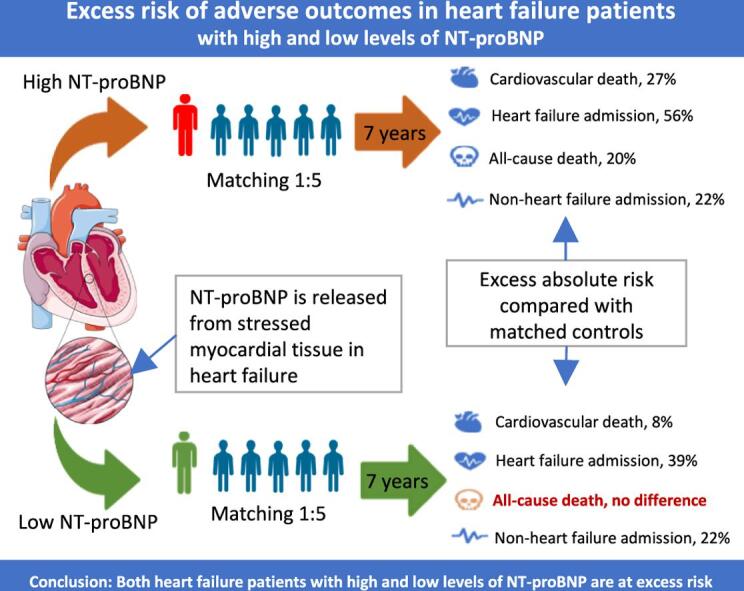
Graphical abstract
Excess risk of adverse outcomes in heart failure with high and low levels of NT-proBNP.
Keywords: Heart failure, NT-proBNP, Excess risk, NorthStar
Abstract
Background
This study investigated excess risk in patients with heart failure with reduced left ventricular ejection fraction (HFrEF) with or without elevated levels of NT-proBNP (N-terminal pro-brain natriuretic peptide).
Methods
Patients with HFrEF from the NorthStar cohort (n = 1120) were matched on age, sex, and presence of AF (atrial fibrillation/flutter) to five controls without HFrEF from The Danish National Patient Registries. Patients were compared with controls before and after stratification according to baseline NT-proBNP levels, with cutoffs defined as </≥ 600 pg/ml in patients with sinus rhythm and </≥ 900 pg/ml in patients with AF. The primary composite endpoint was a 7-year risk of cardiovascular death or HF admission.
Results
In the HFrEF cohort, 704 patients had high NT-proBNP (median age, 73; mean left ventricular ejection fraction (LVEF), 33%). 416 patients had low NT-proBNP (median age, 65; LVEF, 30%). Patients from both groups were in NYHA class I-III. The primary endpoint occurred in 531 patients (75.4%) with HFrEF and elevated NT-proBNP, and 748 controls (21.3%) (risk difference, 54.2%; 95% confidence interval (CI) 50.7–57.6%). In comparison, it occurred in 199 patients (47.9%) with HFrEF and without elevated NT-proBNP, and 185 controls (8,9%) (risk difference, 38.9%; 95% CI 34.0–43.9%). Risk differences for all secondary endpoints were significant, except for overall mortality in the low NT-proBNP group (risk difference, 3.8%; 95% CI, −0.4–8.0%).
Conclusion
This study identified a significant excess risk in patients with HFrEF across various endpoints, which persisted after stratification into high and low levels of NT-proBNP.
1. Introduction
Treatment of patients with heart failure (HF) with reduced left ventricular ejection fraction (HFrEF) has improved substantially over the last decades [1], [2]. Notable recent advances include the introduction of sacubitril/valsartan as an alternative to ACE inhibitors, [3], [4] as well as sodium-glucose co-transporter 2 (SGLT2) inhibitors as an add-on to standard treatment [5], [6]. Yet, implementation of new therapies according to updated clinical guidelines [7], [8]. is a challenge. It has previously been shown that it may take up to 15 years to implement new scientific discoveries, [9] and inertia exists both within the patients due to e.g., habit and/or polypharmacy, as well as within clinicians who may argue that “real-life” patients are either too healthy or too sick to benefit from new treatments.
Clinical trials investigating new therapies such as those mentioned above often require patients with HFrEF to have NT-proBNP levels above a certain threshold [3], [5], [6]. High levels of NT-proBNP are associated with a high risk of mortality and morbidity, [10] but it is unknown whether patients with HFrEF and a low NT-proBNP level, who are well treated with well-established neurohormonal blockade like ACE-inhibitors, beta-blockers and mineralocorticoid receptor antagonists have an excess risk compared to the population without HF. If not, it could be argued that the implementation of new therapies should be reserved for subgroups equal to the patients at higher risk enrolled in the trials.
Therefore, this long-term registry-based follow-up study aimed to investigate the excess risk among patients with HFrEF on optimal guideline-directed therapies. Patients were compared with controls from the Danish population matched on age, sex, and presence of AF before and after stratification into high and low-risk groups according to their baseline NT-proBNP levels. The hypothesis was that both patients with HFrEF with high and low NT-proBNP levels still had a significant excess long-term risk of adverse events compared to patients without HF.
2. Methods
2.1. Study design
The patient case group was the original cohort from the NorthStar trial, which was a multicenter open-label, randomized clinical trial with blinded outcome ascertainment designed to evaluate an HF clinic intervention and a new NT-proBNP monitoring concept in outpatients with HFrEF [11]. At the time of randomization, patients were on optimal guideline-directed therapy and were considered clinically stable. The NorthStar study cohort included 1120 patients with HFrEF from 18 different HF clinics in Denmark from the 21st of November 2005 until the 10th of December 2009. The included patients had LVEF ≤ 45 % and were randomly assigned to either clinical management at their general practitioner (GP) or follow-up at a specialized HF clinic. None of the study interventions proved superior to usual care [12], [13]. After the end of the original study, patients returned to follow up with their GP.
In the present study, patients from the NorthStar cohort were stratified into two groups with and without elevated NT-proBNP groups by using a threshold of ≥ 600 pg/ml in patients with sinus rhythm (SR) and ≥ 900 pg/ml in patients with atrial fibrillation/flutter (AF). Thus, the high-level NT-proBNP patient group corresponds to the patient groups enrolled in the clinical trials leading to the approval of the new HF treatments, sacubitril/valsartan, and the SGLT2-inhibitor dapagliflozin [3], [5]. In that way, long-term risk in patients eligible in recently conducted randomized clinical trials of new therapies for HF could be compared to patients with a presumed lower risk based on NT-proBNP. For each patient, five controls without HF were randomly selected from the Danish population without HF and matched on year of birth, sex, and presence of AF at baseline. Thereby, the excess risk of important clinical events could be evaluated in patients with HFrEF and high or low NT-proBNP levels.
In the patients with HFrEF, comorbidities were assessed by a cardiologist at inclusion into the NorthStar trial. Comorbidities for controls were retrieved from national registries. Before matching, controls were excluded if they had had a hospital contact or admission into a hospital unit with HF as a primary or secondary diagnosis within ten years of inclusion. Specifically, diabetes was defined for controls as having had either a hospital contact or admission with diabetes registered as a primary or secondary cause or having used antidiabetic prescription medicine within 6 months of inclusion. Hypertension was defined as having had either an admission with hypertension registered as a primary or secondary diagnosis or having used ≥ 2 types of antihypertensive prescription medicine within 6 months of inclusion.
2.2. Data sources
Information on outcomes and controls was obtained from the Danish nationwide health registries. All permanent residents in Denmark have a unique personal identification number, linking between registries. All patients were included in the period 2006–2009. Data were obtained from registries through Statistics Denmark.
The Danish National Patients Registry (DNPR) contains data from 1977 onwards on all hospital admissions and outpatient contacts, which are registered with a primary diagnosis and, if relevant, secondary diagnoses at discharge according to the 10th revision of the International Classification of Diseases (ICD-10). Surgical procedures have been registered and coded since 1996 and onwards according to the Nordic Medico‐Statistical Committee Classification of Surgical Procedures. The Danish Civil Registry comprises data on birth date, sex, and residency (i.e., whether a person is residing in Denmark, has emigrated to/from the country, or has disappeared/their residence is unknown to the Danish authorities), along with the date of these events. The Danish National Prescription Registry holds information on the dispensing date, strength, and quantity of all claimed drug prescriptions in Denmark. The Causes of Death registry contains information on the time of death and age at the time, manner of, and causes, from 1970 onwards [14].
2.3. Primary and secondary endpoints
The primary outcome was a composite endpoint of the 7-year risk of CV death or HF admission. Secondary endpoints were risk of CV death, HF admission, non-HF admission, and all-cause mortality. An HF admission was defined as an overnight stay at a hospital with HF as a primary or secondary diagnosis. Non-HF admissions were defined as an overnight stay at a hospital regardless of diagnosis, excluding those with a primary or secondary diagnosis of HF. Patients were followed until the event of interest, migration, death, end of study (31st of December 2018), or 7 years after inclusion, whichever came first.
2.4. Statistical analysis
Survival analyses were performed using the Aalen Johansen estimator. The risk was assessed as the absolute risk of respective endpoints in patients with and without HF after 7 years of follow-up. Absolute risks, risk differences, and risk ratios between cases and controls were reported with 95 % confidence intervals (CI). Time to CV death or HF admission was estimated as a composite endpoint with non-CV death as a competing risk. The risk of CV death, HF admissions, and non-HF admissions were individually estimated using the Aalen-Johansen estimator. Overall mortality was estimated using the Kaplan-Meier estimator. Survival analyses were similarly conducted for all endpoints after stratification into groups with high and low NT-proBNP levels, with both groups being compared with their respective matched controls.
For an age-stratified analysis of the absolute risk of the primary endpoint, CV death or HF admission, cases and controls were stratified into three age categories (<65 years, 65–75 years, >75 years) and compared with controls. Analyses were similarly performed after the stratification of patients into groups with high and low NT-proBNP levels. A supplementary analysis was also conducted using the secondary endpoint of all-cause mortality.
A subgroup of patients who had no prescription of loop diuretics up to 6 months before inclusion was analyzed for time to the first prescription. The analysis was performed before and after stratification according to NT-proBNP. Controls were required to not have a prescription of loop diuretics within 6 months, in addition to being matched to the patients with HFrEF included in this specific analysis. Analysis was performed as a cumulative incidence analysis with death as a competing event.
All statistical analyses were performed using RStudio version 4.2.1. The level of significance was set at 5 %.
2.5. Ethics
NorthStar was approved by the Ethical Committee of Copenhagen (KF 01 2724936). The Danish Data Protection Agency approved this study. In Denmark, registry-based studies, in which individuals cannot be identified, do not require ethical approval.
3. Results
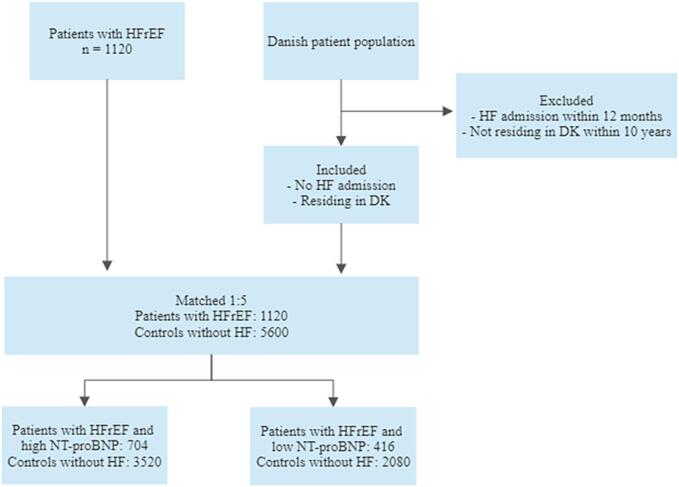
Five matched controls were found for all included patients, Fig. 1. The baseline characteristics of patients with HFrEF and matched controls without HF are shown in Table 1, as well as after stratification for NT-proBNP so that patients are compared with their respective matched controls. The median age was higher in the high NT-proBNP level group than in the low NT-proBNP level group (73 years vs. 65 years). The largest differences in medical history between patients with HFrEF and controls were seen in the medical history of myocardial infarction, stable angina, and admissions (any cause) within 12 months of inclusion. Diabetes and chronic obstructive pulmonary disease were also more frequent in patients with HFrEF than in the matched controls. The proportion of patients with a history of cancer was slightly larger among controls than among cases. As for medication, a very high proportion of patients with HFrEF (>90 %) were treated with RAAS (renin-angiotensin-aldosterone system) inhibitors and beta-blockers, as well as with loop diuretics and statins (>60 %).
Fig. 1.
Flowchart. Inclusion, exclusion, and distribution of patients and matched controls.
Table 1.
Baseline table comparing patients and controls before and after stratification according to baseline NT-proBNP.
|
Baseline table stratified by NT-proBNP | ||||||
|---|---|---|---|---|---|---|
|
Before stratification |
After stratification according to NTproBNP |
|||||
| Patients with HFrEF | Matched controls | Patients w/ HFrEF and high NTproBNP | Controls for patients w/ high NTproBNP | Patients w/ HFrEF and low NTproBNP | Controls for patients w/ low NTproBNP | |
| n | 1120 | 5600 | 704 | 3520 | 416 | 2080 |
| Age, median (25th quant., 75th) | 70 (63, 77) | 70 (63, 77) | 73 (66, 79) | 73 (66, 79) | 65 (58, 71) | 65 (58, 71) |
| Males, no. (%) | 844 (75.4) | 4220 (75.4) | 521 (74.0) | 2605 (74.0) | 323 (77.6) | 1615 (77.6) |
| Clinical features of trial cohort | ||||||
| LVEF (%) | 31 | − | 33 | − | 30 | − |
| NYHA (no.) | ||||||
| − class I | 284 (25.4) | − | 149 (21.2) | − | 135 (32.5) | − |
| − class II | 711 (63.5) | − | 464 (65.9) | − | 247 (59.4) | − |
| − class III | 125 (11.2) | − | 91 (12.9) | − | 34 (8.2) | − |
| NT-proBNP (pg/ml) | 1679 | − | 2487 | − | 312 | − |
| Systolic arterial pressure (mmHg) | 127 | − | 127 | − | 126 | − |
| Diastolic arterial pressure (mmHg) | 80 | − | 83 | − | 75 | − |
| Heart rate (bpm) | 67 | − | 68 | − | 67 | − |
| eGFR (ml/min) | 69 | − | 64 | − | 77 | − |
| Medical history, no. (%) | ||||||
| Atrial fibrilation/-flutter | 383 (34.2) | 1915 (34.2) | 295 (41.9) | 1475 (41.9) | 88 (21.2) | 440 (21.2) |
| Diabetes | 208 (18.6) | 548 (9.8) | 139 (19.7) | 392 (11.1) | 69 (16.6) | 156 (7.5) |
| Hypertension | 456 (40.7) | 1942 (34.7) | 292 (41.5) | 1439 (40.9) | 164 (39.4) | 503 (24.2) |
| Myocardial infarction | 388 (34.6) | 271 (4.8) | 242 (34.4) | 205 (5.8) | 146 (35.1) | 66 (3.2) |
| Stable angina | 376 (33.6) | 492 (8.8) | 240 (34.1) | 367 (10.4) | 136 (32.7) | 125 (6.0) |
| COPD | 140 (12.5) | 290 (5.2) | 88 (12.5) | 221 (6.3) | 52 (12.5) | 69 (3.3) |
| Chronic renal failure | 58 (5.2) | 91 (1.6) | 49 (7.0) | 66 (1.9) | 9 (2.2) | 25 (1.2) |
| Stroke or TCI | 131 (11.7) | 509 (9.1) | 96 (13.6) | 392 (11.1) | 35 (8.4) | 117 (5.6) |
| Cancer | 75 (6.7) | 420 (7.5) | 53 (7.5) | 288 (8.2) | 22 (5.3) | 132 (6.3) |
| Admission (any) within 12 months | 724 (64.6) | 1199 (21.4) | 486 (69.0) | 840 (23.9) | 238 (57.2) | 359 (17.3) |
| Medication, no. (%) | ||||||
| RAAS-inhibitors | 1061 (94.7) | 1474 (26.3) | 661 (93.9) | 1065 (30.3) | 400 (96.2) | 409 (19.7) |
| Beta-blockers | 1028 (91.8) | 1350 (24.1) | 645 (91.6) | 995 (28.3) | 383 (92.1) | 355 (17.1) |
| MRA | 376 (33.6) | 130 (2.3) | 221 (31.4) | 94 (2.7) | 155 (37.6) | 36 (1.7) |
| Triple therapy | 327 (29.2) | 38 (0.7) | 187 (26.6) | 29 (0.8) | 140 (33.7) | 9 (0.4) |
| Loop diuretics | 707 (63.1) | 540 (9.6) | 506 (71.9) | 417 (11.8) | 201 (48.3) | 123 (5.9) |
| Non-loop diuretics | 458 (40.9) | 970 (17.3) | 265 (37.6) | 710 (20.2) | 193 (46.4) | 260 (12.5) |
| Digoxin | 206 (18.4) | 538 (9.6) | 149 (21.2) | 448 (12.7) | 57 (13.7) | 90 (4.3) |
| Amiodarone | 53 (4.7) | 62 (1.1) | 38 (5.4) | 49 (1.4) | 15 (3.6) | 13 (0.6) |
| Calcium antagonists | 96 (8.6) | 980 (17.5) | 51 (7.2) | 707 (20.1) | 45 (10.8) | 273 (13.1) |
| Nitrates | 166 (14.8) | 218 (3.9) | 103 (14.6) | 163 (4.6) | 63 (15.1) | 55 (2.6) |
| Statins | 760 (67.9) | 1368 (24.4) | 471 (66.9) | 976 (27.7) | 289 (69.5) | 392 (18.8) |
| Acetylsalicylic acid | 753 (67.2) | 1363 (24.3) | 458 (65.1) | 996 (28.3) | 295 (70.9) | 367 (17.6) |
| Oral anticoagulants | 363 (32.4) | 973 (17.4) | 275 (39.1) | 746 (21.2) | 88 (21.2) | 227 (10.9) |
| Antidiabetics | 165 (14.7) | 456 (8.1) | 109 (15.5) | 322 (9.1) | 56 (13.5) | 134 (6.4) |
| Antidepressives | 116 (10.4) | 499 (8.9) | 70 (9.9) | 357 (10.1) | 46 (11.1) | 142 (6.8) |
3.1. Primary endpoint
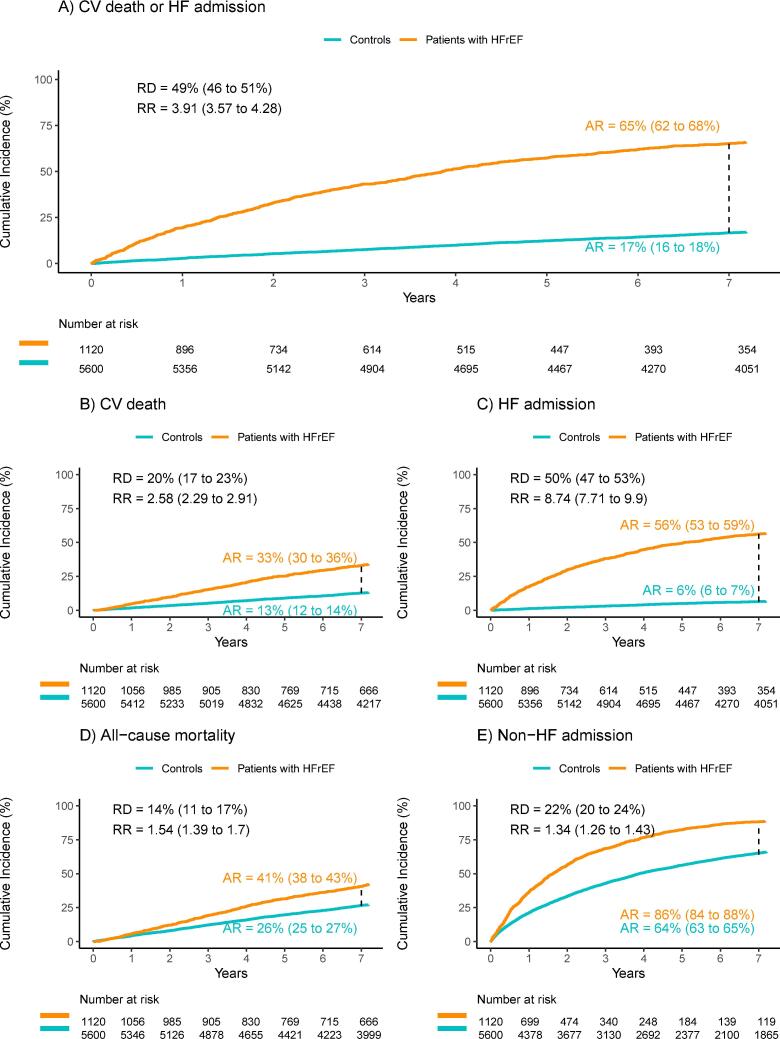
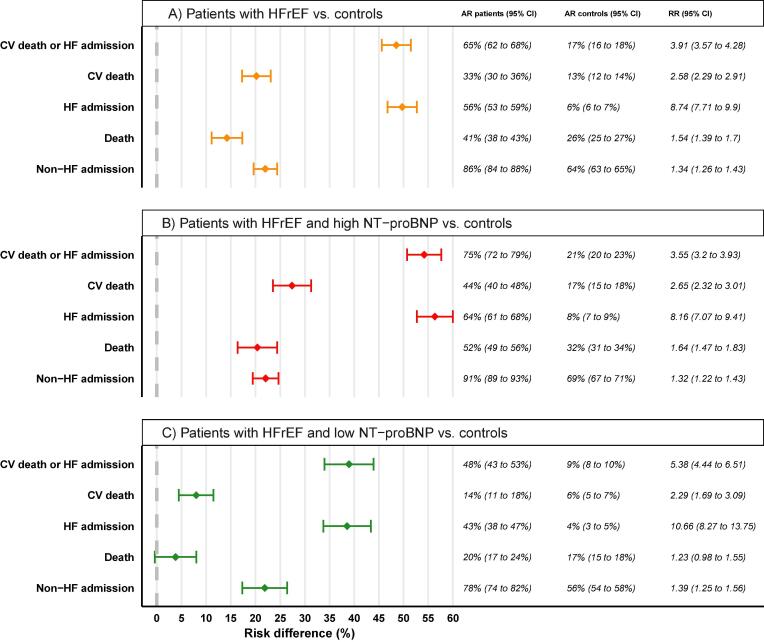
The primary composite endpoint of the 7-year risk of CV death or hospitalization for HF occurred in 730 patients (65.2 %) with HFrEF, and in 933 controls (16.7 %) without HF (Fig. 2a). The absolute risk difference was 48.5 % (95 % CI, 45.6 to 51.5 %). In patients with high NT-proBNP levels, the primary endpoint occurred in 531 patients with HFrEF (75.4 %) and 748 controls (21.3 %) (risk difference, 54.2 %; 95 % CI 50.7 to 57.6 %). In patients with low NT-proBNP levels, the primary endpoint occurred in 199 patients (47.8 %) with HFrEF, and 185 controls (8.9 %) (risk difference, 38.9 %; 95 % CI, 34.0 to 43.9 %). Risk differences for the primary endpoint were thus significantly different for heart failure patients and controls, regardless of NT-proBNP levels, Fig. 3.
Fig. 2.
Cumulative incidences of adverse endpoints. Comparison between patients with HFrEF and matched controls without HF. A) Time to CV death or HF admission. B) Time to CV death. C) Time to HF admission. D) Time to all-cause death. E) Time to non-HF admission. AR: absolute risk; RD: risk difference; RR: relative risk.
Fig. 3.
Forest plot comparing risk differences between patients and controls. A) All patients with HFrEF compared with matched controls without HF. B) Patients with HFrEF and high levels of NT-proBNP compared with matched controls without HF. C) Patients with HFrEF and low levels of NT-proBNP compared with matched controls without HF.
3.2. Secondary endpoints
Fig. 2 shows outcomes for the secondary endpoints for all patients with HFrEF and matched controls including their respective 7-year absolute risks, risk differences, and relative risk. The absolute risk of CV death, HF admission, all-cause death and non-HF admission after 7 years was higher in patients with HFrEF compared to the control group (risk difference 20 %, 50 %, 14 %, and 22 %, respectively; Fig. 2). Following stratification for the NT-proBNP level, the risks were significantly higher in patients with HFrEF across all endpoints in both NT-proBNP groups, albeit not significantly for overall mortality in patients with HFrEF and low NT-proBNP level (risk difference 3.8 %, 95 % CI −0.4 to 8.0 %; Fig. 3).
3.3. Risk of CV death or HF admission according to age
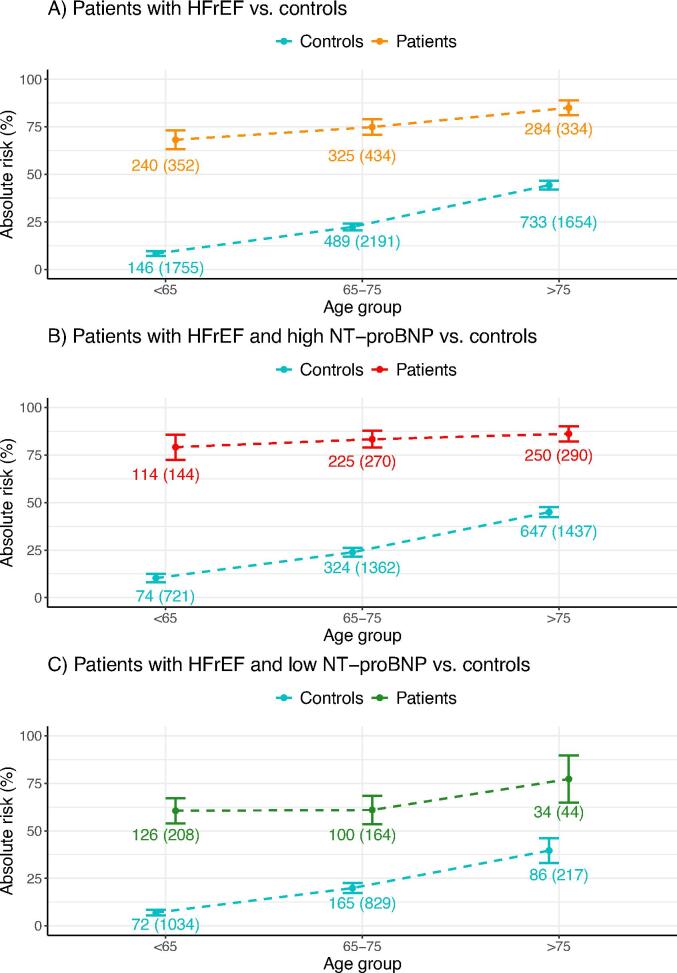
The effect of age was analyzed in an age-stratified analysis (Fig. 4) which compared the risk of the primary endpoint, CV death and HF admissions, in patients compared with matched controls, divided into three age categories (<65 years, 65–75, >75). This was done before and after stratification into groups with high and low NT-proBNP levels. The risk was significantly higher in patients compared to controls within all three age groups, with the greatest difference observed in the group aged < 65 years, where the outcome occurred in 8.3 % of the controls (95 % CI, 7.0 to 9.6 %) and 68.2 % of the patients (95 % CI, 63.3 to 73.1 %). After stratification according to NT-proBNP, the risk was significantly higher within all three age groups in both the high and the low NT-proBNP group, with the risk moderately decreasing with increasing age in both groups.
Fig. 4.
Age-stratified analysis of the risk of the primary endpoint, CV death or HF admission. A) Patients with HFrEF were divided into three age groups (<65, 65–75, >75) and compared with matched controls. B) Patients with HFrEF and high NT-proBNP compared to controls. C) Patients with HFrEF and low NT-proBNP compared to controls.
The effect of age on the secondary endpoint, all-cause mortality, was similarly analyzed (Supplementary Figure S1). Mortality risk was significantly higher in patients compared to controls for all three age groups, with the greatest difference observed in the group aged 65–75, where mortality was 21.1 % in controls (95 % CI, 19.4 to 22.8 %) and 40.1 % in patients (95 % CI, 35.5 to 44.7 %). In the group with high NT-proBNP, the risk of all-cause mortality was significantly higher in all age groups compared to controls, with the risk difference being lowest but still significant in the oldest group. In contrast, the risk difference did not prove significant in any group in the low NT-proBNP level patients.
3.4. Time to first prescription of loop diuretics
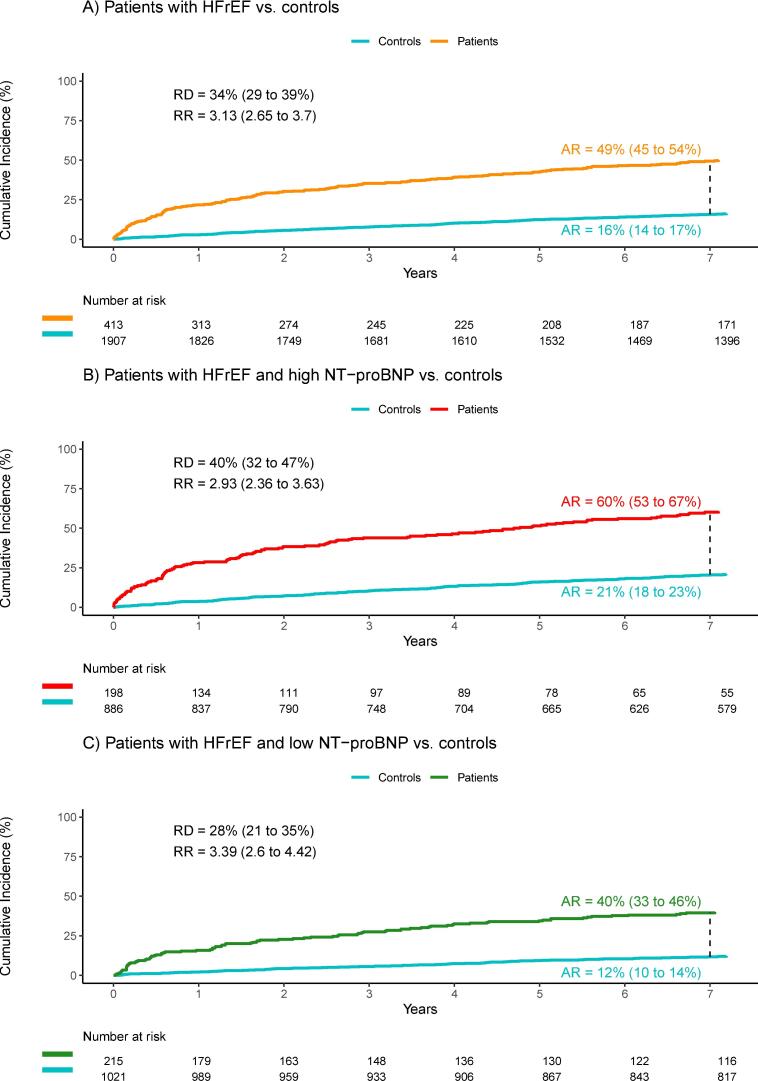
A total of 413 patients and 1907 of their respective matched controls had no loop diuretics prescribed within 6 months of inclusion into the study. Of these, 204 patients (49.4 %) and 301 controls (15.8 %) had loop diuretics prescribed within 7 years (Fig. 5). The relative risk of having loop diuretics prescribed within 7 years was 3.13 (95 % CI, 2.65 to 3.70). In the group with high NT-proBNP level, 198 patients and 886 of their controls were included, and within 7 years, 119 patients (60.1 %) and 182 controls (20.5 %) had a prescription for loop diuretics. Relative risk was significant at 2.93 (95 % CI, 2.93 to 3.63). In the group with low NT-proBNP levels, 85 patients (39.5 %) and 119 controls (11.7 %) used loop diuretics within 7 years. Relative risk was 3.39 (95 % CI, 2.60 to 4.42).
Fig. 5.
Time until the first prescription of loop diuretics. A) All patients with HFrEF compared with matched controls without HF. B) Patients with HFrEF and high levels of NT-proBNP compared with matched controls without HF. C) Patients with HFrEF and low levels of NT-proBNP compared with matched controls without HF.
4. Discussion
4.1. Main findings
In this long-term registry-based follow-up study, we investigated the excess risk of clinical events in Danish patients with HFrEF compared with controls without HF and further used stratification according to NT-proBNP levels at baseline to estimate excess risk in high- and low-risk patients with HFrEF. We observed a significant risk difference between patients with HF and controls without HF in terms of the primary composite endpoint of CV death or HF admissions. The same applies to all secondary endpoints – CV death, HF admissions, all-cause mortality, and non-HF admissions. The same result was observed after stratification according to NT-proBNP with the singular exception of 7-year all-cause mortality in patients with low NT-proBNP levels.
It is well-established that NT-proBNP is a strong independent predictor of adverse outcomes in HF [10]. However, no other studies have used a registry-based matched case-control design to estimate excess risk in patients with HF stratified according to NT-proBNP as in the present study [15], [16]. Though, a very similar study design was used by Tancredi et. al. [17] to assess excess risk in Swedish patients with type 2 diabetes compared to matched controls and stratified according to age, glycemic control, and renal complications. These authors also identified an excess risk in patients with type 2 diabetes.
The high-level NT-proBNP patient group in this study corresponds to the patient groups enrolled in the clinical trials leading to the approval of the before-mentioned new HF treatments, sacubitril/valsartan, and the SGLT2-inhibitor dapagliflozin [3], [5]. The considerable excess risk in high-risk patients with HFrEF across all endpoints found in the present study supports the implementation of the new therapies in this patient group. The low-level NT-proBNP patient group also proved to be at increased risk of CV death and HF admission in combination, as well as CV death, HF-, and non-HF admissions, although not as profoundly increased as in the high-level NT-proBNP group and without proving significant in terms of all-cause mortality. However, due to the higher risk of CV death, this patient group could potentially benefit from the new HF therapies. Further studies of the new treatments in this patient group are therefore warranted, including studies investigating new HF treatments in patients with dilated cardiomyopathies and mild HF symptoms [19]. Future studies should also focus on comorbidities e.g., infections [20], [21] as such endpoints may be dominating in absolute numbers during long-term follow-up.
The risk in the NorthStar cohort may be higher than the placebo arms from more recent HF trials. DAPA-HF had a somewhat similar definition as the patients with HFrEF and a high level of NT-proBNP from the NorthStar trial (sinus rhythm and NT-proBNP > 600 or AF and NT-proBNP > 900). Their primary outcome was similar (CV death or HF hospitalization), and at 24 months we observed a risk of 42 % in the high NT-proBNP level population with HFrEF, whereas the observed risk in DAPA-HF was just above 25 % in the placebo group [5]. Additionally, when comparing the same groups, the use of MRA was lower in the NorthStar trial (31 % vs 72 %), and Sacubitril-valsartan was not used at the index date in the NorthStar trial (11 % in DAPA-HF). The differences in the use of medication may very well be explained by the different calendar timepoints and in part explain the higher risks of the primary outcome in the NorthStar trial. However, when interpreting these numbers, it is also important to note the higher median age in the NorthStar trial (73 years vs 66 years).
4.2. Age stratified analysis
In the analysis of the 7-year risk of the primary endpoint stratified according to three age groups, risk differences were significant across age groups when comparing patients with HFrEF to controls without. After stratification into high- and low-risk groups according to baseline levels of NT-proBNP, absolute excess risks were significant in both groups across all age groups. The differences were greatest in the youngest group (<65 years) and diminished slightly with increasing age in both the high and low NT-proBNP level groups.
In the supplementary analysis, risk differences in all-cause mortality were significant across age groups when comparing patients with HFrEF to non-HF controls even after stratification into high- and low-risk groups according to baseline levels of NT-proBNP. However, in the supplementary analysis on age-stratified mortality risk, no significant difference was seen in any age group for patients with low NT-proBNP levels. Despite having a worse prognosis regarding CV death or HF admission, this patient group does not seem to have higher all-cause mortality than patients without HF regardless of age. Further, for the whole cohort and the group of patients with high NT-proBNP levels, the absolute difference in the 7-year mortality risk decreased with increasing age. Very elderly patients with HFrEF do, therefore, only have a slightly worse prognosis than their matched controls without HF. This should be kept in mind when the implementation of guideline-recommended therapies is organized, and new strategies are developed. It may be speculated that the treatment goal in very elderly patients should concern the quality of life and prevention of re-admissions, instead of focusing on long-term mortality risk [18].
4.3. Time to first prescription of loop diuretics
HF is increasingly diagnosed in an outpatient setting; similarly, worsening HF is often managed without the patients being admitted to the hospital [1], [22]. Therefore, we evaluated the time to first prescription of loop diuretics, and these analyses showed significant risk differences before as well as after stratification according to levels of NT-proBNP. Interestingly, even low-risk patients with HFrEF were over three times more likely to experience worsening in their condition than controls to develop a need for loop diuretics. This analysis supports that patients with HFrEF and a low NT-proBNP level have a chronic disease with a risk of deterioration. It is, therefore, reasonable to conduct further research in this patient group as well as consider the implementation of new treatments.
4.4. Methodological considerations
The present study was based on data from nationwide registries and a clinical trial database. The main strength is the completeness of nationwide registries and the study database with a follow-up time of up to ten years without any individuals lost to follow-up. In addition, all patients from the trial database had echocardiography performed and were evaluated clinically. Therefore, misclassification of the HF diagnosis was minimal, and it was possible to phenotype all the patients with HF as patients with HFrEF according to former clinical guidelines. However, we may have underestimated the excess risk in patients with HFrEF in general, since our cohort represents patients selected for a randomized clinical trial.
Comorbidities for the included patients with HFrEF were originally obtained by medical specialists in HF and cardiology, and the validity of these diagnoses was therefore high (gold standard). In contrast, comorbidities of the matched non-HF controls were obtained through nationwide registries, which were previously validated [23], [24] However, some degree of bias may have been introduced due to the high specificity and lower sensitivity of diagnoses from administrative codes in general [24].
Detailed information on the patients from the trial database was available e.g., body mass index, history of smoking, and quality of life [12]. However, these variables are not accessible in the control group and further subgroup analyses according to these variables were not possible.
Given the inclusion of HFrEF patients in 2009–2012, the HFrEF population may not reflect today’s HFrEF patients, which is underlined by differences in the use of guideline-directed therapy in HFrEF and higher outcome risk compared to more recent trials. However, this is an inevitable drawback when looking at long-term outcomes.
It may be argued that low vs high NT-proBNP levels may reflect normalized and abnormal NT-proBNP levels respectively following medical optimization. Our results may, therefore, reflect that any residual high NT-proBNP level following medical optimization is a risk marker for CV death and HF worsening.
4.5. Perspectives
Our analyses indicate that a substantial excess risk exists independently of NT-proBNP levels in patients HFrEF treated with the traditional neurohormonal blockade. Implementation of new treatments like sacubitril/valsartan and SGLT2 inhibitors may have the potential to further reduce the observed mortality and morbidity risk. More research is needed in patients with HFrEF and high as well as low NT-proBNP levels, but it should be kept in mind that the excess risk in elderly and very elderly patients is less than in younger patients. Finally, the excess risk of non-HF admissions is high and future studies should focus on comorbidities to improve the number of days outside of the hospital.
5. Conclusion
This study observed a significant excess risk in patients with HFrEF compared to matched controls without HF across a broad spectrum of important clinical endpoints. These findings were persistent after stratifying patients with HFrEF into high and low-risk groups according to baseline levels of NT-proBNP.
Funding
The current study was funded internally by the Department of Cardiology at Herlev and Gentofte Hospital in Copenhagen. The original NorthStar trial was funded by unrestricted grants from Roche Diagnostics, Switzerland.
CRediT authorship contribution statement
Anna Tuxen: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Morten Malmborg: Writing – review & editing, Writing – original draft, Visualization, Supervision, Methodology, Investigation, Formal analysis, Data curation. Nina Nouravesh: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation. Lars Videbaek: Writing – review & editing, Writing – original draft. Mariam Malik: Writing – review & editing, Writing – original draft. Deewa Zahir: Writing – review & editing, Writing – original draft. Lars Koeber: Writing – review & editing, Writing – original draft. Camilla F. Andersen: Writing – review & editing, Writing – original draft. Jawad H. Butt: . Jesper Jensen: Writing – review & editing, Writing – original draft. Emil Foesbol: Writing – review & editing, Writing – original draft. Charlotte Andersson: Writing – review & editing, Writing – original draft. Finn Gustafsson: Writing – review & editing, Writing – original draft. Morten Schou: Writing – review & editing, Writing – original draft, Visualization, Supervision, Methodology, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank all patients included in the NorthStar Trial as well as the earlier NorthStar investigators.
Funding
This post hoc study from the NorthStar trial did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors contributed with writing, reviewing and editing the manuscript. NN also contributed with investigation, methodology and supervision. MM with data curation, formal analysis, investigation, methodology, supervision, and visualization. AT and MS contributed to all aspects of the manuscript.
Disclosures
LK: Speaker’s honorarium from Novo, Novartis, AstraZeneca, and Boehringer. FG: Advisor (Bayer, Abbott, Boehringer-Ingelheim, Pfizer, Alnylam, Ionis, Pharmacosmos, Amgen), speakers fee (Orion Pharma, Astra-Zeneca. MS: lecture fee Novo, Bohringer, Astra, Novo.
Trial registration:www.Centerwatch.com: 173491 (NorthStar).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101441.
Appendix.
Supplementary Table S1: Codes and definitions.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Arulmurugananthavadivel A., Holt A., Parveen S., et al. Importance of diagnostic setting in determining mortality in patients with new-onset heart failure: temporal trends in Denmark 1997–2017. Eur. Heart J. Qual. Care Clin. Outcomes. 2021. doi: 10.1093/ehjqcco/qcab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Lueder T.G., Kotecha D., Atar D., Hopper I. Neurohormonal Blockade in Heart Failure. Card. Fail. Rev. 2017;3(1):19–24. doi: 10.15420/cfr.2016:22:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray J.J., Packer M., Desai A.S., et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 4.Packer M., McMurray J.J. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur. J. Heart Fail. 2021;23(6):882–894. doi: 10.1002/ejhf.2149. 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 6.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022-05-03 2022;145(18). [DOI] [PubMed]
- 8.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Rev Esp Cardiol (Engl Ed). 06 2022;75(6):523. [DOI] [PubMed]
- 9.Fang J.C. Heart-failure therapy - new drugs but old habits? N. Engl. J. Med. 2019;381(21):2063–2064. doi: 10.1056/NEJMe1912180. [DOI] [PubMed] [Google Scholar]
- 10.Schou M., Gustafsson F., Corell P., Kistorp C.N., Kjaer A., Hildebrandt P.R. The relationship between N-terminal pro-brain natriuretic peptide and risk for hospitalization and mortality is curvilinear in patients with chronic heart failure. Am. Heart J. 2007;154(1):123–129. doi: 10.1016/j.ahj.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Schou M., Gustafsson F., Videbaek L., et al. Design and methodology of the NorthStar Study: NT-proBNP stratified follow-up in outpatient heart failure clinics – a randomized Danish multicenter study. Am. Heart J. 2008;156(4):649–655. doi: 10.1016/j.ahj.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Schou M., Gustafsson F., Videbaek L., et al. Adding serial N-terminal pro brain natriuretic peptide measurements to optimal clinical management in outpatients with systolic heart failure: a multicentre randomized clinical trial (NorthStar monitoring study) Eur. J. Heart Fail. 2013;15(7):818–827. doi: 10.1093/eurjhf/hft037. [DOI] [PubMed] [Google Scholar]
- 13.Schou M., Gustafsson F., Videbaek L., et al. Extended heart failure clinic follow-up in low-risk patients: a randomized clinical trial (NorthStar) Eur. Heart J. 2013;34(6):432–442. doi: 10.1093/eurheartj/ehs235. [DOI] [PubMed] [Google Scholar]
- 14.Helweg-Larsen K. The Danish register of causes of death. Scand. J. Public Health. 2011;39(7 Suppl):26–29. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 15.Gardner R.S., Ozalp F., Murday A.J., Robb S.D., McDonagh T.A. N-terminal pro-brain natriuretic peptide. a new gold standard in predicting mortality in patients with advanced heart failure. Eur. Heart J. 2003;24(19):1735–1743. doi: 10.1016/j.ehj.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Masson S., Latini R., Anand I.S., et al. Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the Valsartan Heart Failure (Val-HeFT) data. Clin. Chem. 2006;52(8):1528–1538. doi: 10.1373/clinchem.2006.069575. [DOI] [PubMed] [Google Scholar]
- 17.Tancredi M., Rosengren A., Svensson A.M., et al. Excess Mortality among Persons with Type 2 Diabetes. N. Engl. J. Med. 2015;373(18):1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 18.Pfisterer M., Buser P., Rickli H., et al. BNP-guided vs symptom-guided heart failure therapy: the trial of intensified vs standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. J. Am. Med. Assoc. 2009;301(4):383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 19.Repetti G.G., Toepfer C.N., Seidman J.G., Seidman C.E. Novel therapies for prevention and early treatment of cardiomyopathies. Circ. Res. 2019;124(11):1536–1550. doi: 10.1161/CIRCRESAHA.119.313569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modin D., Jørgensen M.E., Gislason G., et al. Influenza vaccine in heart failure. Circulation. 2019;139(5):575–586. doi: 10.1161/CIRCULATIONAHA.118.036788. [DOI] [PubMed] [Google Scholar]
- 21.Shen L., Jhund P.S., Anand I.S., et al. Incidence and outcomes of pneumonia in patients with heart failure. J. Am. Coll. Cardiol. 2021;77(16):1961–1973. doi: 10.1016/j.jacc.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Madelaire C., Gustafsson F., Stevenson L.W., et al. One-year mortality after intensification of outpatient diuretic therapy. J. Am. Heart Assoc. 2020;9(14):e016010. doi: 10.1161/JAHA.119.016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delekta J., Hansen S.M., AlZuhairi K.S., Bork C.S., Joensen A.M. The validity of the diagnosis of heart failure (I50.0-I50.9) in the Danish National Patient Register. Dan. Med. J. 2018;65(4) [PubMed] [Google Scholar]
- 24.Sundbøll J., Adelborg K., Munch T., et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.