance with the ethical standards of the Research Ethics Committee of the Faculty of Medicine, Cairo University (Approval No. N-6-2024). Written informed consent was obtained from all adult patients and from the legal guardian of the patient who is younger than 18 years old, for participation in this study and for publication of the details of their condition and any accompanying images.
Conflict of Interest Statement
All authors certify that they have no conflict of interest in the subject matter or materials discussed in this manuscript.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
I.Y.S., H.H., A.M.K., M.H.E., M.A.E., T.A.M., D.H.H., S.H.S., A.M.A., A.M.N., Y.A.M., S.F.A., L.E., and A.E.F. contributed to the clinical, ancillary, and surgical care of the patients, as well as the retrospective data acquisition and analysis. I.Y.S. drafted the manuscript. T.A.M., A.E.F., and H.H. revised the manuscript. All authors approved the final version.
Funding Statement
This study was not supported by any sponsor or funder.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.
Supplementary Material.
Supplementary Material.
Supplementary Material.
References
- 1. Shields CL, Honavar SG, Shields JA, Cater J, Demirci H. Circumscribed choroidal hemangioma: clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108(12):2237–48. [DOI] [PubMed] [Google Scholar]
- 2. Sen M, Honavar SG. Circumscribed choroidal hemangioma: an overview of clinical manifestation, diagnosis and management. Indian J Ophthalmol. 2019;67(12):1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopez-Caballero C, Saornil MA, de Frutos J, Bianciotto C, Muiños Y, Almaraz A, et al. High-dose iodine-125 episcleral brachytherapy for circumscribed choroidal haemangioma. Br J Ophthalmol. 2010;94(4):470–3. [DOI] [PubMed] [Google Scholar]
- 4. Fionda B, Pagliara MM, Lancellotta V, Caputo CG, Casà C, Sammarco MG, et al. Radiological and clinical findings in uveal melanoma treated by plaque interventional radiotherapy (brachytherapy): visual atlas and literature review on response assessment. J Contemp Brachytherapy. 2022;14(1):96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shields CL, Shields JA, De Potter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol. 1995;79(3):237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rojanaporn D, Kaliki S, Ferenczy SR, Shields CL. Enhanced depth imaging optical coherence tomography of circumscribed choroidal hemangioma in 10 consecutive cases. Middle East Afr J Ophthalmol. 2015;22(2):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heimann H, Jmor F, Damato B. Imaging of retinal and choroidal vascular tumours. Eye. 2013;27(2):208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cennamo G, Rossi C, Breve MA, Velotti N, Farella A, Liuzzi R, et al. Evaluation of vascular changes with optical coherence tomography angiography after ruthenium-106 brachytherapy of circumscribed choroidal hemangioma. Eye. 2018;32(8):1401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanborn GE, Augsburger JJ, Shields JA. Treatment of circumscribed choroidal hemangiomas. Ophthalmology. 1982;89(12):1374–80. [DOI] [PubMed] [Google Scholar]
- 10. Anand R, Augsburger JJ, Shields JA. Circumscribed choroidal hemangiomas. Arch Ophthalmol. 1989;107(9):1338–42. [DOI] [PubMed] [Google Scholar]
- 11. Shields JA. The expanding role of laser photocoagulation for intraocular tumors: the 1993 H. Christian Zweng Memorial Lecture. Retina. 1994;14(4):310–22. [DOI] [PubMed] [Google Scholar]
- 12. García-Arumí J, Sararols Ramsay L, Corcostegui Guraya B. Transpupillary thermotherapy for circumscribed choroidal hemangiomas. Ophthalmology. 2000;107(2):351–6. [DOI] [PubMed] [Google Scholar]
- 13. Gündüz K. Transpupillary thermotherapy in the management of circumscribed choroidal hemangioma. Surv Ophthalmol. 2004;49(3):316–27. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt-Erfurth UM, Michels S, Kusserow C, Jurklies B, Augustin AJ. Photodynamic therapy for symptomatic choroidal hemangioma: visual and anatomic results. Ophthalmology. 2002;109(12):2284–94. [DOI] [PubMed] [Google Scholar]
- 15. Robertson DM. Photodynamic therapy for choroidal hemangioma associated with serous retinal detachment. Arch Ophthalmol. 2002;120(9):1155–61. [DOI] [PubMed] [Google Scholar]
- 16. Porrini G, Giovannini A, Amato G, Ioni A, Pantanetti M. Photodynamic therapy of circumscribed choroidal hemangioma. Ophthalmology. 2003;110(4):674–80. [DOI] [PubMed] [Google Scholar]
- 17. Aizman A, Finger PT, Shabto U, Szechter A, Berson A. Palladium 103 (103Pd) plaque radiation therapy for Circumscribed Choroidal hemangioma with retinal detachment. Arch Ophthalmol. 2004;122(11):1652–6. [DOI] [PubMed] [Google Scholar]
- 18. Ritland J, Eide N, Tausjø J. External beam irradiation therapy for choroidal haemangiomas. Visual and anatomical results after a dose of 20 to 25 Gy. Acta Ophthalmol Scand. 2001;79(2):184–6. [DOI] [PubMed] [Google Scholar]
- 19. Levy-Gabriel C, Rouic LLL, Plancher C, Dendale R, Delacroix S, Asselain B, et al. Long-term results of low-dose proton beam therapy for circumscribed choroidal hemangiomas. Retina. 2009;29(2):170–5. [DOI] [PubMed] [Google Scholar]
- 20. Kong D-S, Lee J-I, Kang S-W. Gamma knife radiosurgery for choroidal hemangioma. Am J Ophthalmol. 2007;144(2):319–22. [DOI] [PubMed] [Google Scholar]
- 21. Pagliara MM, Tagliaferri L, Savino G, Fionda B, D’Aviero A, Lanza A, et al. High-dose-rate interstitial brachytherapy (interventional radiotherapy) for conjunctival melanoma with orbital extension. Ocul Oncol Pathol. 2021;7(3):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schilling H, Sauerwein W, Lommatzsch A, Friedrichs W, Brylak S, Bornfeld N, et al. Long term results after low dose ocular irradiation for choroidal haemangiomas. Br J Ophthalmol. 1997;81(4):267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madreperla SA, Hungerford JL, Plowman PN, Laganowski HC, Gregory PT. Choroidal hemangiomas: visual and anatomic results of treatment by photocoagulation or radiation therapy. Ophthalmology. 1997;104(11):1773–9. [DOI] [PubMed] [Google Scholar]
- 24. Maclean AL, Maumenee E. Hemangioma of the choroid. Am J Ophthalmol. 1960;50(1):3–11. [DOI] [PubMed] [Google Scholar]
- 25. Zografos L, Bercher L, Chamot L, Gailloud C, Raimondi S, Egger E. Cobalt-60 treatment of choroidal hemangiomas. Am J Ophthalmol. 1996;121(2):190–9. [DOI] [PubMed] [Google Scholar]
- 26. Li J, Jin E-Z, Liang J-H. High-dose ruthenium-106 plaque therapy for circumscribed choroidal hemangioma: a retrospective study of 25 Chinese patients. Int J Ophthalmol. 2020;13(3):425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naseripour M, Maleki A, Astaraki A, Sedaghat A, Jaberi R, Lee S, et al. Ruthenium-106 brachytherapy in the treatment of circumscribed choroidal hemangioma. Retina. 2018;38(5):1024–30. [DOI] [PubMed] [Google Scholar]
- 28. Joshi S, Reddy V, Ganesa P, Ali M, Naik M, Honavar S. Ruthenium 106 plaque brachytherapy: indications and outcome in ocular tumors. J Cancer Res Ther. 2009;5(2):S88. [Google Scholar]
- 29. Tagliaferri L, Pagliara MM, Fionda B, Scupola A, Boldrini L, Caputo CG. Uveal melanoma with thickness between 4 and 6 mm treated with two different radioisotopes (I125 or ru106): single institution experience. Turk J Oncol. 2020;35(3). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.