Abstract
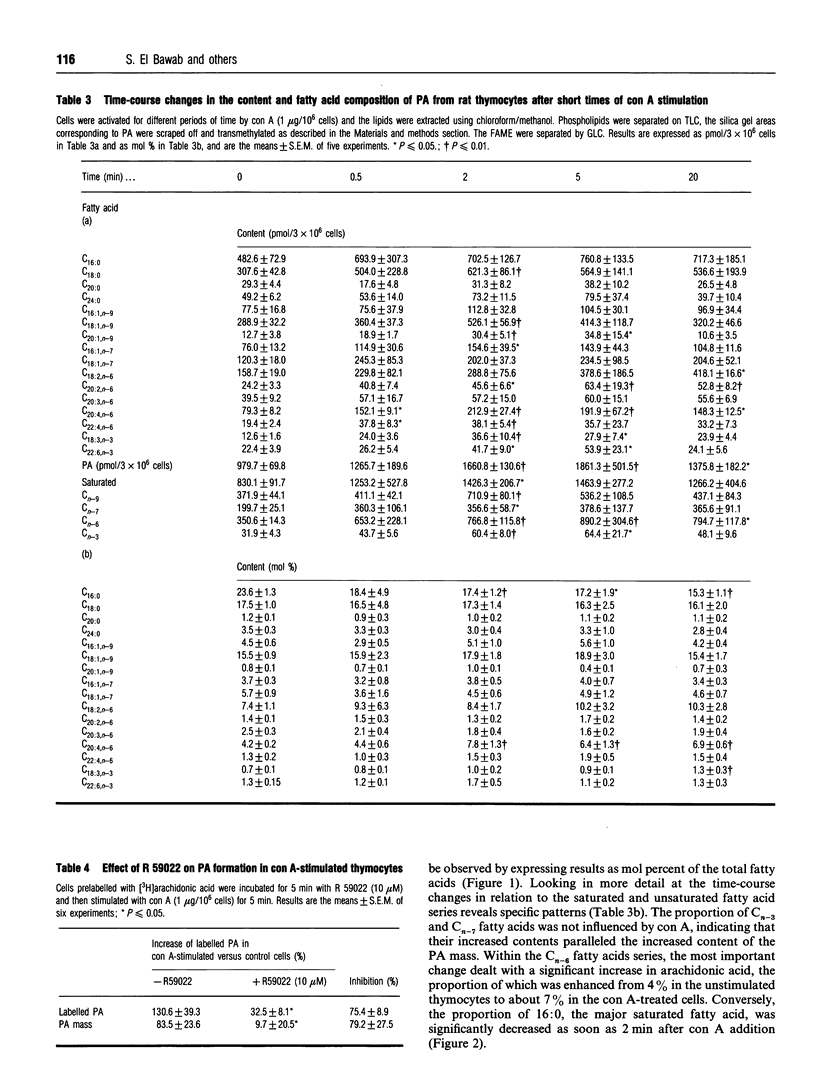
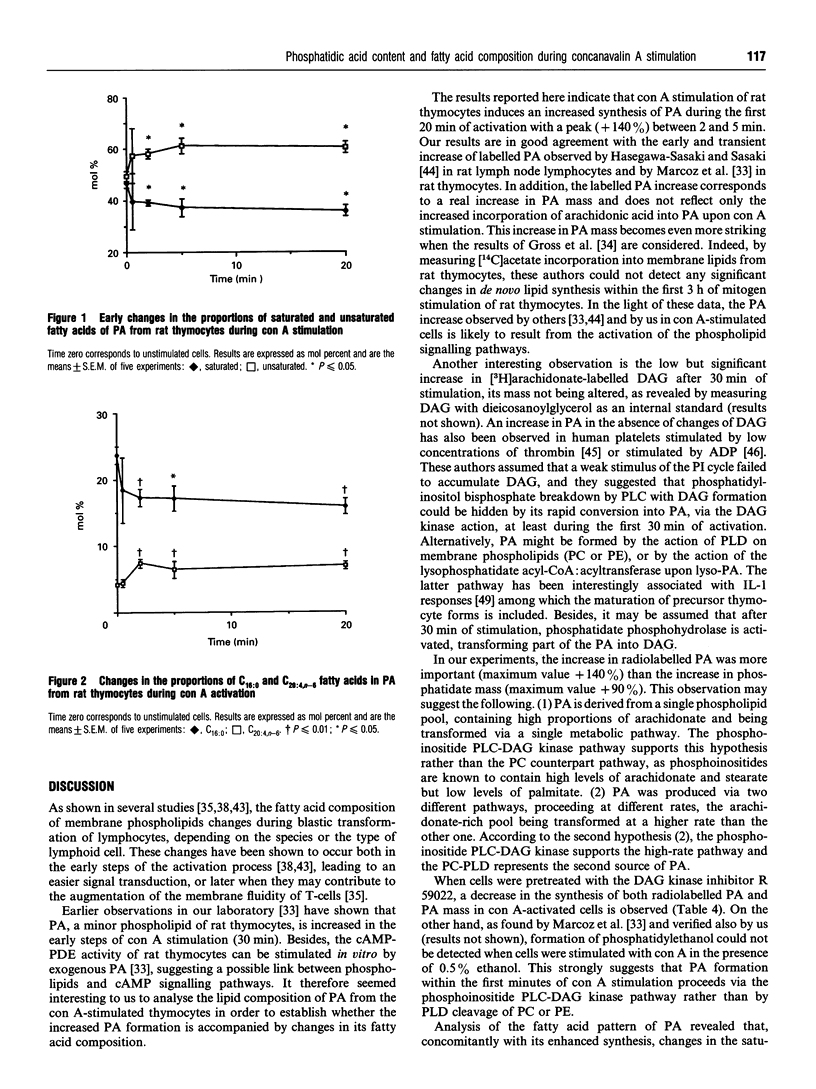
Several studies have shown the potential role of phosphatidic acid (PA) as a second messenger in different cell types. Thus, PA has been shown to mimic physiological agonists leading to various cellular responses, such as neurotransmitter and hormone release, cell proliferation by modulating DNA or RNA synthesis, the expression of several proto-oncogenes and growth factors, and the stimulation of enzyme activities such as phospholipase C (PLC), protein kinases and cyclic AMP (cAMP) phosphodiesterase. Stimulation of [3H]arachidonate-labelled rat thymocytes with the mitogen lectin concanavalin A (con A) resulted in enhanced production of radiolabelled PA after only 5 min of activation. The radiolabelled PA increase corresponded to a real increase in PA mass as determined by GLC quantification of its fatty acid content. In the presence of ethanol (0.5%), formation of phosphatidylethanol was not observed after 5 min of con A activation. Pretreatment of cells with R 59022 (10 microM), a diacylglycerol (DAG) kinase inhibitor, showed an inhibition in the formation of radiolabelled PA and in PA mass. These results suggest that the PLC-DAG kinase may be the pathway for PA synthesis in the first minutes of mitogenic thymocyte activation. A detailed analysis of the fatty acid composition showed that the relative amount of unsaturated fatty acids was increased in PA from stimulated cells concomitantly with a decrease in saturated ones; in particular, arachidonic acid was increased approximately 2-fold only 2 min after con A addition whereas palmitic acid was decreased for the whole period investigated (20 min). These changes favour the hydolysis of phosphoinositides rather than phosphatidylcholines by PLC. As PA remains a minor phospholipid, these changes are unlikely to affect cell membrane fluidity; but PA being now well recognized as a potential second messenger, its increased content as well as its increased unsaturation in the fatty acyl moiety might modulate several signalling pathways or the activity of enzymes such as cyclic nucleotide phosphodiesterase, controlling in this way the cellular level of cAMP, a negative regulator of blastic transformation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anel A., Naval J., González B., Torres J. M., Mishal Z., Uriel J., Piñeiro A. Fatty acid metabolism in human lymphocytes. I. Time-course changes in fatty acid composition and membrane fluidity during blastic transformation of peripheral blood lymphocytes. Biochim Biophys Acta. 1990 Jun 14;1044(3):323–331. doi: 10.1016/0005-2760(90)90076-a. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth G., Theodorou I., Gouy H., Le Gouvello S., Bernard A., Debré P. Cyclic AMP-mediated alteration of the CD2 activation process in human T lymphocytes. Preferential inhibition of the phosphoinositide cycle-related transduction pathway. Eur J Immunol. 1988 Sep;18(9):1351–1357. doi: 10.1002/eji.1830180908. [DOI] [PubMed] [Google Scholar]
- Bocckino S. B., Wilson P. B., Exton J. H. Phosphatidate-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6210–6213. doi: 10.1073/pnas.88.14.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursten S. L., Harris W. E., Bomsztyk K., Lovett D. Interleukin-1 rapidly stimulates lysophosphatidate acyltransferase and phosphatidate phosphohydrolase activities in human mesangial cells. J Biol Chem. 1991 Nov 5;266(31):20732–20743. [PubMed] [Google Scholar]
- Cano E., Muñoz-Fernández M. A., Fresno M. Regulation of interleukin-2 responses by phosphatidic acid. Eur J Immunol. 1992 Jul;22(7):1883–1889. doi: 10.1002/eji.1830220731. [DOI] [PubMed] [Google Scholar]
- Carpenedo F., Floreani M. Stimulation of rat liver microsomal cGMP-inhibited cAMP phosphodiesterase (PDE III) by phospholipase C and D. Biochem Biophys Res Commun. 1993 Jan 29;190(2):609–615. doi: 10.1006/bbrc.1993.1092. [DOI] [PubMed] [Google Scholar]
- Dubois M., Némoz G., Lagarde M., Prigent A. F. Phospholipid metabolism modulates cyclic nucleotide phosphodiesterase activity in rat heart microsomes. Biochem Biophys Res Commun. 1990 Jul 31;170(2):800–809. doi: 10.1016/0006-291x(90)92162-s. [DOI] [PubMed] [Google Scholar]
- Duncan E. M., Tunbridge L. J., Lloyd J. V. An increase in phosphatidic acid in the absence of changes in diacylglycerol in human platelets stimulated with ADP. Int J Biochem. 1993 Jan;25(1):23–27. doi: 10.1016/0020-711x(93)90485-w. [DOI] [PubMed] [Google Scholar]
- Eibl H., Kovatchev S. Preparation of phospholipids and their analogs by phospholipase D. Methods Enzymol. 1981;72:632–639. doi: 10.1016/s0076-6879(81)72055-x. [DOI] [PubMed] [Google Scholar]
- Epstein P. M., Hachisu R. Cyclic nucleotide phosphodiesterase in normal and leukemic human lymphocytes and lymphoblasts. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:303–324. [PubMed] [Google Scholar]
- Exton J. H., Taylor S. J., Augert G., Bocckino S. B. Cell signalling through phospholipid breakdown. 1991 May 29-Jun 12Mol Cell Biochem. 104(1-2):81–86. doi: 10.1007/BF00229807. [DOI] [PubMed] [Google Scholar]
- Ferber E., De Pasquale G. G., Resch K. Phospholipid metabolism of stimulated lymphocytes. Composition of phospholipid fatty acids. Biochim Biophys Acta. 1975 Sep 19;398(3):364–376. doi: 10.1016/0005-2760(75)90187-3. [DOI] [PubMed] [Google Scholar]
- Goppelt-Strübe M., Resch K. Polyunsaturated fatty acids are enriched in the plasma membranes of mitogen-stimulated T-lymphocytes. Biochim Biophys Acta. 1987 Nov 2;904(1):22–28. doi: 10.1016/0005-2736(87)90082-4. [DOI] [PubMed] [Google Scholar]
- Goppelt M., Eichhorn R., Krebs G., Resch K. Lipid composition of functional domains of the lymphocyte plasma membrane. Biochim Biophys Acta. 1986 Jan 29;854(2):184–190. doi: 10.1016/0005-2736(86)90109-4. [DOI] [PubMed] [Google Scholar]
- Goppelt M., Köhler L., Resch K. Functional role of lipid metabolism in activated T-lymphocytes. Biochim Biophys Acta. 1985 Mar 6;833(3):463–472. doi: 10.1016/0005-2760(85)90104-3. [DOI] [PubMed] [Google Scholar]
- Gross G., Danzl M., Fischer W., Brand K. Alterations of cellular lipids in rat thymocytes during cell cycle progression. Biochim Biophys Acta. 1988 Sep 23;962(2):220–226. doi: 10.1016/0005-2760(88)90163-4. [DOI] [PubMed] [Google Scholar]
- Hadden J. W. Transmembrane signals in the activation of T-lymphocytes by lectin mitogens. Mol Immunol. 1988 Nov;25(11):1105–1112. doi: 10.1016/0161-5890(88)90145-9. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Schmidt J., Hitzemann B. A., Hitzemann R. J. Phosphatidate as a molecular link between depolarization and neurotransmitter release in the brain. Science. 1981 Jun 12;212(4500):1290–1291. doi: 10.1126/science.7233220. [DOI] [PubMed] [Google Scholar]
- Hasegawa-Sasaki H., Sasaki T. Rapid breakdown of phosphatidylinositol accompanied by accumulation of phosphatidic acid and diacylglycerol in rat lymphocytes stimulated by concanavalin A. J Biochem. 1982 Feb;91(2):463–468. doi: 10.1093/oxfordjournals.jbchem.a133718. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Stimulation of phosphatidylinositol 4,5-bisphosphate phospholipase C activity by phosphatidic acid. Arch Biochem Biophys. 1989 Feb 1;268(2):516–524. doi: 10.1016/0003-9861(89)90318-4. [DOI] [PubMed] [Google Scholar]
- Johnson K. W., Davis B. H., Smith K. A. cAMP antagonizes interleukin 2-promoted T-cell cycle progression at a discrete point in early G1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6072–6076. doi: 10.1073/pnas.85.16.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kroll M. H., Zavoico G. B., Schafer A. I. Second messenger function of phosphatidic acid in platelet activation. J Cell Physiol. 1989 Jun;139(3):558–564. doi: 10.1002/jcp.1041390315. [DOI] [PubMed] [Google Scholar]
- Lerner A., Jacobson B., Miller R. A. Cyclic AMP concentrations modulate both calcium flux and hydrolysis of phosphatidylinositol phosphates in mouse T lymphocytes. J Immunol. 1988 Feb 1;140(3):936–940. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Marcoz P., Némoz G., Prigent A. F., Lagarde M. Phosphatidic acid stimulates the rolipram-sensitive cyclic nucleotide phosphodiesterase from rat thymocytes. Biochim Biophys Acta. 1993 Mar 10;1176(1-2):129–136. doi: 10.1016/0167-4889(93)90187-t. [DOI] [PubMed] [Google Scholar]
- Marcoz P., Prigent A. F., Lagarde M., Nemoz G. Modulation of rat thymocyte proliferative response through the inhibition of different cyclic nucleotide phosphodiesterase isoforms by means of selective inhibitors and cGMP-elevating agents. Mol Pharmacol. 1993 Nov;44(5):1027–1035. [PubMed] [Google Scholar]
- Meskini N., Hosni M., Nemoz G., Lagarde M., Prigent A. F. Early increase in lymphocyte cyclic nucleotide phosphodiesterase activity upon mitogenic activation of human peripheral blood mononuclear cells. J Cell Physiol. 1992 Jan;150(1):140–148. doi: 10.1002/jcp.1041500119. [DOI] [PubMed] [Google Scholar]
- Metz S. A., Dunlop M. Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem J. 1990 Sep 1;270(2):427–435. doi: 10.1042/bj2700427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Kruijer W., Tilly B. C., Verlaan I., Bierman A. J., de Laat S. W. Growth factor-like action of phosphatidic acid. Nature. 1986 Sep 11;323(6084):171–173. doi: 10.1038/323171a0. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Phosphatidic acid may stimulate membrane receptors mediating adenylate cyclase inhibition and phospholipid breakdown in 3T3 fibroblasts. J Biol Chem. 1987 Apr 25;262(12):5522–5529. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Weiss S. J., Van De Walle C. M., Haddas R. A. Is phosphatidic acid a calcium ionophore under neurohumoral control? Nature. 1980 Mar 27;284(5754):345–347. doi: 10.1038/284345a0. [DOI] [PubMed] [Google Scholar]
- Qian Z., Drewes L. R. Cross-talk between receptor-regulated phospholipase D and phospholipase C in brain. FASEB J. 1991 Mar 1;5(3):315–319. doi: 10.1096/fasebj.5.3.2001791. [DOI] [PubMed] [Google Scholar]
- Robicsek S. A., Blanchard D. K., Djeu J. Y., Krzanowski J. J., Szentivanyi A., Polson J. B. Multiple high-affinity cAMP-phosphodiesterases in human T-lymphocytes. Biochem Pharmacol. 1991 Jul 25;42(4):869–877. doi: 10.1016/0006-2952(91)90047-9. [DOI] [PubMed] [Google Scholar]
- Rode H. N., Szamel M., Schneider S., Resch K. Phospholipid metabolism of stimulated lymphocytes. Preferential incorporation of polyunsaturated fatty acids into plasma membrane phospholipid upon stimulation with concanavalin A. Biochim Biophys Acta. 1982 May 21;688(1):66–74. doi: 10.1016/0005-2736(82)90579-x. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Honeyman T. W. Proposed mechanism of cholinergic action in smooth muscle. Nature. 1980 Mar 27;284(5754):344–345. doi: 10.1038/284344a0. [DOI] [PubMed] [Google Scholar]
- Senisterra G. A., van Gorkom L. C., Epand R. M. Calcium-independent activation of protein kinase C by the dianionic form of phosphatidic acid. Biochem Biophys Res Commun. 1993 Jan 15;190(1):33–36. doi: 10.1006/bbrc.1993.1006. [DOI] [PubMed] [Google Scholar]
- Shenker B. J., Matt W. C. Suppression of human lymphocyte responsiveness by forskolin: reversal by 12-O-tetradecanoyl phorbol 13-acetate, diacylglycerol and ionomycin. Immunopharmacology. 1987 Feb;13(1):73–86. doi: 10.1016/0162-3109(87)90028-2. [DOI] [PubMed] [Google Scholar]
- Szamel M., Schneider S., Resch K. Functional interrelationship between (Na+ + K+)-ATPase and lysolecithin acyltransferase in plasma membranes of mitogen-stimulated rabbit thymocytes. J Biol Chem. 1981 Sep 10;256(17):9198–9204. [PubMed] [Google Scholar]
- Tysnes O. B., Steen V. M., Aarbakke G. M., Holmsen H. Stimulation of human platelets with low concentrations of thrombin: evidence for equimolar accumulation of inositol trisphosphates and phosphatidic acid. Int J Biochem. 1991;23(3):305–310. doi: 10.1016/0020-711x(91)90111-y. [DOI] [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]


