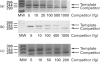
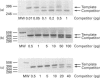
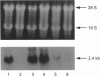
Abstract
The mutual role of glucose and insulin in the regulation of glucokinase and GLUT2 glucose transporter gene expression in pancreatic B-cells and liver has been studied in vivo in the rat. Glucokinase mRNA was quantified by competitive reverse-transcriptase PCR analysis, and GLUT2 mRNA by Northern-blot analysis in total RNA fractions. As in the liver, glucokinase mRNA decreased by 64% in pancreatic B-cells after starvation for 2 days and was induced 3-fold by short-term treatment (1 h) of the rats with oral glucose (4 g/kg body wt.). In contrast the sulphonylurea compound glibenclamide (0.1 mg/kg body wt.) did not significantly stimulate glucokinase gene expression in pancreatic B-cells. But glibenclamide caused a 4-fold increase of glucokinase mRNA in liver which was abolished by concomitant administration of diazoxide, a drug which antagonizes glibenclamide stimulated insulin secretion. GLUT2 gene expression was decreased by 50% in pancreatic B-cells and liver after starvation of the rats for 2 days. Neither short-term treatment (1 h) with glucose nor glibenclamide resulted in a significant increase of GLUT2 gene expression in pancreatic B-cells and liver. The results suggest that it is glucose which stimulates glucokinase gene expression in pancreatic B-cells whereas the transcriptional regulation of the glucokinase gene in liver is directed by insulin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreone T. L., Printz R. L., Pilkis S. J., Magnuson M. A., Granner D. K. The amino acid sequence of rat liver glucokinase deduced from cloned cDNA. J Biol Chem. 1989 Jan 5;264(1):363–369. [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Chen C., Bumbalo L., Leahy J. L. Increased catalytic activity of glucokinase in isolated islets from hyperinsulinemic rats. Diabetes. 1994 May;43(5):684–689. doi: 10.2337/diab.43.5.684. [DOI] [PubMed] [Google Scholar]
- Chen L., Alam T., Johnson J. H., Hughes S., Newgard C. B., Unger R. H. Regulation of beta-cell glucose transporter gene expression. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4088–4092. doi: 10.1073/pnas.87.11.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Efrat S., Leiser M., Wu Y. J., Fusco-DeMane D., Emran O. A., Surana M., Jetton T. L., Magnuson M. A., Weir G., Fleischer N. Ribozyme-mediated attenuation of pancreatic beta-cell glucokinase expression in transgenic mice results in impaired glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2051–2055. doi: 10.1073/pnas.91.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J., Gomis R., Fernández Alvarez J., Casamitjana R., Vilardell E. Signals derived from glucose metabolism are required for glucose regulation of pancreatic islet GLUT2 mRNA and protein. Diabetes. 1993 Sep;42(9):1273–1280. doi: 10.2337/diab.42.9.1273. [DOI] [PubMed] [Google Scholar]
- French S. W. Effect of chronic ethanol ingestion on liver enzyme changes induced by thiamine, riboflavin, pyridoxine, or choline deficiency. J Nutr. 1966 Mar;88(3):291–302. doi: 10.1093/jn/88.3.291. [DOI] [PubMed] [Google Scholar]
- Fölsch U. R., Creutzfeldt W. Pancreatic duct cells in rats: secretory studies in response to secretin, cholecystokinin-pancreozymin, and gastrin in vivo. Gastroenterology. 1977 Nov;73(5):1053–1059. [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg H., De Vos A., Vandercammen A., Van Schaftingen E., Pipeleers D., Schuit F. Heterogeneity in glucose sensitivity among pancreatic beta-cells is correlated to differences in glucose phosphorylation rather than glucose transport. EMBO J. 1993 Jul;12(7):2873–2879. doi: 10.1002/j.1460-2075.1993.tb05949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Yasuda K., Inoue G., Okamoto Y., Yano H., Someya Y., Ohmoto Y., Deguchi K., Imagawa K., Imura H. Glucose as regulator of glucose transport activity and glucose-transporter mRNA in hamster beta-cell line. Diabetes. 1992 May;41(5):592–597. doi: 10.2337/diab.41.5.592. [DOI] [PubMed] [Google Scholar]
- Iwashima Y., Pugh W., Depaoli A. M., Takeda J., Seino S., Bell G. I., Polonsky K. S. Expression of calcium channel mRNAs in rat pancreatic islets and downregulation after glucose infusion. Diabetes. 1993 Jul;42(7):948–955. doi: 10.2337/diab.42.7.948. [DOI] [PubMed] [Google Scholar]
- Iynedjian P. B., Gjinovci A., Renold A. E. Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats. J Biol Chem. 1988 Jan 15;263(2):740–744. [PubMed] [Google Scholar]
- Iynedjian P. B., Jotterand D., Nouspikel T., Asfari M., Pilot P. R. Transcriptional induction of glucokinase gene by insulin in cultured liver cells and its repression by the glucagon-cAMP system. J Biol Chem. 1989 Dec 25;264(36):21824–21829. [PubMed] [Google Scholar]
- Iynedjian P. B. Mammalian glucokinase and its gene. Biochem J. 1993 Jul 1;293(Pt 1):1–13. doi: 10.1042/bj2930001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iynedjian P. B., Pilot P. R., Nouspikel T., Milburn J. L., Quaade C., Hughes S., Ucla C., Newgard C. B. Differential expression and regulation of the glucokinase gene in liver and islets of Langerhans. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7838–7842. doi: 10.1073/pnas.86.20.7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iynedjian P. B., Ucla C., Mach B. Molecular cloning of glucokinase cDNA. Developmental and dietary regulation of glucokinase mRNA in rat liver. J Biol Chem. 1987 May 5;262(13):6032–6038. [PubMed] [Google Scholar]
- Lenzen S., Panten U. Signal recognition by pancreatic B-cells. Biochem Pharmacol. 1988 Feb 1;37(3):371–378. doi: 10.1016/0006-2952(88)90201-8. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Tiedge M., Flatt P. R., Bailey C. J., Panten U. Defective regulation of glucokinase in rat pancreatic islet cell tumours. Acta Endocrinol (Copenh) 1987 Aug;115(4):514–520. doi: 10.1530/acta.0.1150514. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Tiedge M. Regulation of pancreatic B-cell glucokinase and GLUT2 glucose transporter gene expression. Biochem Soc Trans. 1994 Feb;22(1):1–6. doi: 10.1042/bst0220001. [DOI] [PubMed] [Google Scholar]
- Liang Y., Najafi H., Smith R. M., Zimmerman E. C., Magnuson M. A., Tal M., Matschinsky F. M. Concordant glucose induction of glucokinase, glucose usage, and glucose-stimulated insulin release in pancreatic islets maintained in organ culture. Diabetes. 1992 Jul;41(7):792–806. doi: 10.2337/diab.41.7.792. [DOI] [PubMed] [Google Scholar]
- Magnuson M. A., Andreone T. L., Printz R. L., Koch S., Granner D. K. Rat glucokinase gene: structure and regulation by insulin. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4838–4842. doi: 10.1073/pnas.86.13.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson M. A., Shelton K. D. An alternate promoter in the glucokinase gene is active in the pancreatic beta cell. J Biol Chem. 1989 Sep 25;264(27):15936–15942. [PubMed] [Google Scholar]
- Magnuson M. A. Tissue-specific regulation of glucokinase gene expression. J Cell Biochem. 1992 Feb;48(2):115–121. doi: 10.1002/jcb.240480202. [DOI] [PubMed] [Google Scholar]
- Marie S., Diaz-Guerra M. J., Miquerol L., Kahn A., Iynedjian P. B. The pyruvate kinase gene as a model for studies of glucose-dependent regulation of gene expression in the endocrine pancreatic beta-cell type. J Biol Chem. 1993 Nov 15;268(32):23881–23890. [PubMed] [Google Scholar]
- Matschinsky F. M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990 Jun;39(6):647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Noguchi T., Yamada K., Takenaka M., Tanaka T. Regulation of the gene expression of glucokinase and L-type pyruvate kinase in primary cultures of rat hepatocytes by hormones and carbohydrates. J Biochem. 1990 Nov;108(5):778–784. doi: 10.1093/oxfordjournals.jbchem.a123280. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. New perspectives on pancreatic islet glucokinase. Am J Physiol. 1984 Jan;246(1 Pt 1):E1–13. doi: 10.1152/ajpendo.1984.246.1.E1. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Matschinsky F. M. Pancreatic islet glucose metabolism and regulation of insulin secretion. Diabetes Metab Rev. 1986;2(3-4):163–214. doi: 10.1002/dmr.5610020301. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Chiu K. C., Tanizawa Y. Glucokinase and NIDDM. A candidate gene that paid off. Diabetes. 1992 Nov;41(11):1367–1372. doi: 10.2337/diab.41.11.1367. [DOI] [PubMed] [Google Scholar]
- Purrello F., Buscema M., Vetri M., Vinci C., Gatta C., Forte F., Rabuazzo A. M., Vigneri R. Glucose regulates both glucose transport and the glucose transporter gene expression in a hamster-derived pancreatic beta-cell line (HIT). Diabetologia. 1991 May;34(5):366–369. doi: 10.1007/BF00405011. [DOI] [PubMed] [Google Scholar]
- Sako Y., Grill V. E. Coupling of beta-cell desensitization by hyperglycemia to excessive stimulation and circulating insulin in glucose-infused rats. Diabetes. 1990 Dec;39(12):1580–1583. doi: 10.2337/diab.39.12.1580. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. Competitive PCR. Nature. 1992 Oct 8;359(6395):557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- Tal M., Liang Y., Najafi H., Lodish H. F., Matschinsky F. M. Expression and function of GLUT-1 and GLUT-2 glucose transporter isoforms in cells of cultured rat pancreatic islets. J Biol Chem. 1992 Aug 25;267(24):17241–17247. [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Tiedge M., Lenzen S. Regulation of glucokinase and GLUT-2 glucose-transporter gene expression in pancreatic B-cells. Biochem J. 1991 Nov 1;279(Pt 3):899–901. doi: 10.1042/bj2790899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho G., Froguel P., Clement K., Pueyo M. E., Rakotoambinina B., Zouali H., Passa P., Cohen D., Robert J. J. Primary pancreatic beta-cell secretory defect caused by mutations in glucokinase gene in kindreds of maturity onset diabetes of the young. Lancet. 1992 Aug 22;340(8817):444–448. doi: 10.1016/0140-6736(92)91768-4. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C., Sorenson R. L., Kaung H. L. An immunohistochemical, ultrastructural, and physiologic study of pancreatic islets from copper-deficient, penicillamine-treated rats. Diabetes. 1986 Jan;35(1):13–19. doi: 10.2337/diab.35.1.13. [DOI] [PubMed] [Google Scholar]
- Weaver C., Sorenson R. L., Kobienia B. Nonenzymatic isolation and culture of adult islets from atrophic pancreata of copper-deficient rats: a morphologic analysis. In Vitro Cell Dev Biol. 1988 Feb;24(2):108–116. doi: 10.1007/BF02623887. [DOI] [PubMed] [Google Scholar]
- Weir G. C. A defective beta-cell glucose sensor as a cause of diabetes. N Engl J Med. 1993 Mar 11;328(10):729–731. doi: 10.1056/NEJM199303113281012. [DOI] [PubMed] [Google Scholar]