Abstract
Introduction Endoscopic endonasal surgery has globally improved postoperative results in pituitary adenomas.
Material and Methods We retrospectively analyzed 101 patients who underwent endonasal endoscopic surgery for pituitary adenomas in the period from 2016 to 2021. Data on epidemiological variables, preoperative radiological factors including tumor volume, tumor appearance, cavernous sinus invasion (modified Knosp scale), degree of extension according to the SIPAP (stands for the five directions in which a pituitary adenoma can extend: suprasellar, infrasellar, parasellar, anterior, and posterior) classification, and preoperative visualization of the healthy gland on magnetic resonance imaging (MRI) were collected as well as intra- and postoperative cerebrospinal fluid (CSF) leak. As variables of interest, data on the degree of tumoral resection and preservation of hormonal function were collected.
Results Among the preoperative factors related to greater tumoral resection, we found a lesser tumoral extension according to the SIPAP scale, and the absence of a postoperative CSF leak had a statistically significant relation with greater hormonal preservation.
Conclusion The SIPAP classification is a simple-to-measure preoperative radiological variable that could predict the extent of resection, and, conversely, the occurrence of a postoperative CSF leak has been associated with an inferior endocrinological outcome in this type of surgery.
Keywords: pituitary adenoma, endoscopic endonasal approach, hormonal deficiency, CSF leak, sellar barrier, Knosp, SIPAP
Introduction
Pituitary adenomas are a diverse group of tumors originating from the pituitary gland. 1 These are one of the most frequently occurring central nervous system primary neoplasms in adult patients, accounting for 10 to 15% of all intracranial tumors, being the most frequent type of tumor in the sellar area. 2 The prevalence ranges between 80 and 90 cases per 100,000 inhabitants, although some studies based on autopsies and imaging studies show that the prevalence could be up to 16.7%. The average age of presentation is 40 years and there is a slight female predominance (55–65%). 3 4
The use of endoscopy through the endonasal route represents one of the latest advances in this type of surgery, allowing a better exposure of the surgical field with a minimally invasive approach. This technique allows a more complete tumor resection and it also reduces the risk of complications compared with other approaches, which is why, nowadays, it is the route of choice in the vast majority of cases. 5 6 The most common complications of using this approach are cerebrospinal fluid (CSF) leak and hormone dysfunction with good presurgical function, with transient diabetes insipidus being the most common one. 7 One of the most novel concepts in this field is the sellar barrier, which is defined as the interface between the pituitary tumor and the suprasellar cistern, composed of three layers: arachnoid tissue, sellar diaphragm dura mater, and the pituitary gland. The type of sellar barrier can predict the degree of intraoperative CSF leak and therefore point out those patients who may present this complication in the postoperative period. 8
One of the main goals in pituitary surgery is hormonal preservation or restoration of hormonal function altered by tumor effects. The endocrinological results in pituitary surgery are heterogeneous and therefore difficult to compare. 9 10
The preoperative evaluation of each case is extremely important to achieve an optimal postoperative result. This presurgical study is fundamentally based on imaging tests and more specifically on brain magnetic resonance imaging (MRI). There are numerous studies that describe the preoperative factors that predict the extent of resection, among which we find the degree of invasion of the cavernous sinus of Knosp. 11 12 13 14 However, there is little evidence about the preoperative variables that could predict the endocrinological outcome.
Hypotheses and Objectives
Some anatomical and radiological aspects of pituitary adenomas could predict the endocrinological outcome in pituitary endoscopic endonasal surgery. Therefore, the primary objective of this study is to analyze the preoperative anatomical and radiological variables of pituitary adenomas, among which we find tumor volume, cavernous sinus invasion (modified Knosp scale), degree of extension according to the SIPAP classification, preoperative visualization of the healthy gland (adenohypophysis, neurohypophysis, and pituitary stalk), intraoperative and postoperative CSF leak, and its relation to the degree of hormonal preservation as well as to the degree of resection.
Material and Methods
Study Population
It is a retrospective analytical study. Data have been collected from 101 patients diagnosed with pituitary adenoma who underwent endoscopic endonasal pituitary surgery at the General University Hospital of Alicante from January 2016 to December 2021. We established as inclusion criteria the following: clinical diagnosis of pituitary adenoma that involve a hormonal dysfunction of any of the axis of the gland or visual field disturbances due to tumoral growth and imaging confirmation of pituitary adenoma including both micro- and macroadenomas as well as functioning and nonfunctioning adenomas that underwent endoscopic endonasal surgery. The exclusion criteria included pediatric cases and patients who needed a transcranial or combined approach.
Data Collection
Demographic data such as age and sex, structural characteristics of the tumor, differentiating between micro- and macroadenomas, as well as functional characteristics of each adenoma type were collected.
All the patients had an MRI according to the pituitary protocol, in which a series of anatomical and radiological variables were evaluated. The pituitary protocol, based on a 3.0-T scanner, consists of turbo spin echo T1 sequences, both with and without gadolinium centered in the pituitary region. Images were obtained in the three planes: axial, coronal, and sagittal with a thickness of 1 mm. In addition, T2, fluid-attenuated inversion recovery (FLAIR) sequences, diffusion weighted imaging (DWI), and apparent diffusion coefficient (ADC) map were obtained.
In the first place, the tumor volume measured through a software incorporated in the neuronavigation system “Stealth Station” Medtronic was determined ( Fig. 1 ). This tool analyzes the coronal slices of the brain MRI selecting in each two-dimensional slice the surface that represents the adenoma. Once the tumor surface in each section is selected, the software integrates this information with the thickness of the MRI slices, obtaining a three-dimensional model of the tumor with its volume. This is a novel form of surgical planning since previously the tumor volume was calculated by the simplified ellipsoid formula: height × width × depth divided by 2. The current tool allows the tumor volume to be delimited more precisely since not all pituitary adenomas have an ellipsoid shape and can be very irregular and asymmetric. They can also include other structures inside such as the internal carotid artery in its intracavernous portion that can be excluded with the Stealth as opposed to the estimation with the formula. 15
Fig. 1.
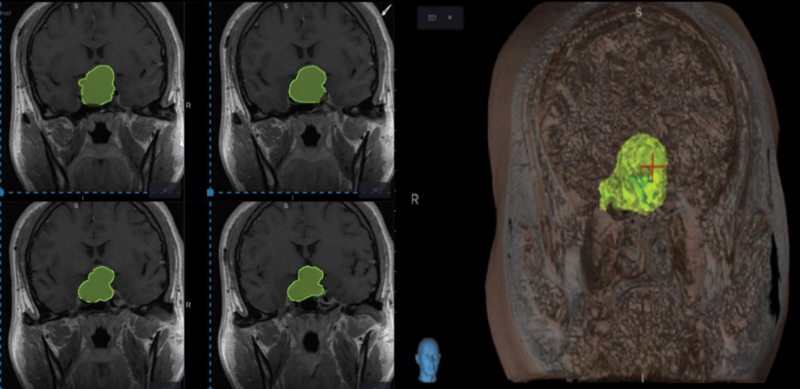
Estimating tumor volume and creating a 3D model of the tumor with neuronavigation system.
On the other hand, the invasion of the cavernous sinus has been evaluated in the coronal section of the brain MRI according to the modified Knosp scale, differentiating in degrees 0, 1, 2, 3A, 3B, and 4. 16 The Knosp classification is one of the widely known systems to describe the cavernous sinus invasion by pituitary adenomas. Based on coronal MRI section, three lines are drawn between the supraclinoid carotid artery: lateral tangent, intercarotid line, and medial tangent. Cavernous sinus invasion is determined by whether the tumor remains medial or exceeds one of these lines. The more lateral the tumor extension goes, the greater the cavernous sinus invasion gets considering grade 4 when a total enclosure of intracavernous internal carotid artery is found. In addition, tumor extension data were collected and classified according to the SIPAP scale. 17 SIPAP is an acronym for the five juxtasellar directions of pituitary adenoma growth near or into adjacent structures of the sella turcica region. This classification takes into account tumoral extension to the Suprasellar, Infrasellar, Parasellar (left and right), Anterior, and Posterior directions.
Likewise, glandular visualization and tumor characteristics on the preoperative MRI data were collected. In coronal sections on T1 sequence enhanced with gadolinium, the visualization of the gland and the aspect of the pituitary stalk have been evaluated, defined as thickened if it exceeded 2 mm in width. In the sagittal plane of the T1 sequence, the neurohypophysis data were collected, which are seen as hyperintense in the posterior part of the gland ( Fig. 2 ). 18 On the other hand, tumor appearance information has been collected, classifying it as solid (isointense in T1 and T2), cystic (hypointense on T1 and hyperintense on T2), and hemorrhagic (hyperintense on T1 and T2; Fig. 3 ).
Fig. 2.
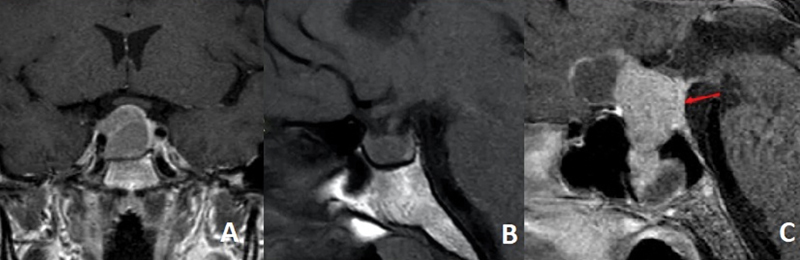
Visualization of pituitary gland in the preoperative MRI. (A) Coronal MRI section T1 with gadolinium. Anterior pituitary gland pushed to the left enhanced. (B) Sagital MRI section T1 without gadolinium. Posterior pituitary gland can be seen hyperintense in the posterior part of the hypofisis. (C) Sagital MRI section T1 with gadolinium. Pituitary stalk is pushed backwards by the tumor and enhanced with gadolinium.
Fig. 3.
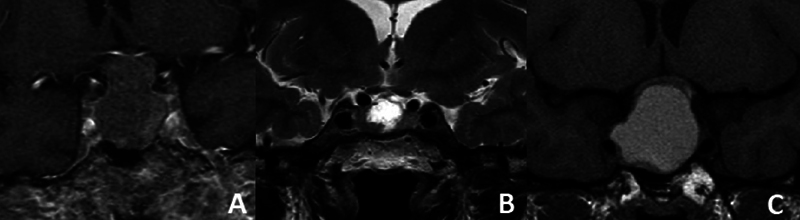
Tumoral appearance at the preoperative MRI. (A) Solid (isointense in T1 and T2). (B) Cystic (hypointense on T1 and hyperintense on T2). (C) Hemorrhagic (hyperintense on T1 and T2).
In addition, we recorded all the cases that presented intraoperative CSF leak regardless of whether or not it persisted after surgery. The intraoperative CSF leak was managed by the otorhinolaryngology team, usually by a Hadad flap and hemostatic agents such as Surgicel, Spongostan, or similar. We considered a postoperative CSF leak when the patient presented symptoms of rhinolicuerrea after the surgery regardless of the time interval between the surgery and its occurrence. The postoperative CSF leak was managed by an external lumbar drainage and antibiotic prophylactic treatment to prevent meningitis.
All the patients present a postoperative study with contrast-enhanced brain MRI performed 3 months after surgery in which the degree of tumor resection was evaluated. “Gross total resection” has been designated to refer to surgical procedures in which the removal of the tumor was complete or 100%, while “subtotal resection” has been used to describe cases where the tumoral tissue removal was less than 100%.
Finally, the status of hormonal function before and after surgery was recorded. In the preoperative period, hormonal deficiency has been defined as an alteration of at least one anterior pituitary hormonal axis as well as diabetes insipidus. After the intervention, postoperative hormonal deficiency has been defined as an alteration of at least one previously functioning anterior pituitary axis as well as diabetes insipidus. Transient diabetes insipidus has not been considered as an added hormonal deficiency.
Statistical Analysis
First, a descriptive study of all the variables (age, sex, tumor volume, tumor appearance, etc.) was performed. For each of the variables, the absolute and relative frequency was calculated in percentage. Next, to study the association between the explanatory variables (age, sex, tumor volume, tumor appearance, etc.) and the incomplete tumor resection and postoperative hormone deficiency, the chi-squared test was used, and to quantify the magnitude of association, the crude odds ratio (OR) and the adjusted odds ratio (ORa) with their 95% confidence intervals (95% CI) using a logistic regression model, in which those variables with a p < 0.20 were introduced, were calculated. The level of statistical significance was p < 0.05. The analysis was performed using IBM SPSS Statistics v.25.0.
Ethical Approval
Due to the retrospective nature of the study, an official waiver of ethical committee approval was obtained. This was because the study did not involve any intervention or interaction with the participants and only relied on the analysis of existing data. The waiver of ethical committee approval was obtained in accordance with the guidelines and regulations established by the responsible authorities.
Results
The mean age of the study population was 54.41 years with a standard deviation of 15.22 years. The female sex was predominant representing 54.45%. Regarding the structural classification, macroadenoma represented 89.9%. Nonfunctioning adenoma was the most prevalent hormonal type representing 75.24%, followed by growth hormone (GH) secreting adenoma with 12.87%, followed by prolactinoma with 6.93%, and finally adrenocorticotropic hormone (ACTH) secreting adenoma with 4.95%. The indication of surgical treatment of a prolactinoma was persistent hormone dysfunction and/or progressive visual field deficit despite dopamine agonist therapy. No thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), or luteinizing hormone (LH) secreting adenomas have been recorded.
The mean tumor volume was 6.02 cm 3 with a standard deviation of 6.06 cm 3 and a median of 3.25 cm 3 . A significant occupation of the cavernous sinus (Knosp grade 3–4) was seen in 45.45% of the cases ( Table 1 ).
Table 1. Demographic data and tumoral characteristics of the sample ( n = 101) .
| Age | Mean | Standard deviation | ||
| 54.51 y | 15.22 y | |||
| Sex | Male | Female | ||
| 46 (45.55%) | 55 (54.45%) | |||
| Size | Microadenoma | Macroadenoma | ||
| 11 (10.89%) | 90 (89.1%) | |||
| Tumor volume | Mean | Standard deviation | ||
| 6.02 cm 3 | 6.06 cm 3 | |||
| Knosp grades | 1–2 | 3–4 | ||
| 55 (54.45%) | 46 (45.55%) | |||
| Functional status | Nonfunctioning | Prolactinoma | GH | ACTH |
| 76 (75.24%) | 7 (6.93%) | 13 (12.87%) | 5 (4.95%) | |
Abbreviations: ACTH, adrenocorticotropic hormone; GH, growth hormone.
Regarding the factors that demonstrated a statistically significant correlation with gross total resection, our analysis revealed that cases with a tumor volume below the median value of 3.25 cm 3 exhibited an OR of 6.0 (95% CI: 2.2–16.6; p < 0.001) for achieving gross total resection, compared with cases with a tumor volume above 3.25 cm 3 .
A lower extension according to the SIPAP scale also showed a statistically significant association with gross total resection, for those cases with SIPAP 0 to 4 (low grade of extension), the OR for gross total resection was 19.1 (95% CI: 5.1–72.5; p < 0.001), and for SIPAP 5 to 8 8.1 (95% CI: 2.3–28.8; p = 0.001) compared with SIPAP 9 or more. Our analysis demonstrated a statistically significant correlation between a lower Knosp cavernous sinus invasion grade and gross total resection, with an OR of 4.0 (95% CI: 1.6–9.8; p = 0.002), compared with cases with a Knosp grade of 3 to 4.
Upon conducting a multivariable analysis, some of the previously identified associations were found to be no longer statistically significant. However, the correlation between a lower SIPAP extension and gross total resection remained significant, with an ORa of 6.6 (95% CI: 5.1–72.5; p = 0.025) for cases with an SIPAP score of 0 to 4, and an ORa of 7.1 (95% CI: 1.9–26.0; p = 0.003) for cases with a SIPAP score of 5 to 8, as compared with those with an SIPAP score of 9 or more. No statistically significant association was observed between patients' age or tumoral characteristics and gross total resection ( Table 2 ).
Table 2. Aspects and their association with gross total resection ( n = 101) .
| Gross total resection % (number) |
OR (CI 95%) | p | ORa (CI 95%) | Pa | ||
|---|---|---|---|---|---|---|
| Age (y) | < 60 | 74.6 (44/59) | 1.5 (0.6–3.5) | 0.387 | – | – |
| ≥60 | 66.7 (28/42) | 1 | ||||
| Volume (cm 3 ) | ≤3.25 | 88.0 (44/50) | 6.0 (2.2–16.6) | < 0.001 | 3.1 (0.8–12.8) | 0.115 |
| > 3.25 | 54.9 (28/51) | 1 | 1 | |||
| SIPAP | 0–4 | 87.2 (41/47) | 19.1 (5.1–72.5) | < 0.001 | 6.6 (5.1–72.5) | 0.025 |
| 5–8 | 74.3 (26/35) | 8.1 (2.3–28.8) | 0.001 | 7.1 (1.9–26.0) | 0.003 | |
| ≥9 | 26.3 (5/19) | 1 | 1 | |||
| Knosp grade | 0–2 | 82.3 (51/62) | 4.0 (1.6–9.8) | 0.002 | 0.5 (0.2–1.6) | 0.303 |
| 3–4 | 53.8 (21/39) | 1 | 1 | |||
| Tumoral aspect | Solid | 72.6 (61/84) | 1.1 (0.2–5.8) | 0.946 | – | – |
| Cystic | 60.6 (6/10) | 0.6 (0.8–4.7) | 0.629 | |||
| Hemorrhagic | 71.4 (5/7) | 1 |
Abbreviations: CI 95%, 95% confidence interval; OR, odds ratio; Ora, adjusted odds ratio; p , level of statistical significance; Pa, adjusted level of statistical significance.
Regarding the association between preoperative factors and postoperative hormonal deficiency, our analysis revealed no statistically significant correlation between any of the tumoral characteristics measured (such as volume, SIPAP score, Knosp grade, and gland visualization, among others) and the occurrence of postoperative hormonal deficiency. The only factor that exhibited a significant association with postoperative hormonal deficiency was the presence of a CSF leak after the surgery. Patients who experienced a postoperative CSF leak exhibited an OR of 38.6 (95% CI: 3.8–384; p < 0.001) for developing an additional hormonal deficiency, as compared with those without a CSF leak ( Table 3 ).
Table 3. Aspects and their association with postoperative hormonal deficit ( n = 101) .
| Postoperative hormonal deficit % (number) |
OR (95% CI) | p | ||
|---|---|---|---|---|
| Age (y) | <60 | 83.1 (49/59) | 0.377 (0.1–1.4) | 0.147 |
| ≥60 | 92.9 (39/42) | 1 | ||
| Volume (cm 3 ) | ≤3.25 | 90.0 (45/50) | 1.674 (0.5–5.5) | 0.394 |
| >3.25 | 84.3 (43/51) | 1 | ||
| SIPAP | 0–4 | 89.4 (42/47) | 1.6 (0.337–7.4) | 0.564 |
| 5–8 | 85.7 (30/35) | 1.1 (0.2–5.3) | 0.882 | |
| ≥9 | 84.2 (16/19) | 1 | ||
| Knosp | 0–2 | 91.9 (57/62) | 2.9 (0.886–9.767) | 0.124 |
| 3–4 | 79.5 (31/39) | 1 | ||
| Anterior pituitary visualization pre-surgical | Yes | 87.7 (71/81) | 1.253 (0.3–5) | 0.718 |
| No | 85 (17/20) | 1 | ||
| Anterior pituitary visualization post-surgical | Yes | 85.5 (71/83) | 0.855 (0.8–0.9) | 0.129 |
| No | 100 (14/14) | 1 | ||
| Postpituitary visualization pre-surgical | Yes | 88.1 (37/42) | 1.161 (0.4–3.8) | 0.807 |
| No | 86.4 (51/59) | 1 | ||
| Postpituitary visualization post-surgical | Yes | 87.2 (34/39) | 0.933 (0.3–3.2) | 0.912 |
| No | 87.9 (51/58) | 1 | ||
| Stalk aspect pre-surgical | Normal | 88.1 (37/42) | 4.44 (0,8–24) | 0.087 |
| Thickened | 90.2 (46/51) | 0.6 (1.1–30) | 0.049 | |
| Not visible | 62.5 (5/8) | 1 | ||
| Stalk aspect post-surgical | Normal | 90.2 (37/41) | 4.62 (0.3–63) | 0.087 |
| Thickened | 86.8 (46/53) | 3.28 (0.2–41) | 0.049 | |
| Not visible | 66.7 (2/3) | 1 | ||
| Tumoral aspect | Solid | 88.1 (74/84) | 0 | 0.999 |
| Cystic | 70 (7/10) | 0 | 0.999 | |
| Hemorrhagic | 100 (7/7) | 1 | ||
| CSF leak | No | 90.6 (87/96) | 38.6 (3.8–384) | <0.001 |
| Yes | 20.0 (1/5) | 1 | ||
| Extent of resection | Complete | 90.3 (65/72) | 2.4 (0.737–8) | 0.136 |
| Incomplete | 79.3 (23/29) | 1 |
Abbreviations: 95% CI, 95% confidence interval; CSF, cerebrospinal fluid; OR, odds ratio; p , level of statistical significance.
Discussion
In this single-center retrospective study, we explored the correlation between anatomical and radiological factors and the extent of resection as well as postoperative hormonal function in pituitary adenomas.
The tumoral volume at first had a correlation with the extent of resection, but this correlation was lost when a multivariate analysis was performed. The observed phenomenon could be elucidated by the progressive growth of the adenoma, which renders the surgical resection more intricate due to its spread beyond the sellar region. This frequently results in the infiltration of the cavernous sinus and entanglement of the carotid arteries. Considering dimensional data of the tumor, the volume is a more reliable measure because it gives data of all three planes comparing it, for example, with cross-sectional diameter. Another important aspect is that tumoral volume should be considered when choosing the approach, endoscopic endonasal, or by craniotomy. So, although the volume is an important factor to consider in surgical planning, the SIPAP extension and Knosp invasion grades could give more information about the possible extent of resection, which is described by many authors. 12 13 15 16 19 20 21 22
Knosp grade of invasion of the cavernous sinus is widely described as one of the most important factors to consider before pituitary adenoma surgery, being unfavorable as the grade goes higher. This invasion represents a negative prognostic factor, reducing the probability of success in surgical treatment, in terms of both complete tumor resection and hormonal remission in functioning tumors. 12 13
Dallapiazza et al found that tumors with Knosp grades 0 to 2 and volumes up to 10 cm 3 had more chance to get a gross total resection, with Knosp grade being the only independent predictor in a multivariate analysis. 21 In our study, Knosp grade had a correlation with the extent of resection, but it did not result as an independent predictor in the multivariate analysis.
Knosp is studied not only as a predictor of the extent of resection but also as a recurrence factor. Meij et al reported that larger tumors present a higher rate of microscopic dural invasion than smaller tumors, which causes a higher recur rate. 23
As for the SIPAP extension grade, its correlation with recurrence of a pituitary tumor after endoscopic endonasal resection was described. Tumors with higher SIPAP values had higher rates of recurrence. This classification can be used to assess patients with pituitary adenoma in terms of predicting the recurrence rates and the probability of a further surgery. 24 In our study, SIPAP grade was the only preoperative factor as an independent predictor of the extent of resection. This could be due to the fact that larger tumors extend in the juxtasellar area, which is more difficult to reach by the endoscopic endonasal approach. Another possible reason is that as the extension goes further, more vital structures can get involved, for example, carotid arteries or optic nerves, making total resection unsafe.
The most described endocrinological complications is transient diabetes insipidus. As for permanent diabetes insipidus, the incidence after pituitary surgery is low, in some series reaching 3%. The deficit of some of the axes of the anterior pituitary gland is found in up to 9% of cases permanently in some series. 12 25 26 27 Biamonte et al found that patients with Knosp grades 3 to 4 presented a higher risk of developing a new hormone deficiency after endoscopic endonasal pituitary surgery. Age did not play a significant role in their study. The restoration of previous pituitary deficiency was present in one-sixth of patients and had a correlation with age. 28
Nomikos et al reported that the rate of an added endocrinological deficit after endoscopic endonasal pituitary adenoma surgery was 1.4%. The most frequent one was persistent diabetes insipidus, which required replacement with oral vasopressin. 29
Standard endoscopic endonasal surgery is associated with a 0.5 to 10% risk of CSF leak. In case of the extended approaches, it increases to 5 to 30%. When CSF leak occurs, the most appropriate step is an early surgical revision (first 48 hours), due to its significant impact on reducing the rate of postsurgical meningitis. 12 In our study, we found a correlation between CSF leak and a new hormonal deficit. We propose three hypotheses regarding this phenomenon. The first is related to the aggressiveness in the surgical act. Highly aggressive surgeries could damage the pituitary gland and the arachnoid, leading to CSF leak. One of the recent concepts in this field is the sellar barrier. As mentioned earlier, it is defined as the interface between a pituitary tumor and suprasellar CSF. It can be classified as weak, mixed, and strong. Villalonga et al report that weak sellar barrier is associated with higher rates of CSF leak. 8 Therefore, it could also have a correlation with postoperative hormonal deficiency. Further investigations should be performed to study this supposition.
The second factor to consider is that CSF leak is frequently associated with meningitis, which can also damage the pituitary gland, resulting in a postoperative hormonal deficiency. Magro et al report that meningitis occurred in 3.3% of patients in their series, and it was strongly associated with intraoperative and postoperative CSF leak. 30
The third explanation is related to hormone requirements. A substitutive hormonal treatment is needed in some cases after a pituitary surgery, especially in those cases that had a hormonal deficit prior to the surgery. 31 CSF leak is a complication that adds biological stress to the patient, especially when complicated with meningitis, and this could lead to a depletion of the hormonal reserve, resulting in a new hormonal deficit.
We consider that this article compiles a wide number of preoperative variables and relates them not only to the degree of tumor resection but also to the hormonal prognosis. However, we are aware of the difficulty of drawing strong conclusions with a limited number of patients, and we are sure that further research is needed to improve the evidence.
Conclusion
The tumoral volume, Knosp grade, and SIPAP grade are measurable anatomical and radiological parameters that show significant correlation with the extent of resection during an endoscopic endonasal surgery of pituitary adenomas. These findings provide valuable insights that should be considered when formulating a surgical plan for individual cases. Furthermore, a link was observed between postoperative CSF leak and added hormonal deficits, although the underlying mechanism remains unclear and warrants further investigation.
Footnotes
Conflict of Interest None declared.
References
- 1.Ezzat S, Asa S L, Couldwell W T et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(03):613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 2.Niveiro de Jaime M, Aranda López F I, Peiró Cabrera G. Patología de los adenomas hipofisarios. Rev Esp Patol. 2003;36(04):357–372. [Google Scholar]
- 3.Fontana E, Gaillard R. [Epidemiology of pituitary adenoma: results of the first Swiss study] Rev Med Suisse. 2009;5(223):2172–2174. [PubMed] [Google Scholar]
- 4.Daly A F, Rixhon M, Adam C, Dempegioti A, Tichomirowa M A, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006;91(12):4769–4775. doi: 10.1210/jc.2006-1668. [DOI] [PubMed] [Google Scholar]
- 5.Dehdashti A R, Ganna A, Karabatsou K, Gentili F.Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series Neurosurgery 200862051006–1015., discussion 1015–1017 [DOI] [PubMed] [Google Scholar]
- 6.Goudakos J K, Markou K D, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: a systematic review and meta-analysis. Clin Otolaryngol. 2011;36(03):212–220. doi: 10.1111/j.1749-4486.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 7.Regmi D, Thapa A, Kc B, Shakya B. Endoscopic endonasal transsphenoidal approach to pituitary adenoma: a multi-disciplinary approach. J Nepal Health Res Counc. 2017;15(02):174–177. doi: 10.3126/jnhrc.v15i2.18209. [DOI] [PubMed] [Google Scholar]
- 8.Villalonga J F, Solari D, Cavallo L M et al. The sellar barrier on preoperative imaging predicts intraoperative cerebrospinal fluid leak: a prospective multicenter cohort study. Pituitary. 2021;24(01):27–37. doi: 10.1007/s11102-020-01082-8. [DOI] [PubMed] [Google Scholar]
- 9.Abellán Galiana P, Fajardo Montañana C, Riesgo Suárez P A, Gómez Vela J, Escrivá C M, Lillo V R. Factores pronósticos de remisión a largo plazo tras cirugía transesfenoidal en la enfermedad de Cushing. Endocrinol Nutr. 2013;60(08):475–482. doi: 10.1016/j.endonu.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Giustina A, Barkan A, Casanueva F F et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab. 2000;85(02):526–529. doi: 10.1210/jcem.85.2.6363. [DOI] [PubMed] [Google Scholar]
- 11.Fang Y, Pei Z, Chen H et al. Diagnostic value of Knosp grade and modified Knosp grade for cavernous sinus invasion in pituitary adenomas: a systematic review and meta-analysis. Pituitary. 2021;24(03):457–464. doi: 10.1007/s11102-020-01122-3. [DOI] [PubMed] [Google Scholar]
- 12.López-García R, Abarca-Olivas J, Monjas-Cánovas I, Picó Alfonso A, Moreno-López P, Gras-Albert J R. Cirugía endoscópica endonasal en adenomas hipofisarios: resultados quirúrgicos en una serie de 86 pacientes consecutivos. Neurocirugia (Astur) 2018;29(04):161–169. doi: 10.1016/j.neucir.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Enseñat J, Ortega A, Topcewski T et al. Valor predictivo de la clasificación de Knosp en el grado de resección quirúrgica de los macroadenomas invasivos. Estudio prospectivo de una serie de 23 casos. Neurocirugia (Astur) 2006;17(06):519–526. [PubMed] [Google Scholar]
- 14.Micko A, Oberndorfer J, Weninger W J et al. Challenging Knosp high-grade pituitary adenomas. J Neurosurg. 2019;132(06):1739–1746. doi: 10.3171/2019.3.JNS19367. [DOI] [PubMed] [Google Scholar]
- 15.Cao L, Chen H, Hong J, Ma M, Zhong Q, Wang S. Magnetic resonance imaging appearance of the medial wall of the cavernous sinus for the assessment of cavernous sinus invasion by pituitary adenomas. J Neuroradiol. 2013;40(04):245–251. doi: 10.1016/j.neurad.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Komotar R J, Starke R M, Raper D MS, Anand V K, Schwartz T H. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary. 2012;15(02):150–159. doi: 10.1007/s11102-011-0359-3. [DOI] [PubMed] [Google Scholar]
- 17.Edal A L, Skjödt K, Nepper-Rasmussen H J. SIPAP: a new MR classification for pituitary adenomas. Suprasellar, infrasellar, parasellar, anterior and posterior. Acta Radiol. 1997;38(01):30–36. doi: 10.1080/02841859709171238. [DOI] [PubMed] [Google Scholar]
- 18.Cho C H, Barkhoudarian G, Hsu L, Bi W L, Zamani A A, Laws E R. Magnetic resonance imaging validation of pituitary gland compression and distortion by typical sellar pathology. J Neurosurg. 2013;119(06):1461–1466. doi: 10.3171/2013.8.JNS13496. [DOI] [PubMed] [Google Scholar]
- 19.Chohan M O, Levin A M, Singh R et al. Three-dimensional volumetric measurements in defining endoscope-guided giant adenoma surgery outcomes. Pituitary. 2016;19(03):311–321. doi: 10.1007/s11102-016-0709-2. [DOI] [PubMed] [Google Scholar]
- 20.Sol Y L, Lee S K, Choi H S, Lee Y H, Kim J, Kim S H. Evaluation of MRI criteria for cavernous sinus invasion in pituitary macroadenoma. J Neuroimaging. 2014;24(05):498–503. doi: 10.1111/j.1552-6569.2012.00710.x. [DOI] [PubMed] [Google Scholar]
- 21.Dallapiazza R F, Grober Y, Starke R M, Laws E R, Jr, Jane J A., JrLong-term results of endonasal endoscopic transsphenoidal resection of nonfunctioning pituitary macroadenomas Neurosurgery 2015760142–52., discussion 52–53 [DOI] [PubMed] [Google Scholar]
- 22.Yano S, Kawano T, Kudo M et al. Endoscopic endonasal transsphenoidal approach through the bilateral nostrils for pituitary adenomas. Neurol Med Chir (Tokyo) 2009;49(01):1–7. doi: 10.2176/nmc.49.1. [DOI] [PubMed] [Google Scholar]
- 23.Meij B P, Lopes M B, Ellegala D B, Alden T D, Laws E R., Jr The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg. 2002;96(02):195–208. doi: 10.3171/jns.2002.96.2.0195. [DOI] [PubMed] [Google Scholar]
- 24.Alahmari M, Lasso A, Banaz Fet al. Utilization of SiPAP classification in prediction of pituitary adenoma recurrence: The Ottawa hospital experiencePaper presented at: Special Virtual Symposium of the North American Skull Base Society; February 13, 2021
- 25.Alexopoulou O, Everard V, Etoa M et al. Outcome of pituitary hormone deficits after surgical treatment of nonfunctioning pituitary macroadenomas. Endocrine. 2021;73(01):166–176. doi: 10.1007/s12020-021-02701-5. [DOI] [PubMed] [Google Scholar]
- 26.Nemergut E C, Zuo Z, Jane J A, Jr, Laws E R., Jr Predictors of diabetes insipidus after transsphenoidal surgery: a review of 881 patients. J Neurosurg. 2005;103(03):448–454. doi: 10.3171/jns.2005.103.3.0448. [DOI] [PubMed] [Google Scholar]
- 27.Gormolysova E. Prevalence of diabetes insipidus and other complications in early period after pituitary surgery: analysis of 259 patients. Biomed J Sci Tech Res. 2022;41(02):32565–32572. [Google Scholar]
- 28.Biamonte E, Betella N, Milani D et al. Impact of age on postsurgical outcomes of nonfunctioning pituitary adenomas. Endocrine. 2021;72(03):915–922. doi: 10.1007/s12020-020-02554-4. [DOI] [PubMed] [Google Scholar]
- 29.Nomikos P, Ladar C, Fahlbusch R, Buchfelder M. Impact of primary surgery on pituitary function in patients with non-functioning pituitary adenomas: a study on 721 patients. Acta Neurochir (Wien) 2004;146(01):27–35. doi: 10.1007/s00701-003-0174-3. [DOI] [PubMed] [Google Scholar]
- 30.Magro E, Graillon T, Lassave J et al. Complications related to the endoscopic endonasal transsphenoidal approach for nonfunctioning pituitary macroadenomas in 300 consecutive patients. World Neurosurg. 2016;89:442–453. doi: 10.1016/j.wneu.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 31.Rojas Z D, Palma F A, Wohllk G N. Manejo de los adenomas hipofisiarios. Rev Chil Neuro-psiquiatr. 2008;46(02):140–147. [Google Scholar]


