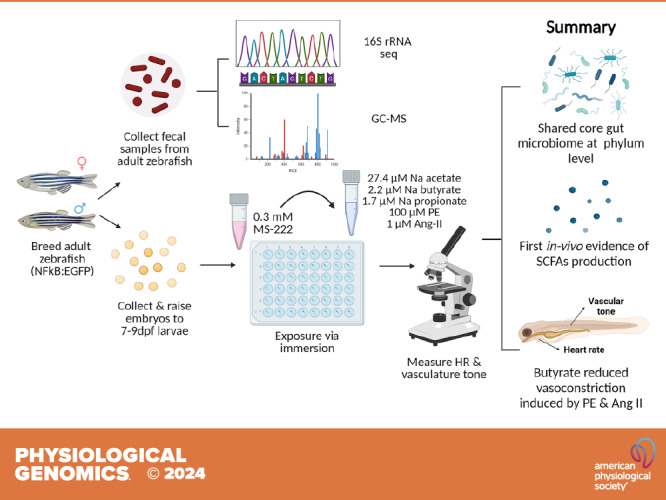
Keywords: acetate, butyrate, cardiovascular, microbiota, zebrafish
Abstract
Short-chain fatty acids (SCFAs) produced by the gut bacteria have been associated with cardiovascular dysfunction in humans and rodents. However, studies exploring effects of SCFAs on cardiovascular parameters in the zebrafish, an increasingly popular model in cardiovascular research, remain limited. Here, we performed fecal bacterial 16S sequencing and gas chromatography/mass spectrometry (GC-MS) to determine the composition and abundance of gut microbiota and SCFAs in adult zebrafish. Following this, the acute effects of major SCFAs on heart rate and vascular tone were measured in anesthetized zebrafish larvae using fecal concentrations of butyrate, acetate, and propionate. Finally, we investigated if coincubation with butyrate may lessen the effects of angiotensin II (ANG II) and phenylephrine (PE) on vascular tone in anesthetized zebrafish larvae. We found that the abundance in Proteobacteria, Firmicutes, and Fusobacteria phyla in the adult zebrafish resembled those reported in rodents and humans. SCFA levels with highest concentration of acetate (27.43 µM), followed by butyrate (2.19 µM) and propionate (1.65 µM) were observed in the fecal samples of adult zebrafish. Immersion in butyrate and acetate produced a ∼20% decrease in heart rate (HR), respectively, with no observed effects of propionate. Butyrate alone also produced an ∼25% decrease in the cross-sectional width of the dorsal aorta (DA) at 60 min (*P < 0.05), suggesting compensatory vasoconstriction, with no effects of either acetate or propionate. In addition, butyrate significantly alleviated the decrease in DA cross-sectional width produced by both ANG II and PE. We demonstrate the potential for zebrafish in investigation of host-microbiota interactions in cardiovascular health.
NEW & NOTEWORTHY We highlight the presence of a core gut microbiota and demonstrate in vivo short-chain fatty acid production in adult zebrafish. In addition, we show cardio-beneficial vasoactive and chronotropic properties of butyrate, and chronotropic properties of acetate in anesthetized zebrafish larvae.
INTRODUCTION
Zebrafish (Danio rerio), a teleost model and a member of the Cyprinidae family, has become an increasingly popular vertebrate model in biomedical research due to advantages that include genome homology, optical clarity, high fecundity and rapid development, high throughput capability, ease of genetic manipulation, and cost efficiency in husbandry (1–3). In addition to these, it is also considered a valuable model in cardiovascular research (4), and a variety of techniques are currently available for physiological hemodynamic measurements in adults and larval zebrafish (5–19).
The gut bacteria produce short-chain fatty acids (SCFAs) by fermentation of resistant starch and dietary fibers in the colon (20–22). Acetate, propionate, and butyrate are three major SCFA metabolites produced in the colon with approximate molar ratio of 3:1:1 in humans (20, 21) and 8:1:1 in rodents (23), but their physiological composition in the zebrafish is unknown. SCFAs can modify host physiology via anti-inflammatory, cardio-, hepato-, and neuro-protective effects (22, 24–26). These include regulation of blood pressure (24, 26–29); however, how the SCFAs may affect the cardiovascular system in the zebrafish is unknown.
Although the composition of the zebrafish microbiota has been reported (30, 31), and the ability of the zebrafish gut bacteria to synthesize three major SCFAs has been demonstrated in vitro (32), to the best of our knowledge, there have been no studies confirming zebrafish SCFAs production in vivo. Studies exploring the effects of SCFAs on cardiovascular parameters in the zebrafish are also lacking. Here, we took advantage of the optical clarity of the zebrafish larvae to investigate the effects of major SCFAs on the heart and vascular tone in vivo. We report similarities in the gut microbiota and SCFA composition and concentration in the zebrafish with that of rodent models and humans. This, coupled with the observed cardiovascular effects of select SCFAs, and the high throughput capability of the zebrafish larvae, suggests a use for zebrafish in understanding host-microbiota interactions in cardiovascular health.
MATERIALS AND METHODS
Animals and Husbandry
Adult Tg(NFkB:EGFP) D. rerio zebrafish were purchased from Zebrafish International Resource Center (Eugene, OR), bred and raised inhouse at the University of Toledo Center for Drug Design and Development Zebrafish Core Facility on a recirculating water system (Tecniplast, 28°C, 14:10-h light:dark cycle). Adult zebrafish were fed live brine shrimp (Artemia salina; 58% crude protein, 4.4% crude fat, 7% crude fiber), and supplemented by Golden Pearl Spheres [300–500 μm, Brine Shrimp Direct; 55% protein, 15% lipids, 12% ash, 2,550 ppm vitamin C, 425 ppm vitamin E, 10 mg/g eicosapentaenoic acid (EPA), and 12 mg/g docosahexaenoic acid (DHA)] once daily. To generate the larvae, male and female adult zebrafish (8–12 mo) were placed together in a breeding tank (1:2 ratio of male to female) overnight. Embryos were collected the following morning and raised in embryo water (sterile distilled water with adjusted salinity, pH 7.6, at 28 ± 1°C). Following experimental procedures, all subjects were euthanized by rapid chilling by submerging in 2–4°C ice-chilled water for 30 min, and death was confirmed by observation of cessation of opercular movement. All experiments and husbandry practices were performed in accordance with the Institutional Animal Care and Use Committee guidelines at the University of Toledo (IACUC No. 400140 and 400099).
Gut Bacterial and Short-Chain Fatty Acid Analyses
For collection of fecal samples, adult zebrafish (n = 10–12/tank/sex, ∼15 mo) were placed in a 1.7-L sloped breeding tank with a perforated internal tank overnight. Fecal samples (100 mg/sample) were collected the following morning and centrifuged (2,000 rpm, 10 min). Excess water was removed from fecal samples using a suction micropipette following centrifugation. Three tanks contained males and the other three tanks were females (n = 10–12 subjects/tank/sample, defined as n = 6 pooled samples). Analysis was done independently of sex. Water sample from the housing tank was used as a control to confirm no contamination. Once collected, samples were stored at −80°C until further processing. For bacterial 16S rRNA gene sequencing, DNA extraction was performed using QIAam PowerFecal DNA kit (Qiagen) as previously described (33). Bacterial 16S rRNA gene sequencing was performed on an Illumina MiSeq platform as previously described (33) and raw 16S data were analyzed using Quantitative Insights Into Microbial Ecology (QIIME2, v.2023.9) and SILVA database (v.132) (34–36). Data from QIIME2 were further filtered to remove amplicon sequence variants (features) with low abundance (<1%) after summing across all samples in RStudio (v.2023.09.0 + 463) for descriptive analyses and presented as bar plots. In the same fecal samples, SCFA analysis was performed by gas chromatography mass spectrometry (GC-MS) (Creative Proteomics, Shirley, NY) (29, 37). Briefly, fecal samples were diluted in water with labeled internal standards for each chain length (C2–C6). The free SCFAs were derivatized using methyl chloroformate in 1-propanol yielding propyl esters before subsequent liquid-liquid extraction into hexane and analysis on a SLB-5ms (30 × 0.25 mm × 1.0 μm) column. Detection was performed using gas chromatography electron ionization mass spectrometry (GC-EI-MS) in selective ion monitoring (SIM) mode and compared with standards. The analytes were quantified using a six-point calibration curves. Final SCFA levels were calculated as µg per g of wet feces.
Effect of Short-Chain Fatty Acids on Heart Rate and Vascular Tone in the Zebrafish Larvae
All experimental procedures used zebrafish larvae that exhibited normal developmental endpoints at 7–9 days postfertilization (dpf) (38, 39). Zebrafish larvae (n = 5–6/treatment) were anesthetized with tricaine (MS-222, Sigma Aldrich; Cat. No. E10521) by immersion (0.3 mM, pH 7.6) for 10 min before measurements. The appropriate tricaine dosage was established in preliminary experiments showing no effects on heart rate (HR) as per Ref. 40. Following baseline measurements, subjects were immersed in embryo water containing either sodium butyrate (2.2 µM, pH 7.6; Sigma-Aldrich; Cat. No. B5887), sodium acetate (27.4 µM, pH 7.6; Sigma-Aldrich; Cat. No. S2889), or sodium propionate (1.7 µM, pH 7.6; Sigma-Aldrich; Cat. No. P1880). These concentrations were determined using GC-MS in zebrafish fecal samples, and final dosages for administration were based on estimated fecal weight (1% of average body weight of the zebrafish larvae) and expressed as µM (41). All compounds were dissolved in embryo water and the pH was adjusted prior to administration. For measurements of effects of SCFAs on HR and vascular tone, larvae were positioned dorsally to allow for optical clarity, and heartbeat was recorded for 10 s every 10 min for an hour and averaged to beats per minute (BPM) in real time under a stereo microscope (Zeiss Stemi 2000-C). To measure effects of SCFA on vascular tone, in anesthetized zebrafish larvae (n = 6–8/treatment), baseline video recordings (representing 0 min timepoint) of dorsal aorta (DA) were taken for 30 s under a phase contrast microscope (Nikon SMZ18, 4× magnification with 1.6× lens). Following administration of SCFAs to the wells containing the larvae, video recordings of the same DA region were taken for 30 s every 10 min for 1 h. For analysis, still images of recordings were exported into ImageJ (v.1.54d). DA cross-sectional width for each treatment was determined by measurements at 10 different points along DA length and averaged for each treatment, timepoint, and larvae.
Effect of Phenylephrine and Angiotensin II on Heart Rate and Vascular Tone in the Zebrafish Larvae
Zebrafish larvae (n = 5–6/treatment) were anesthetized with tricaine by immersion for 10 min before baseline measurements, as previously described. To measure the effects of angiotensin acetate salt II (ANG II) and phenylephrine (PE) on HR, compounds (100 µM of PE, pH 7.6; Sigma-Aldrich; Cat. No. P1240000, or 1 µM of ANG II, pH 7.6; Bachem; Cat. No. 4006473 in embryo water) were administered in separate well plates containing anesthetized larvae. Concentrations of ANG II and PE were based on previous reports of their vasoconstrictive effects (42, 43). HR and vascular tone were imaged as described previously, before and following administration of ANG II or PE to the wells, every 10 min for an hour. Analysis of images was performed as described earlier.
Coincubation of Phenylephrine and Angiotensin II with Sodium Butyrate
Zebrafish larvae (n = 5–10/treatment group) were anesthetized with tricaine by immersion for 10 min before treatments, as previously described. Following baseline measurements, zebrafish were administered with 100 µM of PE, 1 µM of ANG II, and 2.2 µM of sodium butyrate alone or in combination with PE or ANG II. Changes in the vascular tone were analyzed as described previously.
Statistical Analyses
16S rRNA sequencing data was analyzed using QIIME2 (v.2023.9) and SILVA database (v.132) (34–36). GraphPad Prism (v.10.0.3) was used for all other statistical analysis. One-way ANOVA was used to analyze parametric end points followed by Dunnett’s multiple comparison test. Repeated-measures ANOVA was used when variables are assessed over time in the same subjects followed by Dunnett’s multiple comparison test. Two-way ANOVA was used to measure interaction between two independent variables on the dependent variable followed by appropriate post hoc test. Sample size for each treatment group and statistical test used are denoted in the figures. All data are presented as means ± SE.
RESULTS
16S and SCFA Analyses Reveal the Prevalence of Three Major Phyla and SCFAs
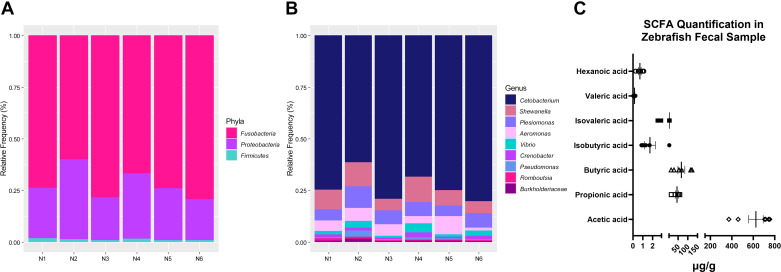
We observed three major phyla in fecal samples of adult zebrafish, namely, the Fusobacteria, Proteobacteria, and Firmicutes (Fig. 1A) that make up the gut microbiota. At the genus level, there was an abundance of Cetobacterium, followed by Shewanella, Plesiomonas, Aeromonas, Vibrio, Crenobacter, Pseudomonas, Romboutsia, and, finally, Burkholderiaceae (Fig. 1B). GC-MS analysis revealed the highest concentration of acetate, followed by butyrate, propionate, isovalerate, isobutyrate, hexanoate, and valerate (Fig. 1C).
Figure 1.
Relative abundance of bacterial taxa and composition of short-chain fatty acids (SCFAs) in adult zebrafish. A and B: 16S rRNA sequencing showing three major phyla (A) and nine genera (B) that make up the gut microbiota in the adult zebrafish (n = 6 pooled samples from 10 to 12 zebrafish/tank/sample). Bar plots show the relative abundance of bacterial taxa in each pooled sample created in RStudio. C: SCFA composition and abundance in fecal samples of adult zebrafish (n = 6 pooled samples from 10 to 12 zebrafish/tank/sample).
Butyrate and Acetate Induced Bradycardia With No Major Effects on Vascular Tone
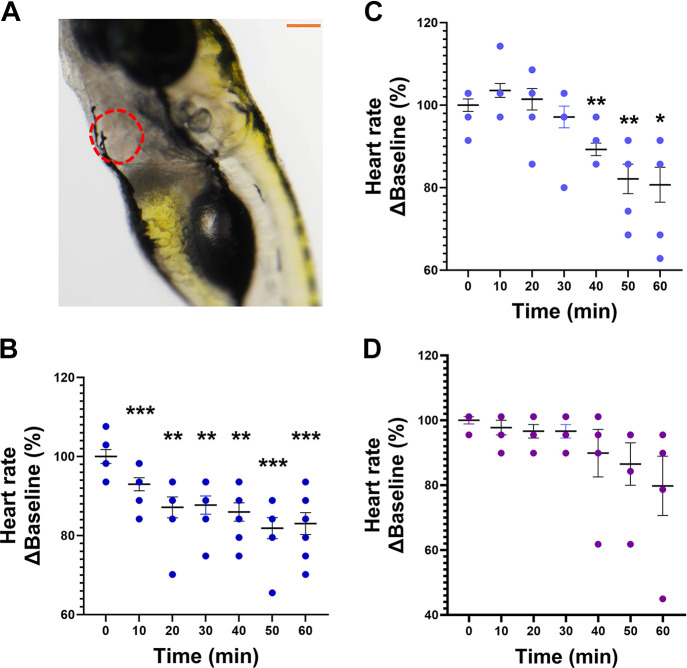
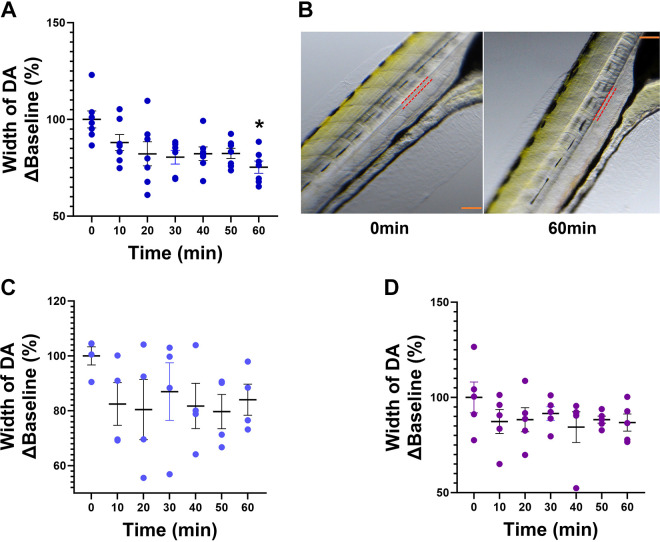
Figure 2A illustrates the optical clarity of zebrafish larvae that allows visualization of the heart under a light microscope. Exposure to sodium butyrate (2.2 µM, n = 8) (Fig. 2B) reduced HR at all time points (**P < 0.01, ***P < 0.001), whereas sodium acetate (27.4 µM, n = 8) produced a significant decrease in HR at 40 min (**P < 0.01), 50 min (**P < 0.01), and 60 min following immersion (*P < 0.05) (Fig. 2C) when compared with baseline (0 min). In contrast, sodium propionate (1.7 µM, n = 5) (Fig. 2D) produced no effect on HR at any time point. Exposure to sodium butyrate (n = 7, *P < 0.05) reduced DA cross-sectional width at 60 min when compared with baseline (0 min) (Fig. 3A). Figure 3B shows representative images of the DA outlined in red dotted lines at baseline (left image) and at 60 min (right image) following butyrate treatment. Exposure to sodium acetate (n = 4) or propionate (n = 5) produced no significant effects on the cross-sectional width of the DA (Fig. 3, C and D).
Figure 2.
Effects of sodium butyrate, sodium acetate, and sodium propionate on heart rate (HR) in anesthetized zebrafish larvae. A: representative image showing optical clarity allowing for visualization of the heart (in red dotted lines). Scale bar in orange = 200 µm. B−D: effects of administration of 2.2 µM butyrate (B), 27.4 µM acetate (C), and 1.7 µM propionate (D) on HR in zebrafish larvae, calculated as %Baseline (0 min). Data are presented as means ± SE (n = 5–8/treatment group). One-way ANOVA with a Dunnett’s post hoc test; *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3.
Effects of sodium butyrate, sodium acetate, and sodium propionate on vascular tone in anesthetized zebrafish larvae. A: 2.2 µM sodium butyrate produced a significant decrease in the cross-sectional width of the dorsal aorta (DA) at 60 min. B: red dotted lines mark the outline of the DA at baseline (0 min, left) and at 60 min (right) following exposure to sodium butyrate. Scale bar in orange = 200 µm. C and D: 27.4 µM acetate (C) and 1.7 µM propionate (D) produced no change. Data are presented as means ± SE and calculated as %Baseline (0 min). n = 5–8/treatment group. Repeated-measures one-way ANOVA with a Dunnett’s post hoc test; *P < 0.05.
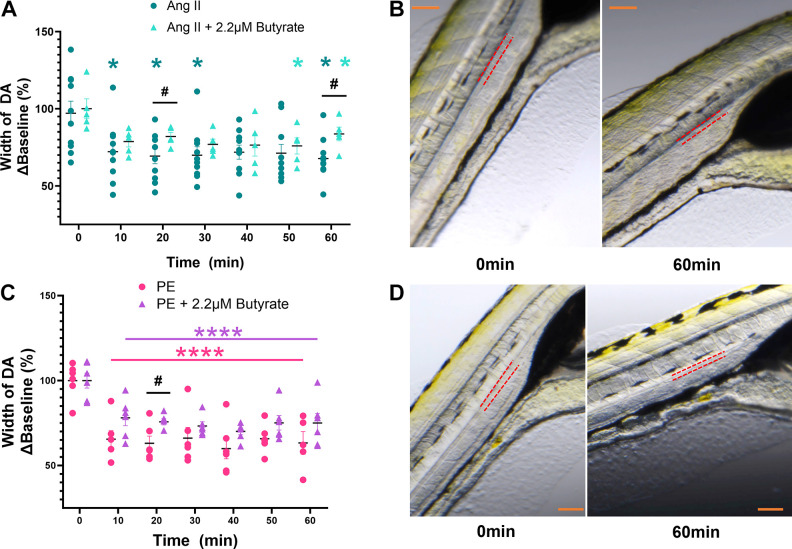
Butyrate Reduced the Effects of PE and ANG II on the Cross-Sectional Width of the Dorsal Aorta
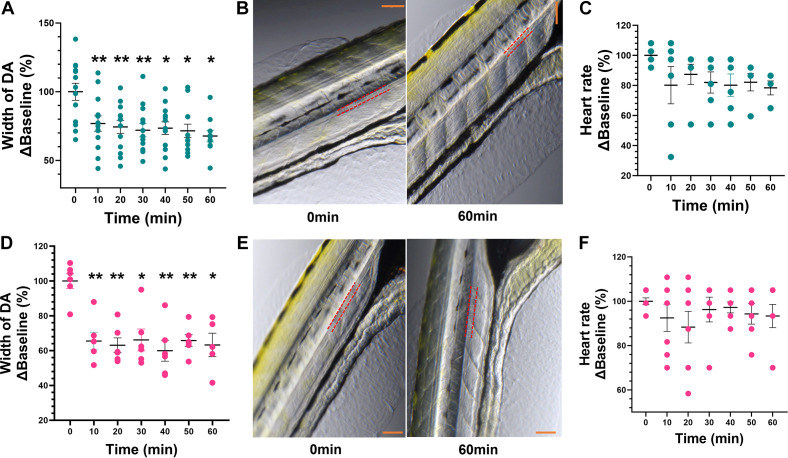
Treatments with 1 µM ANG II (n = 13) and 100 µM PE (n = 6) produced a significant decrease in DA cross-sectional width (∼30%, *P < 0.05, and ∼40%, **P < 0.01, respectively) (Fig. 4, A and D) with no significant effects on HR (n = 6–7/treatment group) when compared with baseline (0 min) (Fig. 4, C and F). Representative images indicate the outline of the DA with red dotted line where measurements were taken, and white lines show the DA cross-sectional width at 10 different points along the length of DA at baseline and 60 min following exposure to ANG II and PE, respectively (Fig. 4, B and E). Coadministration of 2.2 µM butyrate with ANG II produced a significant increase (∼10%) in DA cross-sectional width at 20 and 60 min when compared with ANG II alone (Fig. 5A) (#P < 0.05). In addition, coadministration of 2.2 µM butyrate with PE resulted in ∼15% increase in DA cross-sectional width at 20 min when compared with PE alone (Fig. 5C) (#P < 0.05). Figure 5, B and D, shows representative images of the DA at baseline and at 60 min following exposure to ANG II (top right) and PE with 2.2 µM butyrate (bottom right).
Figure 4.
Effects of phenylephrine and angiotensin II (ANG II) on vascular tone and heart rate in anesthetized zebrafish larvae. A and D: administration of 1 µM ANG II (A) and 100 µM phenylephrine (PE) (D) produced a decrease in the cross-sectional width of dorsal aorta (DA) at all time points when compared with baseline (0 min). B and E: representative images at baseline (0 min) and the 60-min timepoint for ANG II (top) and PE (bottom), with red dotted lines marking the outline of the DA. Scale bar in orange = 200 µm. C and F: ANG II (C) and (F) PE had no effect on heart rate (beats/min). Data are presented as means ± SE (n = 6–7 PE and 6–13 ANG II) and calculated as %Baseline (0 min). Repeated-measures one-way ANOVA with a Dunnett’s post hoc test; *P < 0.05, **P < 0.01.
Figure 5.
Effect of coadministration of sodium butyrate with phenylephrine (PE) and angiotensin II (ANG II) on vascular tone. A: 1 µM ANG II alone (in dark teal) significantly decreased dorsal aorta (DA) cross-sectional width at several time points compared with baseline (0 min). Coadministration of sodium butyrate (in light teal) reduced the effects of ANG II at 20 and 60 min. B: representative DA images at 0 and 60 min for ANG II, with red dotted lines marking the outline of the DA. Scale bar in orange = 200 µm. C: 100 µM PE alone (in pink) produced a significant decrease in DA cross-sectional width at all timepoints when compared with baseline (0 min). Coadministration of sodium butyrate (in purple) significantly reduced the effects of PE at 20 min. D: representative DA images at 0 and 60 min for PE, with red dotted lines marking the outline of the DA. Scale bar in orange = 200 µm. Data are presented as means ± SE and calculated as %Baseline (0 min). n = 5–10/treatment group. Two-way ANOVA with a Dunnett’s and Sidak’s multiple comparison test; *P < 0.05, ****P < 0.0001: within-group comparison to baseline (0 min). #P < 0.05 comparisons between treatment groups.
DISCUSSION
We uncovered several novel findings. We first determined the levels of SCFAs in fecal samples of adult zebrafish in vivo. We found that acetate, followed by butyrate and propionate, were the three major SCFAs present at highest concentrations in adult zebrafish. In humans, the approximate molar ratio of intestinal SCFAs is reportedly 3:1:1 (acetate:propionate:butyrate) (20, 21, 26, 44), and in rodents these ratios are ∼8:1:1 (23, 45). Thus, the relative trend of abundance of the three major SCFAs are similar in zebrafish (16:1:1), rodents, and humans. An approximate 1:1 ratio of propionate:butyrate is found across all three species. In addition, we found that Fusobacteria, Proteobacteria, and Firmicutes were the three most abundant phyla in adult zebrafish, consistent with previous reports (46–48). These phyla are shared with rodents and complex mammals (49). In addition, at the genus level, Aeromonas and Vibrio are reportedly the two most predominant genera in the zebrafish (50), consistent with our current findings. It is expected that the composition and abundance of the gut bacteria will differ between species owing to many internal and external factors; however, our findings highlight that significant conservation in the gut bacteria and their major SCFA products exists between the zebrafish, rodents, and humans, justifying the use of the zebrafish in investigation of host-microbiota interactions. As SCFAs are metabolic byproducts of dietary fibers and resistant starch, diet can play a role in modifying SCFAs and gut bacterial community. The larval zebrafish rely on the yolk sac for nutrition up to 7 dpf (51), therefore modification of nutrient intake in the young larvae may be challenging. In the adult zebrafish, the dietary nutrients in the feed primarily consist of proteins, with smaller percentages of fat, lipids, fiber, and vitamins. The dietary proteins are crucial for growth and metabolism throughout the zebrafish development (52, 53) making the dietary studies challenging. Despite this, several studies have investigated dietary effects on zebrafish gut microbiota and physiology (9, 54, 55) but studies of zebrafish SCFAs is lacking. The developments in gnotobiotic husbandry in the zebrafish (56–59) provide an accessible avenue for investigation of host-microbiota interactions to researchers limited by the lack of rodent germ-free facilities.
We next investigated the effects of the three most abundant SCFAs (acetate, propionate, and butyrate) on HR and vascular tone in the larvae. We chose to use the larval zebrafish at 7–9 dpf due to their optical clarity at this developmental stage, which enabled us to monitor the HR and vascular effects of SCFAs by light microscopy. Anatomical positioning were aided by study from Fritsche et al. (60), where digital motion analysis investigated the effects of sodium nitroprusside and epinephrine, as well as by the vascular anatomy atlas of the embryonic and early larval development in the zebrafish (61). We chose to focus on the DA as it is the main artery that runs along the trunk and carries oxygenated blood from the gills throughout the body (62, 63). We show that immersion in sodium acetate and butyrate produced a significant bradycardia at several time points within an hour, with no effects of sodium propionate. The effects of sodium butyrate on the heart are consistent with those reported in rats, where activation of GPR41/43 receptors and modulation of the nervous system is reported to play a role (27, 60, 64–67). However, there have not been many studies investigating SCFA receptors in regulation of cardiovascular variables in the zebrafish. One study identified and validated 10 coding sequences for common carp (Cyprinus carpio) gpr40L gene as it is closely related to the mammalian GPR43 (68). Cholan et al. (32) studied the role of hcar1 ortholog in reducing inflammatory responses following a tail wound injury in the zebrafish, while a recent study showed evidence of expression of free fatty acid receptor 2 (FFAR2,also known as GPR43) that can be activated by SCFAs in the zebrafish gut-brain axis in cadmium-induced neurodevelopmental toxicity (69). Interestingly, we observed no major effects of SCFAs on the vascular tone when administered alone, contrary to the reports in rodents (70–72) and human colonic arteries (73, 74). We measured the changes in the vascular tone by the increase and decrease in the cross-sectional width of the DA, which were considered as indicators of vasodilation and vasoconstriction respectively, similar to a study by Fritsche et al. (60). A significant decrease in the DA cross-sectional width following 1 h immersion with butyrate, indicative of xpotential vasoconstriction, was attributed to a potential compensatory response to butyrate-induced bradycardia to maintain hemodynamic homeostasis.
Next, we explored whether butyrate may lessen the effects of ANG II and PE, two vasoconstricting agents associated with elevated blood pressure in rodent models of hypertension (75–81). Indeed, we show that both PE and ANG II produced a significant decrease in the cross-sectional width of the DA, suggesting vasoconstrictive properties previously shown in rodents (75–81). The lack of effects of PE and ANG II on zebrafish HR, despite the reported importance of adrenergic receptors and ANG II in regulation of HR in the zebrafish (42, 82–87), may be due to the mode and/or duration of compound delivery in the current study. Interestingly, when coadministered with PE and ANG II, sodium butyrate was able to reduce the vasoconstrictive effects of ANG II and PE but only at select time points. Vasodilating properties of butyrate have been shown in rat mesenteric arteries following vasoconstriction by PE (64); however, to the best of our knowledge, there are no studies reporting vascular effects of butyrate in the zebrafish. Higher doses of butyrate delivered orally may be used in future studies for more pronounced cardiovascular effects.
Our study demonstrates a role for the zebrafish in investigation of host-microbiota interactions in cardiovascular health. We show that the core gut bacterial phyla and major SCFAs are similar between the zebrafish, rodent models, and humans, and that the zebrafish larvae may present as a useful tool in investigation of chronotropic and vasoactive properties of bacterial metabolites associated with cardiovascular health. Furthermore, we demonstrate that butyrate may benefit both the cardiac and vascular function to promote cardiovascular health.
Limitations
One potential limitation was the use of anesthetizing agent, which can influence cardiovascular measurements. However, tricaine is widely used (40) as an alternative to other immobilization techniques (88), and produced no HR effects in our preliminary measurements. In addition, administration of compounds via immersion relies mostly on passive diffusion and/or absorption, which may have guided the timing and extent of our responses. Moreover, there may be a difference in the concentration of fecal SCFAs between larval and adult zebrafish, as well as between fecal and circulating SCFAs. It is technically challenging to obtain sufficient larval fecal or blood sample or adult zebrafish blood sample (89) for SCFA detection. Thus, we utilized concentrations obtained from adult zebrafish fecal samples for a proof-of-concept experiment in the larvae. Future studies should use SCFA heart and vascular studies in adult zebrafish with direct blood pressure measurements (12, 13) and oral administration of SCFAs to increase specificity.
Conclusions and Perspectives
Our study provides the first in vivo evidence, to the best of our knowledge, demonstrating the production of SCFAs by the zebrafish gut bacteria similarly to those reported in rodents and humans. We also highlight a shared core gut microbiota between these species. The bradycardic effects of the major SCFAs were consistent with findings reported in rodent models. In addition, our research reveals that butyrate may lessen the PE- and ANG II-induced vasoconstriction, similarly to what has been reported in rodents (64). These findings underscore significant conservation between the zebrafish, rodents, and humans. Coupled with its inherent advantages (90), zebrafish may present a valuable tool in elucidating the intricate interactions of host-microbiota in regulation of cardiovascular health, thus reducing our reliance on complex mammalian systems.
DATA AVAILABILITY
The data sets generated following 16S rRNA gene sequencing are openly available at https://doi.org/10.6084/m9.figshare.25055612.v2.
GRANTS
This work was supported by National Institutes of Health Grant HL152162 and University of Toledo startup funds (to J.Z.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Z. conceived and designed research; H.S.K. performed experiments; H.S.K. and A.A.P. analyzed data; H.S.K. interpreted results of experiments; H.S.K. prepared figures; H.S.K. and J.Z. drafted manuscript; H.S.K., A.S.W., I.T.S., A.A.P., and J.Z. edited and revised manuscript; H.S.K., A.S.W., I.T.S., A.A.P., and J.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank the University of Toledo Center for Drug Design and Discovery (CD3), the zebrafish animal core facility, and the University of Toledo Microbiome Consortium in the Department of Physiology and Pharmacology for technical support with 16S sequencing. The authors also thank Dr. Wissam Abou-Alaiwi and Dr. Frederick Williams in the Department of Pharmacology and Experimental Therapeutics at the University of Toledo College of Pharmacy and Pharmaceutical Sciences for technical support with imaging. The graphical abstract was created with a licensed version of BioRender.com.
REFERENCES
- 1. Reed B, Jennings M. Guidance on the Housing and Care of Zebrafish Danio rerio [Online]. www.rspca.org.uk.
- 2. Choi TY, Choi TI, Lee YR, Choe SK, Kim CH. Zebrafish as an animal model for biomedical research. Exp Mol Med 53: 310–317, 2021. doi: 10.1038/s12276-021-00571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M , et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498–503, 2013. [Erratum in Nature 505: 248, 2014]. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakkers J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res 91: 279–288, 2011. doi: 10.1093/cvr/cvr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng SH, Chan PK, Wu RS. The use of microangiography in detecting aberrant vasculature in zebrafish embryos exposed to cadmium. Aquat Toxicol 52: 61–71, 2001. doi: 10.1016/s0166-445x(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 6. Schmitt CE, Holland MB, Jin SW. Visualizing vascular networks in zebrafish: an introduction to microangiography. Methods Mol Biol 843: 59–67, 2012. doi: 10.1007/978-1-61779-523-7_6. [DOI] [PubMed] [Google Scholar]
- 7. Hoffmann S, Mullins L, Rider S, Brown C, Buckley CB, Assmus A, Li Z, Sierra Beltran M, Henderson N, Del Pozo J, De Goes Martini A, Sequeira-Lopez MLS, Gomez RA, Mullins J. Comparative studies of renin-null zebrafish and mice provide new functional insights. Hypertension 79: e56–e66, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ye L, Bae M, Cassilly CD, Jabba SV, Thorpe DW, Martin AM, Lu H-Y, Wang J, Thompson JD, Lickwar CR, Poss KD, Keating DJ, Jordt S-E, Clardy J, Liddle RA, Rawls JF. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe 29: 179–196.e9, 2021. doi: 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ye L, Mueller O, Bagwell J, Bagnat M, Liddle RA, Rawls JF. High fat diet induces microbiota-dependent silencing of enteroendocrine cells. eLife 8: 8, 2019. doi: 10.7554/eLife.48479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taormina MJ, Jemielita M, Stephens WZ, Burns AR, Troll JV, Parthasarathy R, Guillemin K. Investigating bacterial-animal symbioses with light sheet microscopy. Biol Bull 223: 7–20, 2012. doi: 10.1086/BBLv223n1p7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Vito G, Turrini L, Müllenbroich C, Ricci P, Sancataldo G, Mazzamuto G, Tiso N, Sacconi L, Fanelli D, Silvestri L, Vanzi F, Pavone FS. Fast whole-brain imaging of seizures in zebrafish larvae by two-photon light-sheet microscopy. Biomed Opt Express 13: 1516–1536, 2022. doi: 10.1364/BOE.434146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pelster B, Burggren WW. Disruption of hemoglobin oxygen transport does not impact oxygen-dependent physiological processes in developing embryos of zebra fish (Danio rerio). Circ Res 79: 358–362, 1996. doi: 10.1161/01.res.79.2.358. [DOI] [PubMed] [Google Scholar]
- 13. Kopp R, Schwerte T, Pelster B. Cardiac performance in the zebrafish breakdance mutant. J Exp Biol 208: 2123–2134, 2005. doi: 10.1242/jeb.01620. [DOI] [PubMed] [Google Scholar]
- 14. Parker T, Libourel P-A, Hetheridge MJ, Cumming RI, Sutcliffe TP, Goonesinghe AC, Ball JS, Owen SF, Chomis Y, Winter MJ. A multi-endpoint in vivo larval zebrafish (Danio rerio) model for the assessment of integrated cardiovascular function. J Pharmacol Toxicol Methods 69: 30–38, 2014. doi: 10.1016/j.vascn.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 15. Jamison RA, Samarage CR, Bryson-Richardson RJ, Fouras A. In vivo wall shear measurements within the developing zebrafish heart. PLoS One 8: e75722, 2013. doi: 10.1371/journal.pone.0075722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan PK, Lin CC, Cheng SH. Noninvasive technique for measurement of heartbeat regularity in zebrafish (Danio rerio) embryos. BMC Biotechnol 9: 11, 2009. doi: 10.1186/1472-6750-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shin JT, Pomerantsev EV, Mably JD, MacRae CA. High-resolution cardiovascular function confirms functional orthology of myocardial contractility pathways in zebrafish. Physiol Genomics 42: 300–309, 2010. doi: 10.1152/physiolgenomics.00206.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwerte T, Pelster B. Digital motion analysis as a tool for analysing the shape and performance of the circulatory system in transparent animals. J Exp Biol 203: 1659–1669, 2000. doi: 10.1242/jeb.203.11.1659. [DOI] [PubMed] [Google Scholar]
- 19. De Luca E, Zaccaria GM, Hadhoud M, Rizzo G, Ponzini R, Morbiducci U, Santoro MM. ZebraBeat: a flexible platform for the analysis of the cardiac rate in zebrafish embryos. Sci Rep 4: 4898, 2014. doi: 10.1038/srep04898. [DOI] [Google Scholar]
- 20. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340, 2013. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev 69: 245–258, 2011. doi: 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- 22. Xiong R-G, Zhou D-D, Wu S-X, Huang S-Y, Saimaiti A, Yang Z-J, Shang A, Zhao C-N, Gan R-Y, Li H-B. Health benefits and side effects of short-chain fatty acids. Foods 11: 2863, 2022. doi: 10.3390/foods11182863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagpal R, Wang S, Solberg Woods LC, Seshie O, Chung ST, Shively CA, Register TC, Craft S, McClain DA, Yadav H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front Microbiol 9: 2897, 2018. doi: 10.3389/fmicb.2018.02897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pluznick JL. Renal and cardiovascular sensory receptors and blood pressure regulation. Am J Physiol Renal Physiol 305: F439–F444, 2013. doi: 10.1152/ajprenal.00252.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165: 1332–1345, 2016. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 26. Lu Y, Zhang Y, Zhao X, Shang C, Xiang M, Li L, Cui X. Microbiota-derived short-chain fatty acids: implications for cardiovascular and metabolic disease. Front Cardiovasc Med 9: 900381, 2022. doi: 10.3389/fcvm.2022.900381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poll BG, Xu J, Jun S, Sanchez J, Zaidman NA, He X, Lester L, Berkowitz DE, Paolocci N, Gao WD, Pluznick JL. Acetate, a short-chain fatty acid, acutely lowers heart rate and cardiac contractility along with blood pressure. J Pharmacol Exp Ther 377: 39–50, 2021. doi: 10.1124/jpet.120.000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poll BG, Cheema MU, Pluznick JL. Gut microbial metabolites and blood pressure regulation: focus on SCFAs and TMAO. Physiology (Bethesda) 35: 275–284, 2020. doi: 10.1152/physiol.00004.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang T, Magee KL, Colon-Perez LM, Larkin R, Liao YS, Balazic E, Cowart JR, Arocha R, Redler T, Febo M, Vickroy T, Martyniuk CJ, Reznikov LR, Zubcevic J. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol (Oxf) 226: e13256, 2019. doi: 10.1111/apha.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xia H, Chen H, Cheng X, Yin M, Yao X, Ma J, Huang M, Chen G, Liu H. Zebrafish: an efficient vertebrate model for understanding role of gut microbiota. Mol Med 28: 161, 2022. doi: 10.1186/s10020-022-00579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martyniuk CJ, Buerger AN, Vespalcova H, Rudzanova B, Sohag SR, Hanlon AT, Ginn PE, Craft SL, Smetanova S, Budinska E, Bisesi JH, Adamovsky O. Sex-dependent host-microbiome dynamics in zebrafish: implications for toxicology and gastrointestinal physiology. Comp Biochem Physiol Part D Genomics Proteomics 42: 100993, 2022. doi: 10.1016/j.cbd.2022.100993. [DOI] [PubMed] [Google Scholar]
- 32. Cholan PM, Han A, Woodie BR, Watchon M, Kurz AR, Laird AS, Britton WJ, Ye L, Holmes ZC, McCann JR, David LA, Rawls JF, Oehlers SH. Conserved anti-inflammatory effects and sensing of butyrate in zebrafish. Gut Microbes 12: 1–11, 2020. doi: 10.1080/19490976.2020.1824563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chakraborty S, Galla S, Cheng X, Yeo J-Y, Mell B, Singh V, Yeoh BSan, Saha P, Mathew AV, Vijay-Kumar M, Joe B. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep 25: 677–689.e4, 2018. doi: 10.1016/j.celrep.2018.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang T, Mei X, Tackie-Yarboi E, Akere MT, Kyoung J, Mell B, Yeo J-Y, Cheng X, Zubcevic J, Richards EM, Pepine CJ, Raizada MK, Schiefer IT, Joe B. Identification of a gut commensal that compromises the blood pressure-lowering effect of ester angiotensin-converting enzyme inhibitors. Hypertension 79: 1591–1601, 2022. doi: 10.1161/HYPERTENSIONAHA.121.18711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, , et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37: 852–857, 2019. [Erratum in Nat Biotechnol 37: 1091, 2019]. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41: D590–D596, 2013. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zubcevic J, Watkins J, Lin C, Bautista B, Hatch HM, Tevosian SG, Hayward LF. Nicotine exposure during rodent pregnancy alters the composition of maternal gut microbiota and abundance of maternal and amniotic short chain fatty acids. Metabolites 12: 735, 2022. doi: 10.3390/metabo12080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn 238: 2975–3015, 2009. doi: 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thitinarongwate W, Mektrirat R, Nimlamool W, Khonsung P, Pikulkaew S, Okonogi S, Kunanusorn P. Phytochemical and safety evaluations of Zingiber ottensii valeton essential oil in zebrafish embryos and rats. Toxics 9: 102, 2021. doi: 10.3390/toxics9050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang X, Giusti A, Ny A, de Witte PA. Nephrotoxic effects in zebrafish after prolonged exposure to aristolochic acid. Toxins 12: 217, 2020. doi: 10.3390/toxins12040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kirla KT, Groh KJ, Poetzsch M, Banote RK, Stadnicka-Michalak J, Eggen RIL, Schirmer K, Kraemer T. Importance of toxicokinetics to assess the utility of zebrafish larvae as model for psychoactive drug screening using meta-chlorophenylpiperazine (mCPP) as example. Front Pharmacol 9: 414, 2018. doi: 10.3389/fphar.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steele SL, Yang X, Debiais-Thibaud M, Schwerte T, Pelster B, Ekker M, Tiberi M, Perry SF. In vivo and in vitro assessment of cardiac β-adrenergic receptors in larval zebrafish (Danio rerio). J Exp Biol 214: 1445–1457, 2011. doi: 10.1242/jeb.052803. [DOI] [PubMed] [Google Scholar]
- 43. Kumai Y, Bernier NJ, Perry SF. Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water. J Endocrinol 220: 195–205, 2014. doi: 10.1530/JOE-13-0374. [DOI] [PubMed] [Google Scholar]
- 44. Hsu YL, Chen CC, Lin YT, Wu WK, Chang LC, Lai CH, Wu MS, Kuo CH. Evaluation and optimization of sample handling methods for quantification of short-chain fatty acids in human fecal samples by GC–MS. J Proteome Res 18: 1948–1957, 2019. doi: 10.1021/acs.jproteome.8b00536. [DOI] [PubMed] [Google Scholar]
- 45. Holota Y, Dovbynchuk T, Kaji I, Vareniuk I, Dzyubenko N, Chervinska T, Zakordonets L, Stetska V, Ostapchenko L, Serhiychuk T, Tolstanova G. The long-term consequences of antibiotic therapy: role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS One 14: e0220642, 2019. doi: 10.1371/journal.pone.0220642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu H, Li P, Huang X, Wang CH, Li M, Xu ZZ. Zebrafish model for human gut microbiome-related studies: advantages and limitations. Med Microecol 8: 100042, 2021. doi: 10.1016/j.medmic.2021.100042. [DOI] [Google Scholar]
- 47. Kanther M, Rawls JF. Host-microbe interactions in the developing zebrafish. Curr Opin Immunol 22: 10–19, 2010. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. Evidence for a core gut microbiota in the zebrafish. ISME J 5: 1595–1608, 2011. doi: 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Abreu MS, Giacomini ACVV, Sysoev M, Demin KA, Alekseeva PA, Spagnoli ST, Kalueff AV. Modeling gut-brain interactions in zebrafish. Brain Res Bull 148: 55–62, 2019. doi: 10.1016/j.brainresbull.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 50. Phelps D, Brinkman NE, Keely SP, Anneken EM, Catron TR, Betancourt D, Wood CE, Espenschied ST, Rawls JF, Tal T. Microbial colonization is required for normal neurobehavioral development in zebrafish. Sci Rep 7: 11244, 2017. doi: 10.1038/s41598-017-10517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schwartz AV, Sant KE, Navarrete J, George UZ. Mathematical modeling of the interaction between yolk utilization and fish growth in zebrafish, Danio rerio. Development 148: dev193508, 2021. doi: 10.1242/dev.193508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Watts SA, D'Abramo LR. Standardized reference diets for zebrafish: addressing nutritional control in experimental methodology. Annu Rev Nutr 41: 511–527, 2021. doi: 10.1146/annurev-nutr-120420-034809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Watts SA, Powell M, D'Abramo LR. Fundamental approaches to the study of zebrafish nutrition. ILAR J 53: 144–160, 2012. doi: 10.1093/ilar.53.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buerger AN, Dillon DT, Schmidt J, Yang T, Zubcevic J, Martyniuk CJ, Bisesi JH Jr.. Gastrointestinal dysbiosis following diethylhexyl phthalate exposure in zebrafish (Danio rerio): altered microbial diversity, functionality, and network connectivity. Environ Pollut 265: 114496, 2020. doi: 10.1016/j.envpol.2020.114496. [DOI] [PubMed] [Google Scholar]
- 55. Vargas R, Vásquez IC. Cardiac and somatic parameters in zebrafish: tools for the evaluation of cardiovascular function. Fish Physiol Biochem 42: 569–577, 2016. doi: 10.1007/s10695-015-0160-8. [DOI] [PubMed] [Google Scholar]
- 56. Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA 101: 4596–4601, 2004. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Milligan-Myhre K, Charette JR, Phennicie RT, Stephens WZ, Rawls JF, Guillemin K, Kim CH. Study of host-microbe interactions in zebrafish. Methods Cell Biol 105: 87–116, 2011. doi: 10.1016/B978-0-12-381320-6.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Melancon E, Gomez De La Torre Canny S, Sichel S, Kelly M, Wiles TJ, Rawls JF, Eisen JS, Guillemin K. Best practices for germ-free derivation and gnotobiotic zebrafish husbandry. Methods Cell Biol 138: 61–100, 2017. doi: 10.1016/bs.mcb.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pham LN, Kanther M, Semova I, Rawls JF. Methods for generating and colonizing gnotobiotic zebrafish. Nat Protoc 3: 1862–1875, 2008. doi: 10.1038/nprot.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fritsche R, Schwerte T, Pelster B. Nitric oxide and vascular reactivity in developing zebrafish, Danio rerio. Am J Physiol Regul Integr Comp Physiol 279: R2200–R2207, 2000. doi: 10.1152/ajpregu.2000.279.6.R2200. [DOI] [PubMed] [Google Scholar]
- 61. Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol 230: 278–301, 2001. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- 62. Campinho P, Lamperti P, Boselli F, Vilfan A, Vermot J. Blood flow limits endothelial cell extrusion in the zebrafish dorsal aorta. Cell Rep 31: 107505, 2020. doi: 10.1016/j.celrep.2020.03.069. [DOI] [PubMed] [Google Scholar]
- 63. Eberlein J, Herdt L, Malchow J, Rittershaus A, Baumeister S, Helker CS. Molecular and cellular mechanisms of vascular development in zebrafish. Life (Basel) 11: 1088, 2021. doi: 10.3390/life11101088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E, Samborowska E, Ufnal M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch 471: 1441–1453, 2019. doi: 10.1007/s00424-019-02322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rolig AS, Mittge EK, Ganz J, Troll JV, Melancon E, Wiles TJ, Alligood K, Stephens WZ, Eisen JS, Guillemin K. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol 15: e2000689, 2017. doi: 10.1371/journal.pbio.2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108: 8030–8035, 2011. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miyamoto J, Kasubuchi M, Nakajima A, Irie J, Itoh H, Kimura I. The role of short-chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens 25: 379–383, 2016. doi: 10.1097/MNH.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 68. Petit J, Wiegertjes GF. Conservation of members of the free fatty acid receptor gene family in common carp. Dev Comp Immunol 126: 104240, 2022. doi: 10.1016/j.dci.2021.104240. [DOI] [PubMed] [Google Scholar]
- 69. Yang J, Li J, Zhang X, Zhou Q, Wang J, Chen Q, Meng X, Xia Y. Effects of ecologically relevant concentrations of cadmium on the microbiota, short-chain fatty acids, and FFAR2 expression in zebrafish. Metabolites 13: 657, 2023. doi: 10.3390/metabo13050657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nutting CW, Islam S, Daugirdas JT. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am J Physiol Heart Circ Physiol 261: H561–H567, 1991. doi: 10.1152/ajpheart.1991.261.2.H561. [DOI] [PubMed] [Google Scholar]
- 71. Knock G, Psaroudakis D, Abbot S, Aaronson PI. Propionate-induced relaxation in rat mesenteric arteries: a role for endothelium-derived hyperpolarising factor. J Physiol 538: 879–890, 2002. doi: 10.1113/jphysiol.2001.013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nutting CW, Islam S, Ye MH, Batlle DC, Daugirdas JT. The vasorelaxant effects of acetate: role of adenosine, glycolysis, lyotropism, and pHi and Cai2+. Kidney Int 41: 166–174, 1992. doi: 10.1038/ki.1992.23. [DOI] [PubMed] [Google Scholar]
- 73. Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 31: 1391–1394, 1990. doi: 10.1136/gut.31.12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu J, Moore BN, Pluznick JL. Short-chain fatty acid receptors and blood pressure regulation: Council on Hypertension Mid-Career Award for Research Excellence 2021. Hypertension 79: 2127–2137, 2022. doi: 10.1161/HYPERTENSIONAHA.122.18558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Loiola RA, Fernandes L, Eichler R, Passaglia RdeCT, Fortes ZB, de Carvalho MHC. Vascular mechanisms involved in angiotensin II-induced venoconstriction in hypertensive rats. Peptides 32: 2116–2121, 2011. doi: 10.1016/j.peptides.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 76. Simon G, Cserep G, Limas C. Development of structural vascular changes with subpressor angiotensin II administration in rats. Am J Hypertens 8: 67–73, 1995. doi: 10.1016/0895-7061(94)00192-E. [DOI] [PubMed] [Google Scholar]
- 77. Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: new insights from genetic models. J Cereb Blood Flow Metab 26: 449–455, 2006. doi: 10.1038/sj.jcbfm.9600204. [DOI] [PubMed] [Google Scholar]
- 78. Chies AB, de Oliveira AM, Pereira FC, de Andrade CR, Corrêa FMA. Phenylephrine-induced vasoconstriction of the rat superior mesenteric artery is decreased after repeated swimming. J Smooth Muscle Res 40: 249–258, 2004. doi: 10.1540/jsmr.40.249. [DOI] [PubMed] [Google Scholar]
- 79. Liao LM, Zhou L, Wang CR, Hu JY, Lu YJ, Huang S. Opposing responses of the rat pulmonary artery and vein to phenylephrine and other agents in vitro. BMC Pulm Med 21: 189, 2021. doi: 10.1186/s12890-021-01558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li N, Li Y, Gao Q, Li D, Tang J, Sun M, Zhang P, Liu B, Mao C, Xu Z. Chronic fetal exposure to caffeine altered resistance vessel functions via RyRs-BKCa down-regulation in rat offspring. Sci Rep 5: 13225, 2015. doi: 10.1038/srep13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gustafsson F, Holstein-Rathlou NH. Angiotensin II modulates conducted vasoconstriction to norepinephrine and local electrical stimulation in rat mesenteric arterioles. Cardiovasc Res 44: 176–184, 1999. doi: 10.1016/s0008-6363(99)00174-1. [DOI] [PubMed] [Google Scholar]
- 82. Jiang D, Matsuzaki M, Kawagoe Y, Kitamura K, Tsuruda T, Kaikita K, Asada Y, Kato J. Analysis of mechanisms for increased blood pressure variability in rats continuously infused with angiotensin II. J Renin Angiotensin Aldosterone Syst 2023: 4201342, 2023. doi: 10.1155/2023/4201342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bivalacqua TJ, Champion HC, Hyman AL, McNamara DB, Kadowitz PJ. Analysis of responses to angiotensin II in the mouse. J Renin Angiotensin Aldosterone Syst 2: S48–S53, 2001. doi: 10.1177/14703203010020010801. [DOI] [PubMed] [Google Scholar]
- 84. Machado BH, Salgado HC. Intrinsic heart rate after infusion of angiotensin II in rats with sino-aortic deafferentation. Braz J Med Biol Res 23: 337–341, 1990. [PubMed] [Google Scholar]
- 85. Gembardt F, Heringer-Walther S, van Esch JHM, Sterner-Kock A, van Veghel R, Le TH, Garrelds IM, Coffman TM, Danser AHJ, Schultheiss H-P, Walther T. Cardiovascular phenotype of mice lacking all three subtypes of angiotensin II receptors. FASEB J 22: 3068–3077, 2008. doi: 10.1096/fj.08-108316. [DOI] [PubMed] [Google Scholar]
- 86. Vollmer RR, Meyers-Schoy SA, Marinelli RR. Mechanisms involved in angiotensin II induced increases in cardiac output in pithed rats. Clin Exp Hypertens A 13: 1433–1445, 1991. doi: 10.3109/10641969109048803. [DOI] [PubMed] [Google Scholar]
- 87. Filice M, Barca A, Amelio D, Leo S, Mazzei A, Del Vecchio G, Verri T, Cerra MC, Imbrogno S. Morpho-functional remodelling of the adult zebrafish (Danio rerio) heart in response to waterborne angiotensin II exposure. Gen Comp Endocrinol 301: 113663, 2021. doi: 10.1016/j.ygcen.2020.113663. [DOI] [PubMed] [Google Scholar]
- 88. Leyden C, Brüggemann T, Debinski F, Simacek CA, Dehmelt FA, Arrenberg AB. Efficacy of tricaine (MS-222) and hypothermia as anesthetic agents for blocking sensorimotor responses in larval zebrafish. Front Vet Sci 9: 864573, 2022. doi: 10.3389/fvets.2022.864573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pedroso GL, Hammes TO, Escobar TDC, Fracasso LB, Forgiarini LF, da Silveira TR. Blood collection for biochemical analysis in adult zebrafish. J Vis Exp 2012: e3865, 2012. doi: 10.3791/3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sree Kumar H, Wisner AS, Refsnider JM, Martyniuk CJ, Zubcevic J. Small fish, big discoveries: zebrafish shed light on microbial biomarkers for neuro-immune-cardiovascular health. Front Physiol 14: 1186645, 2023. doi: 10.3389/fphys.2023.1186645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated following 16S rRNA gene sequencing are openly available at https://doi.org/10.6084/m9.figshare.25055612.v2.