Abstract
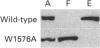
We determined the amino acid sequence responsible for the calmodulin (CaM)-binding ability of mouse type 1 Ins(1,4,5)P3 receptor (IP3R1). We expressed various parts of IP3R1 from deleted cDNA and examined their CaM-binding ability. It was shown that the sequence stretching from Lys-1564 to Arg-1585 is necessary for the binding. The full-length IP3R1 with replacement of Trp-1576 by Ala lost its CaM-binding ability. Antibody against residues 1564-1585 of IP3R1 inhibited cerebellar IP3R1 from binding CaM. The fluorescence spectrum of the peptide that corresponds to residues 1564-1585 shifted when Ca(2+)-CaM was added. From the change in the fluorescence spectrum, we estimated the dissociation constant (KD) between the peptide and CaM to be 0.7 microM. The submicromolar value of KD suggests an actual interaction between CaM and IP3R1 within cells. The CaM-binding ability of other types of IP3Rs was also examined. A part of the type 2IP3R, including the region showing sequence identity with the CaM-binding domain of IP3R1, also bound CaM, while the expressed full-length type 3 IP3R did not.
Full text
PDF




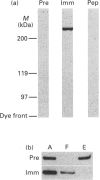
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi I. C., Huang Q. H., Means A. R. Identification of amino acids essential for calmodulin binding and activation of smooth muscle myosin light chain kinase. J Biol Chem. 1992 Feb 15;267(5):3024–3029. [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J Biol Chem. 1993 May 25;268(15):11356–11363. [PubMed] [Google Scholar]
- Caceres A., Bender P., Snavely L., Rebhun L. I., Steward O. Distribution and subcellular localization of calmodulin in adult and developing brain tissue. Neuroscience. 1983 Oct;10(2):449–461. doi: 10.1016/0306-4522(83)90145-8. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Cameron A. M., Bredt D. S., Huganir R. L., Snyder S. H. Inositol 1,4,5-trisphosphate receptor is phosphorylated by cyclic AMP-dependent protein kinase at serines 1755 and 1589. Biochem Biophys Res Commun. 1991 Feb 28;175(1):192–198. doi: 10.1016/s0006-291x(05)81219-7. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Shiota C., Mikoshiba K. Distribution of inositol 1,4,5-trisphosphate receptor mRNA in mouse tissues. FEBS Lett. 1990 Jul 2;267(1):85–88. doi: 10.1016/0014-5793(90)80294-s. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Glaser P., Elmaoglou-Lazaridou A., Krin E., Ladant D., Bârzu O., Danchin A. Identification of residues essential for catalysis and binding of calmodulin in Bordetella pertussis adenylate cyclase by site-directed mutagenesis. EMBO J. 1989 Mar;8(3):967–972. doi: 10.1002/j.1460-2075.1989.tb03459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. D., Campos-Gonzalez R., Kindmark H., Boynton A. L. Inhibition of inositol trisphosphate-stimulated calcium mobilization by calmodulin antagonists in rat liver epithelial cells. J Biol Chem. 1988 Nov 5;263(31):16479–16484. [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Ryan S. V. Phosphorylation of the inositol trisphosphate receptor in isolated rat hepatocytes. J Biol Chem. 1993 Nov 5;268(31):23059–23065. [PubMed] [Google Scholar]
- Kakiuchi S., Yasuda S., Yamazaki R., Teshima Y., Kanda K., Kakiuchi R., Sobue K. Quantitative determinations of calmodulin in the supernatant and particulate fractions of mammalian tissues. J Biochem. 1982 Oct;92(4):1041–1048. doi: 10.1093/oxfordjournals.jbchem.a134019. [DOI] [PubMed] [Google Scholar]
- Lin C. T., Dedman J. R., Brinkley B. R., Means A. R. Localization of calmodulin in rat cerebellum by immunoelectron microscopy. J Cell Biol. 1980 May;85(2):473–480. doi: 10.1083/jcb.85.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas T. J., Burgess W. H., Prendergast F. G., Lau W., Watterson D. M. Calmodulin binding domains: characterization of a phosphorylation and calmodulin binding site from myosin light chain kinase. Biochemistry. 1986 Mar 25;25(6):1458–1464. doi: 10.1021/bi00354a041. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kawasaki T., Nakade S., Yokota N., Taguchi T., Kasai M., Mikoshiba K. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J Biol Chem. 1991 Jan 15;266(2):1109–1116. [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J. 1990 Jan;9(1):61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto A. R. Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J Biol Chem. 1994 Jan 14;269(2):1222–1230. [PubMed] [Google Scholar]
- Marshall I. C., Taylor C. W. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem J. 1994 Jul 15;301(Pt 2):591–598. doi: 10.1042/bj3010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Jul 25;265(21):12679–12685. [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989 Nov 9;342(6246):192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990 Dec;9(12):3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A., Furuichi T., Maeda N., Mikoshiba K. Expressed cerebellar-type inositol 1,4,5-trisphosphate receptor, P400, has calcium release activity in a fibroblast L cell line. Neuron. 1990 Jul;5(1):11–18. doi: 10.1016/0896-6273(90)90029-f. [DOI] [PubMed] [Google Scholar]
- Miyawaki A., Furuichi T., Ryou Y., Yoshikawa S., Nakagawa T., Saitoh T., Mikoshiba K. Structure-function relationships of the mouse inositol 1,4,5-trisphosphate receptor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4911–4915. doi: 10.1073/pnas.88.11.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade S., Maeda N., Mikoshiba K. Involvement of the C-terminus of the inositol 1,4,5-trisphosphate receptor in Ca2+ release analysed using region-specific monoclonal antibodies. Biochem J. 1991 Jul 1;277(Pt 1):125–131. doi: 10.1042/bj2770125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakade S., Rhee S. K., Hamanaka H., Mikoshiba K. Cyclic AMP-dependent phosphorylation of an immunoaffinity-purified homotetrameric inositol 1,4,5-trisphosphate receptor (type I) increases Ca2+ flux in reconstituted lipid vesicles. J Biol Chem. 1994 Mar 4;269(9):6735–6742. [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Otsu H., Yamamoto A., Maeda N., Mikoshiba K., Tashiro Y. Immunogold localization of inositol 1, 4, 5-trisphosphate (InsP3) receptor in mouse cerebellar Purkinje cells using three monoclonal antibodies. Cell Struct Funct. 1990 Jun;15(3):163–173. doi: 10.1247/csf.15.163. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Danoff S. K., Schell M. J., Snyder S. H., Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Ross C. A., Villa A., Supattapone S., Pozzan T., Snyder S. H., Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990 Aug;111(2):615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber W. E., Sasagawa T., Titani K., Wade R. D., Malencik D., Fischer E. H. Human brain calmodulin: isolation, characterization, and sequence of a half-molecule fragment. Biochemistry. 1981 Sep 1;20(18):5239–5245. doi: 10.1021/bi00521a022. [DOI] [PubMed] [Google Scholar]
- Somogyi R., Stucki J. W. Hormone-induced calcium oscillations in liver cells can be explained by a simple one pool model. J Biol Chem. 1991 Jun 15;266(17):11068–11077. [PubMed] [Google Scholar]
- Sugiyama T., Furuya A., Monkawa T., Yamamoto-Hino M., Satoh S., Ohmori K., Miyawaki A., Hanai N., Mikoshiba K., Hasegawa M. Monoclonal antibodies distinctively recognizing the subtypes of inositol 1,4,5-trisphosphate receptor: application to the studies on inflammatory cells. FEBS Lett. 1994 Nov 7;354(2):149–154. doi: 10.1016/0014-5793(94)01099-4. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Yamamoto-Hino M., Miyawaki A., Furuichi T., Mikoshiba K., Hasegawa M. Subtypes of inositol 1,4,5-trisphosphate receptor in human hematopoietic cell lines: dynamic aspects of their cell-type specific expression. FEBS Lett. 1994 Aug 1;349(2):191–196. doi: 10.1016/0014-5793(94)00662-8. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Südhof T. C., Newton C. L., Archer B. T., 3rd, Ushkaryov Y. A., Mignery G. A. Structure of a novel InsP3 receptor. EMBO J. 1991 Nov;10(11):3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa K., Erneux C. Identification of residues essential for catalysis and binding of calmodulin in rat brain inositol 1,4,5-trisphosphate 3-kinase. Biochem J. 1991 Nov 15;280(Pt 1):125–129. doi: 10.1042/bj2800125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K., Stukenbrok H., Metcalf A., Mignery G. A., Südhof T. C., Volpe P., De Camilli P. Ca2+ stores in Purkinje neurons: endoplasmic reticulum subcompartments demonstrated by the heterogeneous distribution of the InsP3 receptor, Ca(2+)-ATPase, and calsequestrin. J Neurosci. 1992 Feb;12(2):489–505. doi: 10.1523/JNEUROSCI.12-02-00489.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Makino Y., Clark R. A., Pearson D. W., Mattei M. G., Guénet J. L., Ohama E., Fujino I., Miyawaki A., Furuichi T. Human inositol 1,4,5-trisphosphate type-1 receptor, InsP3R1: structure, function, regulation of expression and chromosomal localization. Biochem J. 1994 Sep 15;302(Pt 3):781–790. doi: 10.1042/bj3020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Miyawaki A., Kawano H., Sugiyama T., Furuichi T., Hasegawa M., Mikoshiba K. Immunohistochemical study of inositol 1,4,5-trisphosphate receptor type 3 in rat central nervous system. Neuroreport. 1995 Jan 26;6(2):273–276. doi: 10.1097/00001756-199501000-00012. [DOI] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Sugiyama T., Hikichi K., Mattei M. G., Hasegawa K., Sekine S., Sakurada K., Miyawaki A., Furuichi T., Hasegawa M. Cloning and characterization of human type 2 and type 3 inositol 1,4,5-trisphosphate receptors. Receptors Channels. 1994;2(1):9–22. [PubMed] [Google Scholar]
- Yamamoto A., Otsu H., Yoshimori T., Maeda N., Mikoshiba K., Tashiro Y. Stacks of flattened smooth endoplasmic reticulum highly enriched in inositol 1,4,5-trisphosphate (InsP3) receptor in mouse cerebellar Purkinje cells. Cell Struct Funct. 1991 Oct;16(5):419–432. doi: 10.1247/csf.16.419. [DOI] [PubMed] [Google Scholar]
- Yuzaki M., Mikoshiba K. Pharmacological and immunocytochemical characterization of metabotropic glutamate receptors in cultured Purkinje cells. J Neurosci. 1992 Nov;12(11):4253–4263. doi: 10.1523/JNEUROSCI.12-11-04253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B. X., Zhao H., Muallem S. Ca(2+)-dependent kinase and phosphatase control inositol 1,4,5-trisphosphate-mediated Ca2+ release. Modification by agonist stimulation. J Biol Chem. 1993 May 25;268(15):10997–11001. [PubMed] [Google Scholar]