Abstract
Background Prospective studies comparing quality-of-life and olfaction in patients undergoing endoscopic uni-nostril versus bi-nostril trans-sphenoidal pituitary surgery have not been published.
Methods We prospectively compared olfaction and quality-of-life at baseline and at 3 to 6 months follow-up using the Anterior Skull Base Nasal Inventory-12 (ASK-12) questionnaire, composite olfaction score, and Lund–Kennedy Endoscopic Score (LKES) in 43 patients who underwent endoscopic excision of pituitary adenoma with either a uni-nostril (24 patients) or a bi-nostril (19 patients) approach.
Results Baseline data for both groups were comparable. In the uni-nostril group, ASK-12 and LKES scores were not significantly different at follow-up when compared with the preoperative scores. In the bi-nostril group, there was a significant postoperative worsening of ASK-12 scores (mean: 3.2 vs. 5.3; p = 0.04) and the LKES (mean: 2.9 vs. 6.6; p = 0.01). Composite olfaction score was not significantly affected postoperatively with either approach. Nasal complications were also more in the bi-nostril group (5/18, 27.8% vs. 1/23, 4.3%) but this was not statistically significant ( p = 0.07).
Conclusion Both approaches preserve olfactory function but the uni-nostril approach is associated with better postoperative quality-of-life and endoscopic scores and subjective olfaction outcomes. At least in short term, the postoperative morbidity is higher in the bi-nostril approach compared with the uni-nostril approach. Although preference for a particular approach is related to a surgeon's preference, preoperative counselling of the patients regarding sinonasal morbidity is important.
Keywords: endoscopic surgery, olfaction, quality of life, trans-sphenoidal surgery
Introduction
Over the past two decades, endoscopic endonasal approaches have become the gold standard for excision of pituitary adenomas. 1 2 3 4 The endoscope provides a panoramic view of the surgical field with improved magnification and illumination and the ability to look around corners using angled lenses when compared with the microsurgical approach. 1 3 5 6 A systematic review of 806 patients comparing microscopic and endoscopic techniques for pituitary surgery found that the endoscopic approach resulted in fewer nasal complications. 5 7 8 9 However, operative trauma to nasal mucosa and olfactory epithelium with transient or permanent reduction in olfaction have been reported in endoscopic approaches. 10 11 12 13 14 15 16 Using specific instruments such as the Anterior Skull Base Nasal Inventory-12 (ASK-12) and Sinonasal Outcome Test 22 (SNOT-22) questionnaires, reduction in quality of life (QoL) following endoscopic pituitary surgery has been reported particularly when resection of nasal mucosa with resultant crusting, nasal block, sinusitis, and epistaxis occurs. 14 17 18 19 20 The two major endoscopic techniques in current practice are the uni-nostril and bi-nostril approaches, 3 6 10 21 both are safe and effective in pituitary surgery, although the bi-nostril approach may be preferred when an extended approach is required. 3 6 21 While the deterioration in QoL is probably transient, there are no prospective studies comparing the nasal outcomes between the two approaches. The present study was specifically designed to prospectively compare the QoL, olfaction status, and nasal endoscopic scores between patients undergoing uni-nostril and bi-nostril approaches for endoscopic trans-sphenoidal surgery for pituitary adenomas.
Methods
Patients
Sixty-eight patients diagnosed with pituitary adenoma were prospectively recruited to this non-randomized study between January 2018 and December 2019. Of these, 34 patients underwent a uni-nostril endoscopic approach to the pituitary fossa, while the other 34 underwent a bi-nostril approach. Informed consent was obtained from all individual participants of the study. Patient recruitment and follow-up are depicted in the flow chart in Fig. 1 .
Fig. 1.
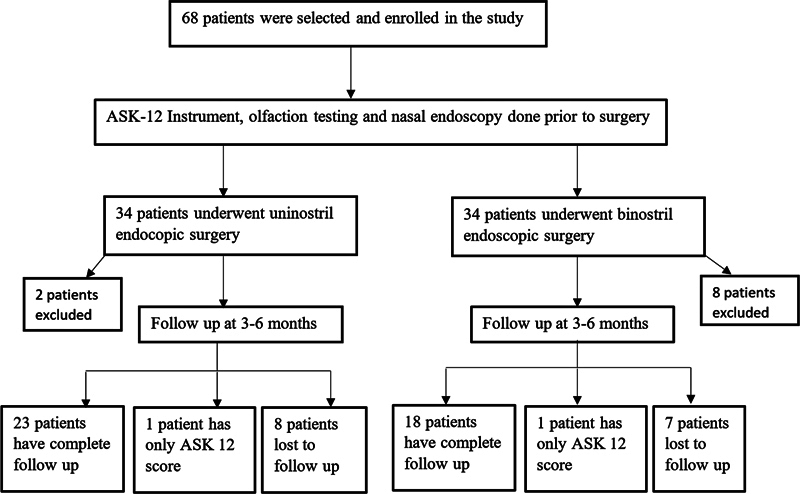
Flow chart showing patient recruitment and follow-up.
Exclusion Criteria
The following patients were excluded from the study: patients who were unable to complete the ASK-12 questionnaire due to altered mental status, those who had previously undergone nasal or trans-sphenoidal surgeries, patients with preoperative co-existing sinonasal diseases like sinusitis or nasal polyps, patients with extension of the tumor into the nasal cavity, and patients planned for extended endoscopic trans-nasal transsphenoidal surgery and combined trans-nasal and trans-cranial surgery. Patients requiring re-exploration and those in whom nasoseptal grafts were used for cerebrospinal fluid (CSF) leak repairs or in whom the middle turbinate was removed were also excluded from the analysis.
Preoperative Assessment
All patients were administered the well-validated, site-specific ASK-12 questionnaire, which included 12 questions with a maximum of 5 points for each answer. 22 Patients then underwent rigid nasal endoscopy and the Lund–Kennedy Endoscopic Score (LKES) was calculated for each nostril. 23 Finally, each patient underwent olfaction assessment using the butanol threshold test and smell identification test. 24
Anterior Skull Base Nasal Inventory-12
The total score ranged from a minimum of 0 to a maximum of 60, with a higher score indicating severe symptoms. The test questionnaire was filled by the patient under the guidance of the first author. The minimal clinically important difference (MCID) for ASK-12 questionnaire is 4.8. 25 26
Olfaction Testing
Olfaction testing consisted of two components: odor threshold test using n -butyl alcohol (1-butanol) as the odorant and odor identification test using common household odorants. 24 The outcomes of these two components were combined and expressed as a composite score out of 7. A diagnosis of anosmia (0–1.75), severe hyposmia (2–3.75), moderate hyposmia (4–4.75), mild hyposmia (5–5.75), and normosmia (6–7) was made, depending on the composite score obtained. Each nostril was tested and scored separately. The olfaction test was administered by a trained olfaction testing nurse who was blinded to the surgery done.
The olfaction testing was coordinated by the first and second authors.
Nasal Endoscopy
Rigid nasal endoscopy was performed by ENT surgeons to specifically assess the nasal cavity for the presence of polyps, discharge, and scarring. The nasal endoscopy findings were objectively scored on a modified LKES for a total score of 33. 23 A higher score indicates worse outcomes.
Surgical Technique
One author (V.R.) performed all the uni-nostril approaches, while the other (A.G.C.) performed all the bi-nostril approaches. Both surgeons have been using these approaches for excision of pituitary adenomas for over 10 years and have performed >400 endoscopic pituitary surgeries individually. Both surgeons were blinded to the results of the tests performed at baseline and after the surgery till the end of the study.
Uni-nostril Approach
The surgery was performed entirely using the endoscope. Initially a hand held 0 degree 18-cm endoscope was used but once the sellar floor was reached, a 0 degree long telescope (30 cm) replaced the hand-held scope. The long telescope was placed in a sheath with an irrigation port and was held in a table-mounted telescope holder (Karl Storz, Germany). The endoscope holder was used only for the uni-nostril approach, wherein the main surgeon was responsible for moving the endoscope into the desired position. Through a right nasal approach, a vertical incision was made in the posterior septal mucosa and mucosal flaps were elevated on both sides of the septum and the rostrum of the sphenoid sinus. The contralateral nasal mucosa and most of the ipsilateral nasal mucosa as well as adjacent structures are preserved. The posterior nasal septum was partially excised and a thin blade tapered, self-retaining Killian's nasal speculum was used to keep the septal flaps apart. The sphenoid rostrum was removed widely to expose the sella. At this point the long telescope was introduced and the rest of the surgery was performed with bi-manual instrumentation. At the end of the surgery, the sella and sphenoid sinus were packed with abdominal fat and oxidized cellulose and gelatin sponge. The mucosal flaps were allowed to fall back into place and the mucosal incision was covered with gelatin sponge. Nasal packing was not used.
Bi-nostril Approach
The surgery was performed entirely using the endoscope. The sphenoid ostia were widened bilaterally followed by a small posterior septectomy in which the posterior part of the bony nasal septum and the overlying mucosa were excised. The bi-nostril approach was a three-handed approach, wherein the endoscope was held and moved by the first assistant and placed in the right nostril, while the surgeon had both hands free to introduce instruments through both nostrils. This was followed by the removal of vomer and partial excision of the superior turbinate on the right side and opening into the posterior ethmoids. The anterior wall of the sphenoid sinus was excised through both the nostrils. At the end of the surgery, the sella and sphenoid sinus were packed with abdominal fat and oxidized cellulose and gelatin sponge. Anterior nasal packs (Ivalon nasal packing with string—First Aid Bandage Company, New London, Connecticut, United States) were applied bilaterally.
Postoperative Care
All patients were started on saline nasal drops/spray postoperatively, which was continued for 1 week. All patients undergoing the bi-nostril approach had their packs removed 24 to 48 hours after surgery and underwent nasal endoscopy and cleaning a week following surgery. If the patient had nasal crusting, then the saline nasal drops/sprays were continued beyond that period. Ten patients in the bi-nostril group were advised to continue saline nasal spray (Isotonic nasal spray—Solspre, Abbott India Limited) for 3 months postoperatively due to extensive crusting at 1 week follow-up. Douching was not routinely recommended, but one patient in the bi-nostril group was advised douching at 3 months follow-up as saline nasal spray was not helpful in reducing the crusting.
Follow-Up
At 3 to 6 months following surgery, patients were reassessed using the ASK-12 questionnaire, olfaction testing, and nasal endoscopy. In addition to calculating the LKES, the presence of specific abnormalities like excessive crusting, septal perforation, atrophic rhinitis, purulence, and nasal synechiae were noted.
Patients also underwent hormonal function evaluation and a gadolinium-enhanced magnetic resonance (MR) scanning was performed to determine the extent of resection which was assessed using volumetric measurements and categorized as gross total resection (GTR; if no tumor was visible on the follow-up MR) or near-total resection (NTR; if <10% of the preoperative tumor volume was visible on the follow-up MR).
Statistical Methods
Sample Size Calculations
The difference between preoperative and postoperative ASK-12 scores was considered as the primary outcome measure. With the α error was set at 0.05, and power was set at 0.9, the sample size calculated using a two-tailed Student's t -test for paired samples was 52. Allowing for possible dropouts, a final sample size of 68 patients, with 34 in each arm, was obtained.
Statistical Analysis
The results are presented as mean ± standard deviation for quantitative variables and numbers with percentages for categorical variables. Fisher's exact test and Student's t -test were applied for comparison of categorical and continuous variables, respectively. The preoperative and follow-up scores were compared within each group (uni-nostril and bi-nostril) and between the two groups, using the paired t -test. A p -value of <0.05 was considered significant.
Results
We recruited 58 patients in the study; however, only 24 patients in the uni-nostril group and 19 patients in the bi-nostril group reported for follow-up. Patients who were lost to follow-up (15 patients) were excluded from the analysis. This was largely related their inability to travel because of the COVID-19 (coronavirus disease 2019) pandemic. The recruitment of the patients had to be stopped because of the COVID-19 pandemic when all elective trans-nasal surgeries were suspended for a considerable period of time.
The demographic data, Hardy's grade of the tumor, and preoperative nasal QoL indicators are shown in Table 1 . Both groups of patients were homogeneous in terms of baseline demographic data. There were 22 male and 21 female patients. Most of the pituitary adenomas were Hardy's grade C in both groups.
Table 1. Demographics and baseline data.
| Characteristics | Uni-nostril group, N = 24 (%) |
Bi-nostril group, N = 19 (%) |
p -Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 45.2 ± 13.9 | 42.9 ± 10.9 | 0.6 | |
| Sex (male, %) | 12 (50) | 10 (52.6) | 1.0 | |
| ASK 12 (score out of 60) |
2.9 ± 2.3 | 3.2 ± 3.1 | 0.9 | |
| Olfaction testing (scored out of 7) |
Left | 5.5 ± 1.0 | 5.2 ± 1.1 | 0.3 |
| Right | 5.3 ± 1.1 | 5.0 ± 1.2 | 0.6 | |
| Endoscopy score (scored out of 27) |
3.4 ± 2.2 | 2.9 ± 1.3 | 0.4 | |
| Hardy's grade | A | 1 (4.2) | 1 (5.3) | |
| B | 6 (25) | 4 (21) | ||
| C | 16 (66.6) | 14 (73.7) | ||
| D | 1 (4.2) | 0 | ||
| E | 6 (25) | 2 (13.6) | ||
| Gross and near-total resection | 16 (66.6) | 13 (68.4) | 1.0 | |
Abbreviation: SD, standard deviation.
Cavernous sinus invasion was seen in 6 (25%) patients in the uni-nostril group and 2 (13.6%) patients in the bi-nostril group ( p = 0.3).
The GTR/NTR rates were 66.1% in the uni-nostril and 68.4% in the bi-nostril group and the difference was not statistically significant ( p = 1). The intraoperative CSF leak rates were 29.2 and 5.3% in the uni-nostril and bi-nostril groups, respectively. None of the patients with intra-operative CSF leak had postoperative CSF leak. The difference in the rate of intra-operative CSF leak was not statistically significant ( p = 0.06).
Early Nasal Complications
The most common complaint in the early postoperative period was nasal obstruction. Two patients in the uni-nostril group and four patients in the bi-nostril group complained of nasal obstruction in the early postoperative period.
Two patients, one from each group, had mild blood-stained nasal discharge for 1 week following surgery. None of the patients had any major nasal complications in the early postoperative period.
Follow-Up
The median duration of follow-up was 3 (interquartile range [IQR]: 3–4) months for the uni-nostril group compared with 4 (IQR: 3–4) months for the bi-nostril group ( p = 0.08).
Sinonasal Outcome
Tables 2 and 3 present the analysis of the nasal QoL indicators for the two groups at baseline and at follow-up. Baseline analysis showed no difference between the uni-nostril and bi-nostril groups in terms of demography, ASK-12, olfaction score, LKES.
Table 2. Nasal function before and after surgery.
| Indicator | Uni-nostril group | Bi-nostril group | ||||
|---|---|---|---|---|---|---|
| Baseline, mean ± SD |
Follow-up, mean ± SD |
p -Value | Baseline, mean ± SD |
Follow-up, mean ± SD |
p -Value | |
| ASK-12 (scored out of 60) | n = 24 | n = 19 | ||||
| 2.9 ± 2.3 | 2.1 ± 2.1 | 0.1 | 3.2 ± 3.1 | 5.3 ± 4.3 | 0.04 | |
| Olfaction testing (scored out of 7) |
n = 23 | n = 18 | ||||
| Left | 5.5 ± 1.0 | 5.1 ± 1.4 | 0.3 | 5.2 ± 1.1 | 5.3 ± 1.5 | 0.7 |
| Right | 5.3 ± 1.1 | 5.1 ± 1.4 | 0.9 | 5.1 ± 1.2 | 5.3 ± 1.4 | 0.5 |
| Endoscopy score (scored out of 27) |
n = 23 | n = 18 | ||||
| 3.4 ± 2.2 | 2.4 ± 2.1 | 0.1 | 2.9 ± 1.3 | 6.6 ± 5.7 | 0.01 | |
Abbreviation: SD, standard deviation.
Note: Statistically significant p -values are indicated in bold.
Table 3. Comparing nasal function at follow-up in the two groups.
| Indicator | Uni-nostril group, mean ± SD |
Bi-nostril group, mean ± SD |
p -Value |
|---|---|---|---|
| ASK-12 (scored out of 60) |
n = 24 | n = 19 | |
| 2.1 ± 2.1 | 5.3 ± 4.3 | 0.002 | |
| Olfaction testing (scored out of 7) Left Right |
n = 23 | n = 18 | |
| 5.1 ± 1.4 | 5.3 ± 1.5 | 0.5 | |
| 5.2 ± 1.4 | 5.3 ± 1.4 | 0.7 | |
| Endoscopy score (scored out of 27) |
n = 23 | n = 18 | |
| 2.4 ± 2.1 | 6.6 ± 5.7 | <0.001 |
Abbreviation: SD, standard deviation.
Note: Statistically significant p -values are indicated in bold.
Preoperative versus Follow-Up Scores
There was no statistically significant difference between the preoperative and postoperative ASK-12 scores ( p = 0.1), olfaction scores ( p > 0.05), and LKES ( p = 0.1) in the uni-nostril group. In the bi-nostril group, there was a significant worsening, at follow-up, of the ASK-12 score ( p = 0.04) and LKES ( p = 0.01) but not the olfaction scores.
Follow-Up Scores: Uni-nostril versus Bi-nostril
A comparison of the three postoperative scores in the uni-nostril and bi-nostril groups showed a statistically significant worsening of ASK-12 scores in the bi-nostril group compared with the uni-nostril group at follow-up ( p = 0.002). In the bi-nostril group, LKES was also significantly worse compared with the uni-nostril group ( p < 0.001). No significant difference in olfaction status was noted between both groups.
Three patients (15.8%) in the bi-nostril group and one patient in the uni-nostril group (4.2%) had worsening in the ASK-12 score, which reached the MCID (≥4.8). However, the difference in the preoperative and follow-up mean ASK-12 scores did not reach the MCID in either group.
Olfactory Function
There was no significant change in the olfaction composite score compared with the baseline with either of the endoscopic approaches ( Tables 2 and 3 ). Subgroup analysis of the smell function in the follow-up ASK-12 questionnaire showed that 5 (5/19, 26.3%) patients in the bi-nostril group had subjective impairment of smell compared with only 1 (1/24, 4.2%) patient in the uni-nostril group, but even this difference was not statistically significant ( p = 0.07).
Nasal Complications at Follow-Up
A higher proportion of patients had nasal complications in the bi-nostril group (27.8%) compared with the uni-nostril group (4.3%). But the difference was not statistically significant ( p = 0.07) ( Table 4 ).
Table 4. Nasal complications at follow-up.
| Nasal complication | Uni-nostril group, n = 23 (%) |
Bi-nostril group, n = 18 (%) |
|---|---|---|
| Septal abscess | 0 | 1 (5.5) |
| Septal perforation | 1 (4.3) | 0 |
| Severe synechiae requiring intervention | 0 | 1 (5.5) |
| Extensive nasal crusting | 0 | 2 (11.1) |
| Developed sleep apnea postoperatively | 0 | 1 (5.5) |
| Total complications a | 1 (4.3) | 5 (27.8) |
p- Value: 0.07.
Discussion
Effect of Surgical Approach on Olfaction
The uni-nostril endoscopic trans-septal approach is considered to be less injurious to the nasal and olfactory mucosa compared with the bi-nostril approach, because in the former approach, the contralateral nasal mucosa and most of the ipsilateral nasal mucosa as well as adjacent structures are preserved. 16 21 27 The olfactory area in the nose comprises an area of approximately 2 cm 2 covering the cribriform plate, mucosa over the superior portion of the nasal septum and superior turbinate. Endoscopic endonasal approaches to the sella may result in trauma to the superior septum and turbinate either because of resection (as occurs in the bi-nostril approach) or pressure (as occurs in the uni-nostril approach when a nasal speculum is used). Other causes for worsening of olfactory function in the early postoperative period could include crusting within the nasal cavities, mucosal edema, scarring, and unrecognized damage to the nasal mucosa. 28
Many studies have shown transient worsening of olfaction in the early postoperative period. 10 11 12 13 15 29 30
Zhu et al 31 in a systematic review of olfactory outcomes after endonasal skull base surgery, showed the incidence of decrease in the olfactory function following endoscopic surgery to be 20%. They did not find any evidence to suggest that middle turbinectomy was detrimental to recovery of olfactory function. They suggested testing olfaction ≥3 months following surgery, as recovery of ciliary function takes approximately 3 months.
Yin et al 32 in a meta-analysis of 29 articles, showed no difference in olfactory outcomes following endoscopic sellar/parasellar surgery. The authors found great variation in the surgical technique used, as well as differences in the techniques used, to evaluate olfaction and suggested a need for prospective studies using validated objective measures of olfaction.
A retrospective study comparing olfactory outcomes between uni-nostril and bi-nostril approaches using only a subjective assessment of olfaction found no difference in smell disturbances and permanent hyposmia in ≤10% in both groups. 3 The use of a nasoseptal flap is believed by some to increase the likelihood of mucosal damage and resultant postoperative hyposmia, 16 but not by others. 33 34 Zhu et al 31 and Greig et al 35 in their systematic review found that elevation of nasoseptal flap leads to impairment in olfactory function. Methods to reduce such damage by reducing the extent of posterior septectomy and avoiding resection of the lower part of superior turbinate even when using a bi-nostril approach have been suggested by some. 16 36
In the present study, subgroup analysis of smell in the ASK-12 questionnaire showed that 26.3% of patients in the bi-nostril group had worsening of olfaction/parosmia compared with 4.2% in the uni-nostril group but the difference failed to reach statistical significance ( p = 0.07). Upon objective olfaction testing, there was no significant worsening of olfaction at 3 to 6 months compared with the baseline in either uni-nostril or bi-nostril group. Subjective reduction of smell following the bi-nostril approach has been reported previously, 16 although studies using objective testing have mostly failed to show any significant worsening of the olfactory function at 3 to 6 months ( Table 5 ). 10 15 16 19 28 29 37 38 39 40
Table 5. Published studies on olfactory function following endoscopic trans-nasal trans-sphenoidal surgery.
| Author/year | Endoscopic approach | Cases included in the study | Test used to assess olfaction | Time of evaluation | Outcome |
|---|---|---|---|---|---|
| Prospective studies | |||||
| Kim et al/2013 | Bi-nostril | Various skull base tumors | VAS score | Preoperative and postoperative at 6 months | Worsened |
| Sowerby et al/2013 | Uni-nostril | Pituitary adenoma, Rathke's cleft cyst | UPSIT | Preoperative, postoperative 1 to 3 months | No change |
| Wu et al/2018 | Bi-nostril | Various skull base tumors | Subjective | Preoperative, postoperative at 0–1, 2–4, and >5 months | Worse at 0–1 month follow-up but returned to baseline thereafter |
| Cingoz et al/2018 | Bi-nostril | Pituitary adenoma | Sniffin' Sticks Test | Preoperative and postoperative at 8 weeks | No change |
| Kawabata et al/2019 | Uni-nostril | Various skull base tumors | Toyota and Takagi olfactometer test and venous olfaction test | Preoperative, postoperative 1 and at 3 months | Slight worsening at 1 month follow-up but returned to baseline thereafter |
| Schreiber et al/2019 | Bi-nostril | Various skull base tumors | UPSIT | Preoperative and postoperative at 6 months | No change |
| Carvalho et al/2022 a | Not mentioned | Various skull base tumors | CCCRC | Preoperative, postoperative 1, 3, and 6 months | Worse at 1 month follow-up but returned to baseline thereafter |
| Conrad et al/2023 | Both uni-nostril and bi-nostril | Various skull base tumors | Sniffin' Sticks Test | Preoperative and postoperative at 6 months | No change |
| Retrospective studies | |||||
| Hong et al/2016 | Bi-nostril | Pituitary adenoma | VAS score/CCSIT | Preoperative, postoperative 1 month and at 3 months | VAS score worse at 1 and 3 months but no change on CCSIT |
| Kim et al/2016 | Bi-nostril | Various skull base tumors | CCCRC, CCSIT | Preoperative and postoperative at 6 months | Worsened |
| Linsler et al/2018 | Uni-nostril | Various skull base tumors | Subjective | Postoperative 3 and 6 months, 1 year, and 2 years | Worse |
| Kuwata et al/2020 | Bi-nostril | Pituitary adenoma | Toyota and Takagi olfactometer test | Preoperative and postoperative at 6 months | Improved |
| Ting et al/2019 b | Bi-nostril | Various skull base tumors | Sniffin' Sticks Test | Preoperative and postoperative at 3 months | No change if nasoseptal flap used and improved if not used |
| Raikundalia et al/2021 | Bi-nostril | Various skull base tumors | UPSIT, ASOF | Preoperative and postoperative at 3 months | Worse |
| Tao et al/2021 | Both uni-nostril and bi-nostril | Pituitary adenoma | VAS score | Preoperative and postoperative at 1 year | No change |
| Ting et al/2019 b | Bi-nostril | Various skull base tumors | Sniffin' Sticks Test | Preoperative and postoperative at 3 months | No change if nasoseptal flap used and improved if not used |
| Raikundalia et al/2021 | Bi-nostril | Various skull base tumors | UPSIT, ASOF | Preoperative and postoperative at 3 months | Worse |
| Tao et al/2021 | Both uni-nostril and bi-nostril | Pituitary adenoma | VAS score | Preoperative and postoperative at 1 year | No change |
Abbreviations: ASOF, Assessment of Self-Reported Olfactory Functioning questionnaire; CCCRC, Connecticut Chemosensory Clinical Research Center test; CCSIT, Cross-Cultural Smell Identification Test; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analogue scale.
Nasoseptal flap was used in all cases.
Nasoseptal flap was used in 37% of cases.
Assessing Sinonasal Outcome
Instruments that have been utilized for assessment of QoL following endoscopic pituitary surgery include ASK-12, 22 41 SNOT-22, 14 18 19 20 41 SNOT-20, 13 VAS (visual analogue scale), 13 SF-36, 42 and WHO-QoL BREF. 43 The ASK-12 is a site-specific 12-question inventory which focuses on sinonasal morbidity and evaluates all likely symptoms related to impaired nasal function, taste, airway, tearing, and ear function. This instrument is also specific for postoperative skull base surgery unlike the SNOT-22, which is more suited to postoperative assessment of endoscopic sinus surgery and the Nasal Obstruction Symptom Evaluation (NOSE) inventory which is more suited to septal surgery. 44 45 In the present study, we utilized ASK-12 inventory to get a more complete assessment of sinonasal QoL changes following endoscopic pituitary surgery.
Many authors have shown that the sinonasal QoL worsens and reaches a nadir at 2 to 3 weeks following surgery followed by recovery by 6 weeks to 3 months ( Table 6 ). 14 17 20 41 46 Postoperative follow-up at 3 to 6 months was chosen in the present study as it has been shown to be adequate to achieve complete healing of the nasal cavities and most studies reporting sinonasal outcomes beyond 3 months of surgery do not document any significant further worsening of QoL. 16 28 37 47
Table 6. Published studies on sinonasal outcome following endoscopic trans-nasal trans-sphenoidal surgery.
| Author/year | Endoscopic approach | Cases included in the study | Sinonasal outcome indicator used | Time of evaluation | Outcome |
|---|---|---|---|---|---|
| Prospective studies | |||||
| Kim et al/2013 | Bi-nostril | Various skull base tumors | NOSE, SNOT-20 | Preoperative and postoperative at 6 months | No change |
| Sowerby et al/2013 | Bi-nostril | Pituitary adenoma, Rathke's cleft cyst | Modified SNOT-22, LKES | Preoperative, postoperative 1 to 3 months | No change in Modified SNOT-22 but worse LKES |
| Wu et al/2018 | Bi-nostril | Various skull base tumors | SNOT-22 | Preoperative, postoperative at 0–1, 2–4, and >5 months | Worse at 0–1 month follow-up but returned to baseline thereafter |
| Riley et al/2019 a | Not mentioned | Various skull base tumors | SNOT-22, LMS | Preoperative and postoperative at >3 months | No change in SNOT-22 but worse LMS |
| Schreiber et al/2019 | Bi-nostril | Various skull base tumors | SNOT-22, ASK-12 | Preoperative and postoperative at 6 months | No change |
| Bryl et al/2020 | Bi-nostril | Pituitary adenoma | SNOT-22 | Preoperative, postoperative between 3rd and 6th day and at 3 months | No change |
| Hallén et al/2021 | Bi-nostril | Various skull base tumors | SNOT-22 | Preoperative and postoperative at 6 months | No change overall but rhinologic domain of SNOT-22 worsened |
| Novák et al/2021 | Bi-nostril | Various skull base tumors | SNOT-22 | Preoperative and postoperative at 6 months | No change |
| Castle-Kirszbaum et al/2022 | Not mentioned | Pituitary adenoma | SNOT-22 | Preoperative, postoperative at 1, 3, and 7 days, 3 and 6 weeks, and 3, 6, and 12 months | Worse in first 3 weeks, returned to baseline at 6 weeks and improved above baseline thereafter |
| Sunil et al/2022 | Bi-nostril | Pituitary adenoma | ASK-12 | Preoperative and postoperative at 2 weeks, 3 months, and 1 year | No change overall, only at 2 weeks had worsening in “nasal crusting” and “thick nasal discharge” components and improvement in headache component |
| Conrad et al/2023 | Both uni-nostril and bi-nostril | Various skull base tumors | SNOT-22 | Preoperative, postoperative between 2nd and 3rd day and at 6 months | Worse at 2–3 days but returned to baseline at 6 months |
| Retrospective studies | |||||
| Hong et al/2016 | Bi-nostril | Pituitary adenoma | SNOT-20, ASK | Preoperative, postoperative 1 month and at 3 months | No change |
| Kim et al/2016 | Bi-nostril | Various skull base tumors | NOSE, SNOT-20 | Preoperative and postoperative at 6 months | No change |
| Choi et al/2019 | Bi-nostril | Various skull base tumors | SNOT-22 | Preoperative, postoperative 3 months and at 6 months | No change |
| Linsler et al/2018 | Uni-nostril | Various skull base tumors | Subjective sinonasal complaints | Postoperative 3 and 6 months, 1 year, and 2 years | New complaints postoperative majority of which improved by 1 year |
| Tao et al/2021 | Both uni-nostril and bi-nostril | Pituitary adenoma | SNOT-22 | Preoperative and postoperative at 1 year | No change |
Abbreviations: LKES, Lund–Kennedy Endoscopic Score; LMS, Lund–Mackay scores; NOSE, Nasal Obstruction Symptoms Evaluation; SNOT, Sinonasal Outcome Test.
Nasoseptal flap was used in all cases.
Previous Studies on Sinonasal Quality of Life
Bhenswala et al 48 in a meta-analysis of sinonasal QoL outcomes after endoscopic endonasal skull base surgery using SNOT-22, demonstrated a temporary worsening of sinonasal QoL at ≤4 weeks after surgery in all patients but they returned to their preoperative sinonasal status within 3 months. In patients with impaired preoperative sinonasal QoL, they demonstrated a continued improvement beyond the baseline after 3 months reaching a peak at 1 year postoperatively.
Wang and Zhu 49 in a meta-analysis of patient-reported QoL after endoscopic surgery for pituitary lesions, showed no difference in the QoL after 1 month postoperatively although there was transient worsening of the sinonasal outcome postoperatively.
Kirkman et al 50 in a systematic review of QoL after anterior skull base surgery, showed improvement in QoL within several months of surgery, mainly if the endoscopic approach is used, with no long-term adverse effects on sinonasal outcome.
Unique Features of Present Study
There is considerable literature on the sinonasal outcomes and olfactory specific QoL following endoscopic trans-sphenoidal surgery, some which we have summarized in Tables 5 and 6 . However, many of these studies are retrospective studies. 16 30 34 40 47 51 52 53 Our study is a prospective study. Also, most of the studies are focused on the bi-nostril technique. 13 16 19 30 34 37 38 43 52 53 54 55 56 Finally, the studies report on patients undergoing both the extended and standard versions of the surgery. Thus, the indications for the endoscopic surgery include both pituitary adenomas and other pathologies such as craniopharyngiomas and meningiomas for which an extended approach is used and typically the resection of nasal mucosa is more extensive. 10 13 15 18 19 30 34 37 39 40 47 51 53 54 55 The present study is the only prospective study in the literature designed to compare sinonasal outcomes in patients undergoing uni-nostril and bi-nostril endoscopic pituitary surgery, using subjective and objective nasal QoL indicators.
Differences in Sinonasal Outcomes: Uni-nostril versus Bi-nostril
We found no significant worsening of the sinonasal QoL in the uni-nostril group at 3 to 6 months while the sinonasal QoL in the bi-nostril group was found to be significantly worse when compared with the baseline. There was also a greater percentage (23.8%) of complications in the bi-nostril group, mainly synechiae and nasal crusting, compared with the uni-nostril group (4.3%). We excluded patients in the bi-nostril group who had a nasoseptal flap or middle turbinectomy to avoid the confounding effect of these procedures on the nasal outcomes and provide a homogenous group to compare with the uni-nostril group. In spite of excluding patients who had procedures which have been suggested to contribute to nasal complications, the bi-nostril group had worse outcomes. Although the follow-up was slightly longer in patients in the bi-nostril group, they had worse nasal QoL and LKES. Nasal morbidity following trans-sphenoidal surgery is generally worse soon after surgery and improves over several months after surgery. However, since the group with the longer follow-up has the worse nasal outcomes, the marginal difference in follow-up duration cannot explain the difference in nasal outcomes between the two groups.
Thus, performing posterior septectomy and using both nostrils for the surgery themselves cause more nasal mucosal trauma and consequently worse nasal outcomes than the uni-nostril trans-septal approach.
The worsening in the mean ASK-12 score in the bi-nostril group, although statistically significant, did not reach the reported MCID of 4.8. But a larger proportion of patients in the bi-nostril group (22.7%) reached the MCID than that in the uni-nostril group (4.2%).
Our findings suggest that, at least in short term, the postoperative morbidity might be higher in the bi-nostril approach compared with the uni-nostril approach. Although preference for a particular approach is related to a surgeon's preference, preoperative counselling of the patients regarding sinonasal morbidity is advisable.
The complications in the bi-nostril group which were not there in the uni-nostril group were synechiae and crusting, both of which are known to occur when there is greater mucosal injury. Synechiae formation occurs when there is mucosal injury on apposing mucosal surfaces like the septum and lateral wall of nose. Crusting occurs when mucosa is sacrificed. Both of these phenomena were therefore seen in the bi-nostril approach alone.
Factors besides the differences in the technique could also be responsible for the differences in the nasal outcomes between the uni-nostril and bi-nostril groups. One readily identifiable cause is the routine use of nasal packing for 24 to 48 hours postoperatively in patients undergoing the bi-nostril approach.
Since we did not study the long-term nasal outcomes beyond 6 months, we are unable to compare the long-term nasal outcomes between the two groups. It is possible that the nasal QoL and other parameters of nasal function might improve in both groups and particularly the bi-nostril group over a period of 1 year following surgery.
Conclusion
Olfactory function was retained at preoperative levels in patients undergoing either uni-nostril or bi-nostril endoscopic pituitary surgery. However, at 3 to 6 months following surgery, nasal QoL and endoscopic status is better preserved in patients who undergo the uni-nostril trans-septal trans-sphenoidal approach. Preference for a particular approach may be related to a surgeon's preference rather than concern for postoperative sinonasal morbidity. However, preoperative counselling of patients regarding postoperative sinonasal morbidity is advisable.
Conflict of Interest None declared.
Ethical Approval
We obtained approval from the institutional review board and ethics committee of the institution for the conduct of this study (IRB number 11113).
References
- 1.Cappabianca P, Cavallo L M, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002;97(02):293–298. doi: 10.3171/jns.2002.97.2.0293. [DOI] [PubMed] [Google Scholar]
- 2.Dehdashti A R, Ganna A, Karabatsou K, Gentili F.Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series Neurosurgery 200862051006–1015., discussion 1015–1017 [DOI] [PubMed] [Google Scholar]
- 3.Conrad J, Ayyad A, Wüster C et al. Binostril versus mononostril approaches in endoscopic transsphenoidal pituitary surgery: clinical evaluation and cadaver study. J Neurosurg. 2016;125(02):334–345. doi: 10.3171/2015.6.JNS142637. [DOI] [PubMed] [Google Scholar]
- 4.Baussart B, Declerck A, Gaillard S. Mononostril endoscopic endonasal approach for pituitary surgery. Acta Neurochir (Wien) 2021;163(03):655–659. doi: 10.1007/s00701-020-04542-z. [DOI] [PubMed] [Google Scholar]
- 5.Goudakos J K, Markou K D, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: a systematic review and meta-analysis. Clin Otolaryngol. 2011;36(03):212–220. doi: 10.1111/j.1749-4486.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 6.Elhadi A M, Hardesty D A, Zaidi H Aet al. Evaluation of surgical freedom for microscopic and endoscopic transsphenoidal approaches to the sella Neurosurgery 2015110269–78., discussion 78–79 [DOI] [PubMed] [Google Scholar]
- 7.Kennedy D W, Cohn E S, Papel I D, Holliday M J. Transsphenoidal approach to the sella: the Johns Hopkins experience. Laryngoscope. 1984;94(08):1066–1074. doi: 10.1288/00005537-198408000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda K, Watanabe K, Suzuki H et al. Nasal airway resistance and olfactory acuity following transsphenoidal pituitary surgery. Am J Rhinol. 1999;13(01):45–48. doi: 10.2500/105065899781389957. [DOI] [PubMed] [Google Scholar]
- 9.Kahilogullari G, Beton S, Al-Beyati E S et al. Olfactory functions after transsphenoidal pituitary surgery: endoscopic versus microscopic approach. Laryngoscope. 2013;123(09):2112–2119. doi: 10.1002/lary.24037. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho A CM, Dolci R LL, Rickli J CK et al. Evaluation of olfactory function in patients undergoing endoscopic skull base surgery with nasoseptal flap. Rev Bras Otorrinolaringol (Engl Ed) 2022;88(01):15–21. doi: 10.1016/j.bjorl.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart C K, Theodosopoulos P V, Zimmer L A. Olfactory changes after endoscopic pituitary tumor resection. Otolaryngol Head Neck Surg. 2010;142(01):95–97. doi: 10.1016/j.otohns.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Rotenberg B W, Saunders S, Duggal N. Olfactory outcomes after endoscopic transsphenoidal pituitary surgery. Laryngoscope. 2011;121(08):1611–1613. doi: 10.1002/lary.21890. [DOI] [PubMed] [Google Scholar]
- 13.Kim B Y, Kang S G, Kim S W et al. Olfactory changes after endoscopic endonasal transsphenoidal approach for skull base tumors. Laryngoscope. 2014;124(11):2470–2475. doi: 10.1002/lary.24674. [DOI] [PubMed] [Google Scholar]
- 14.Zimmer L A, Shah O, Theodosopoulos P V. Short-term quality-of-life changes after endoscopic pituitary surgery rated with SNOT-22. J Neurol Surg B Skull Base. 2014;75(04):288–292. doi: 10.1055/s-0034-1372464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawabata T, Takeuchi K, Nagata Y et al. Preservation of olfactory function following endoscopic single-nostril transseptal transsphenoidal surgery. World Neurosurg. 2019;132:e665–e669. doi: 10.1016/j.wneu.2019.08.051. [DOI] [PubMed] [Google Scholar]
- 16.Hong S D, Nam D H, Kong D S, Kim H Y, Chung S K, Dhong H J. Endoscopic modified transseptal transsphenoidal approach for maximal preservation of sinonasal quality of life and olfaction. World Neurosurg. 2016;87:162–169. doi: 10.1016/j.wneu.2015.12.050. [DOI] [PubMed] [Google Scholar]
- 17.Little A S, Kelly D, Milligan J et al. Predictors of sinonasal quality of life and nasal morbidity after fully endoscopic transsphenoidal surgery. J Neurosurg. 2015;122(06):1458–1465. doi: 10.3171/2014.10.JNS141624. [DOI] [PubMed] [Google Scholar]
- 18.Riley C A, Tabaee A, Conley L et al. Long-term sinonasal outcomes after endoscopic skull base surgery with nasoseptal flap reconstruction. Laryngoscope. 2019;129(05):1035–1040. doi: 10.1002/lary.27637. [DOI] [PubMed] [Google Scholar]
- 19.Wu V, Cusimano M D, Lee J M. Extent of surgery in endoscopic transsphenoidal skull base approaches and the effects on sinonasal morbidity. Am J Rhinol Allergy. 2018;32(01):52–56. doi: 10.2500/ajra.2018.32.4499. [DOI] [PubMed] [Google Scholar]
- 20.Rimmer R A, Vimawala S, Chitguppi C et al. Rate of rhinosinusitis and sinus surgery following a minimally destructive approach to endoscopic transsphenoidal hypophysectomy. Int Forum Allergy Rhinol. 2020;10(03):405–411. doi: 10.1002/alr.22482. [DOI] [PubMed] [Google Scholar]
- 21.Wen G, Tang C, Zhong C et al. Mononostril versus binostril endoscopic transsphenoidal approach for pituitary adenomas: a systematic review and meta-analysis. PLoS One. 2016;11(04):e0153397. doi: 10.1371/journal.pone.0153397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little A S, Kelly D, Milligan J et al. Prospective validation of a patient-reported nasal quality-of-life tool for endonasal skull base surgery: the Anterior Skull Base Nasal Inventory-12. J Neurosurg. 2013;119(04):1068–1074. doi: 10.3171/2013.3.JNS122032. [DOI] [PubMed] [Google Scholar]
- 23.The Staging and Therapy Group . Lund V J, Kennedy D W. Quantification for staging sinusitis. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 24.Cain W S, Gent J F, Goodspeed R B, Leonard G. Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center. Laryngoscope. 1988;98(01):83–88. doi: 10.1288/00005537-198801000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Little A S, Kshettry V R, Rosen M R et al. Postoperative oral antibiotics and sinonasal outcomes following endoscopic transsphenoidal surgery for pituitary tumors study: a multicenter, prospective, randomized, double-blinded, placebo-controlled study. Neurosurgery. 2021;89(05):769–776. doi: 10.1093/neuros/nyab301. [DOI] [PubMed] [Google Scholar]
- 26.Gravbrot N, Kelly D F, Milligan J et al. The minimal clinically important difference of the anterior skull base nasal inventory-12. Neurosurgery. 2018;83(02):277–280. doi: 10.1093/neuros/nyx401. [DOI] [PubMed] [Google Scholar]
- 27.Berhouma M, Messerer M, Jouanneau E. Occam's razor in minimally invasive pituitary surgery: tailoring the endoscopic endonasal uninostril trans-sphenoidal approach to sella turcica. Acta Neurochir (Wien) 2012;154(12):2257–2265. doi: 10.1007/s00701-012-1510-2. [DOI] [PubMed] [Google Scholar]
- 28.Kim B Y, Son H L, Kang S G et al. Postoperative nasal symptoms associated with an endoscopic endonasal transsphenoidal approach. Eur Arch Otorhinolaryngol. 2013;270(04):1355–1359. doi: 10.1007/s00405-012-2226-x. [DOI] [PubMed] [Google Scholar]
- 29.Cingoz I D, Kizmazoglu C, Guvenc G, Sayin M, Imre A, Yuceer N. Evaluation of the olfactory function with the “sniffin' sticks” test after endoscopic transsphenoidal pituitary surgery. J Craniofac Surg. 2018;29(04):1002–1005. doi: 10.1097/SCS.0000000000004398. [DOI] [PubMed] [Google Scholar]
- 30.Raikundalia M D, Huang R J, Chan Let al. Olfactory-specific quality of life outcomes after endoscopic endonasal surgery of the sella Allergy Rhinol (Providence) 20211221526567211045041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Feng K, Tang C et al. Olfactory outcomes after endonasal skull base surgery: a systematic review. Neurosurg Rev. 2021;44(04):1805–1814. doi: 10.1007/s10143-020-01385-1. [DOI] [PubMed] [Google Scholar]
- 32.Yin L X, Low C M, Puccinelli C L et al. Olfactory outcomes after endoscopic skull base surgery: a systematic review and meta-analysis. Laryngoscope. 2019;129(09):1998–2007. doi: 10.1002/lary.28003. [DOI] [PubMed] [Google Scholar]
- 33.Chaaban M R, Chaudhry A L, Riley K O, Woodworth B A. Objective assessment of olfaction after transsphenoidal pituitary surgery. Am J Rhinol Allergy. 2015;29(05):365–368. doi: 10.2500/ajra.2015.29.4206. [DOI] [PubMed] [Google Scholar]
- 34.Ting K C, Wang W H, Kuan E C, Lin Y Y, Lan M Y. Bilateral smell preservation is routinely possible following endoscopic endonasal approach for sellar/suprasellar lesions. J Neurol Surg B Skull Base. 2019;82(04):410–416. doi: 10.1055/s-0039-3400751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greig S R, Cooper T J, Sommer D D, Nair S, Wright E D. Objective sinonasal functional outcomes in endoscopic anterior skull-base surgery: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6(10):1040–1046. doi: 10.1002/alr.21760. [DOI] [PubMed] [Google Scholar]
- 36.Stamm A C, Pignatari S, Vellutini E, Harvey R J, Nogueira J F., Jr A novel approach allowing binostril work to the sphenoid sinus. Otolaryngol Head Neck Surg. 2008;138(04):531–532. doi: 10.1016/j.otohns.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber A, Bertazzoni G, Ferrari M et al. Nasal morbidity and quality of life after endoscopic transsphenoidal surgery: a single-center prospective study. World Neurosurg. 2019;123:e557–e565. doi: 10.1016/j.wneu.2018.11.212. [DOI] [PubMed] [Google Scholar]
- 38.Sowerby L J, Gross M, Broad R, Wright E D. Olfactory and sinonasal outcomes in endoscopic transsphenoidal skull-base surgery. Int Forum Allergy Rhinol. 2013;3(03):217–220. doi: 10.1002/alr.21103. [DOI] [PubMed] [Google Scholar]
- 39.Conrad J, Blaese M, Becker S, Huppertz T, Ayyad A, Ringel F. Sinonasal outcome after endoscopic transnasal surgery-a prospective rhinological study. Oper Neurosurg (Hagerstown) 2023;24(03):223–231. doi: 10.1227/ons.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 40.Tao C, Cheng G, Chen Y, Gu P, Hu W. Early outcomes of endoscopic endonasal approach pituitary adenomas resection with minimal nasal injury. Medicine (Baltimore) 2021;100(46):e27843. doi: 10.1097/MD.0000000000027843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCoul E D, Anand V K, Bedrosian J C, Schwartz T H. Endoscopic skull base surgery and its impact on sinonasal-related quality of life. Int Forum Allergy Rhinol. 2012;2(02):174–181. doi: 10.1002/alr.21008. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa T, Takeuchi K, Nagatani T et al. Quality of life changes before and after transsphenoidal surgery for sellar and parasellar lesions. World Neurosurg. 2019;122:e1202–e1210. doi: 10.1016/j.wneu.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Bryl M, Woźniak J, Dudek K, Czapiga B, Tabakow P. The quality of life after transnasal microsurgical and endoscopic resection of nonfunctioning pituitary adenoma. Adv Clin Exp Med. 2020;29(08):921–928. doi: 10.17219/acem/123351. [DOI] [PubMed] [Google Scholar]
- 44.Hopkins C, Gillett S, Slack R, Lund V J, Browne J P. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(05):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 45.Stewart M G, Witsell D L, Smith T L, Weaver E M, Yueh B, Hannley M T. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(02):157–163. doi: 10.1016/j.otohns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Castle-Kirszbaum M, Wang Y Y, King J, Goldschlager T. Quality of life after endoscopic surgical management of pituitary adenomas. Neurosurgery. 2022;90(01):81–91. doi: 10.1227/NEU.0000000000001740. [DOI] [PubMed] [Google Scholar]
- 47.Choi K J, Ackall F Y, Truong T et al. Sinonasal quality of life outcomes after extended endonasal approaches to the skull base. J Neurol Surg B Skull Base. 2019;80(04):416–423. doi: 10.1055/s-0038-1675592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhenswala P N, Schlosser R J, Nguyen S A, Munawar S, Rowan N R. Sinonasal quality-of-life outcomes after endoscopic endonasal skull base surgery. Int Forum Allergy Rhinol. 2019;9(10):1105–1118. doi: 10.1002/alr.22398. [DOI] [PubMed] [Google Scholar]
- 49.Wang C H, Zhu N. Patient-reported quality of life after endoscopic surgery for pituitary lesions: a meta-analysis. J Biol Regul Homeost Agents. 2018;32(05):1151–1156. [PubMed] [Google Scholar]
- 50.Kirkman M A, Borg A, Al-Mousa A, Haliasos N, Choi D. Quality-of-life after anterior skull base surgery: a systematic review. J Neurol Surg B Skull Base. 2014;75(02):73–89. doi: 10.1055/s-0033-1359303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linsler S, Prokein B, Hendrix P, Oertel J. Sinonasal outcome after endoscopic mononostril transsphenoidal surgery: a single center cohort study. J Clin Neurosci. 2018;53:92–99. doi: 10.1016/j.jocn.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Kuwata F, Kikuchi M, Ishikawa M et al. Long-term olfactory function outcomes after pituitary surgery by endoscopic endonasal transsphenoidal approach. Auris Nasus Larynx. 2020;47(02):227–232. doi: 10.1016/j.anl.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Kim D H, Hong Y K, Jeun S S et al. Intranasal volume changes caused by the endoscopic endonasal transsphenoidal approach and their effects on nasal functions. PLoS One. 2016;11(03):e0151531. doi: 10.1371/journal.pone.0151531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hallén T, Olsson D S, Farahmand D et al. Sinonasal symptoms and self-reported health before and after endoscopic pituitary surgery-a prospective study. J Neurol Surg B Skull Base. 2021;83 02:e160–e168. doi: 10.1055/s-0041-1722929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novák V, Hrabálek L, Hoza J, Pohlodek D, Macura J. Sinonasal quality of life in patients after an endoscopic endonasal surgery of a sellar tumour. Sci Rep. 2021;11(01):23351. doi: 10.1038/s41598-021-02747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sunil A, Thakar S, Aryan S, Hegde A S. Changes in sinonasal and overall quality of life following endoscopic endonasal surgery for non-functioning pituitary adenomas: results of a prospective observational study. Neurol India. 2022;70(06):2357–2365. doi: 10.4103/0028-3886.364068. [DOI] [PubMed] [Google Scholar]


