Abstract
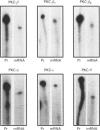
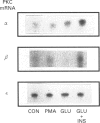
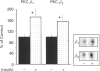
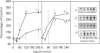
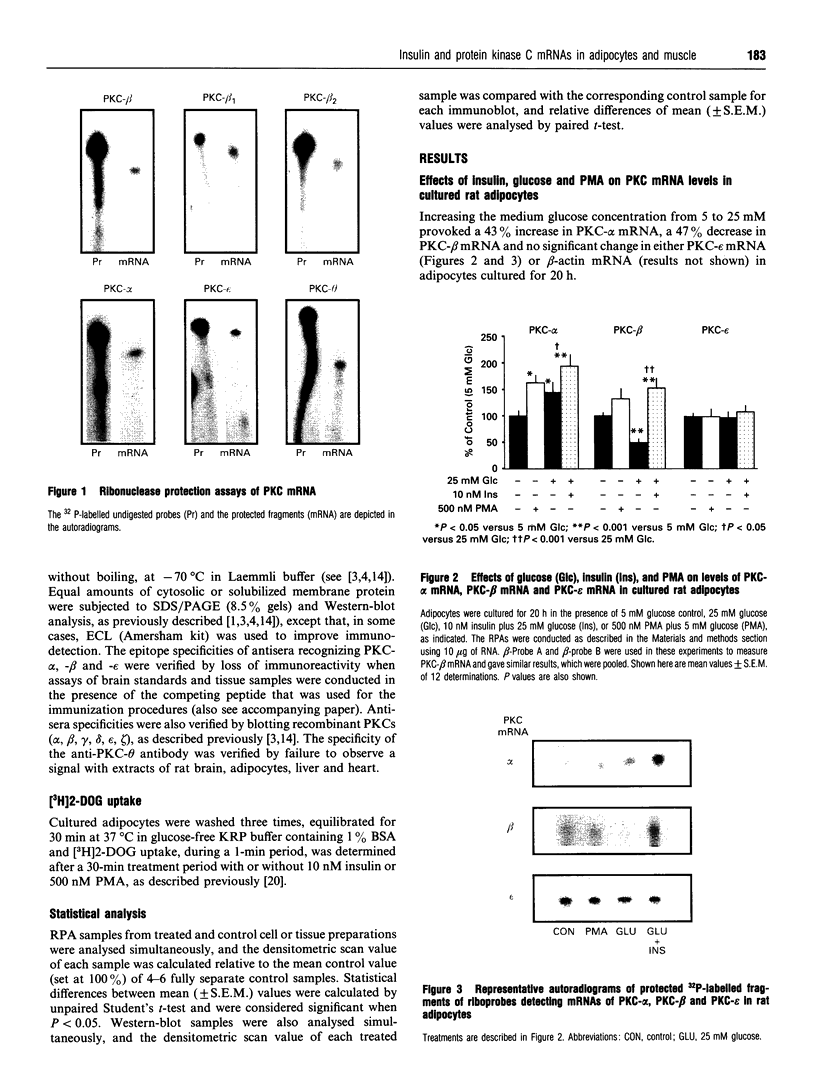
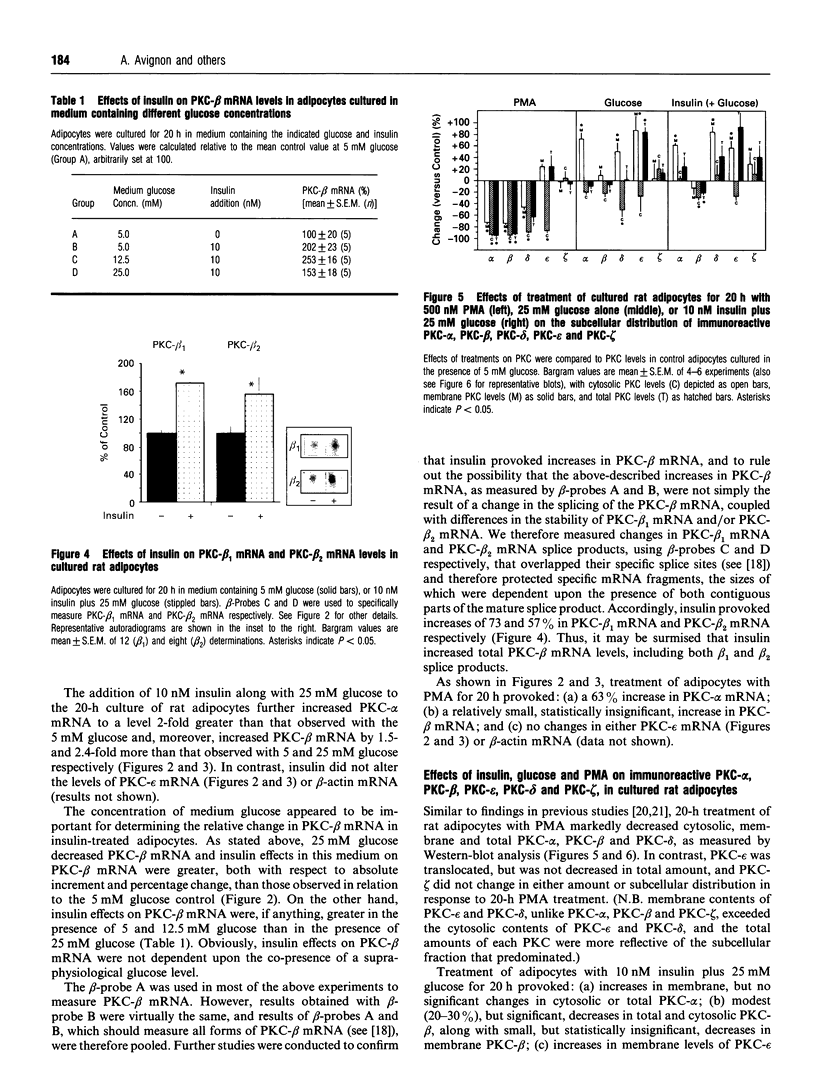
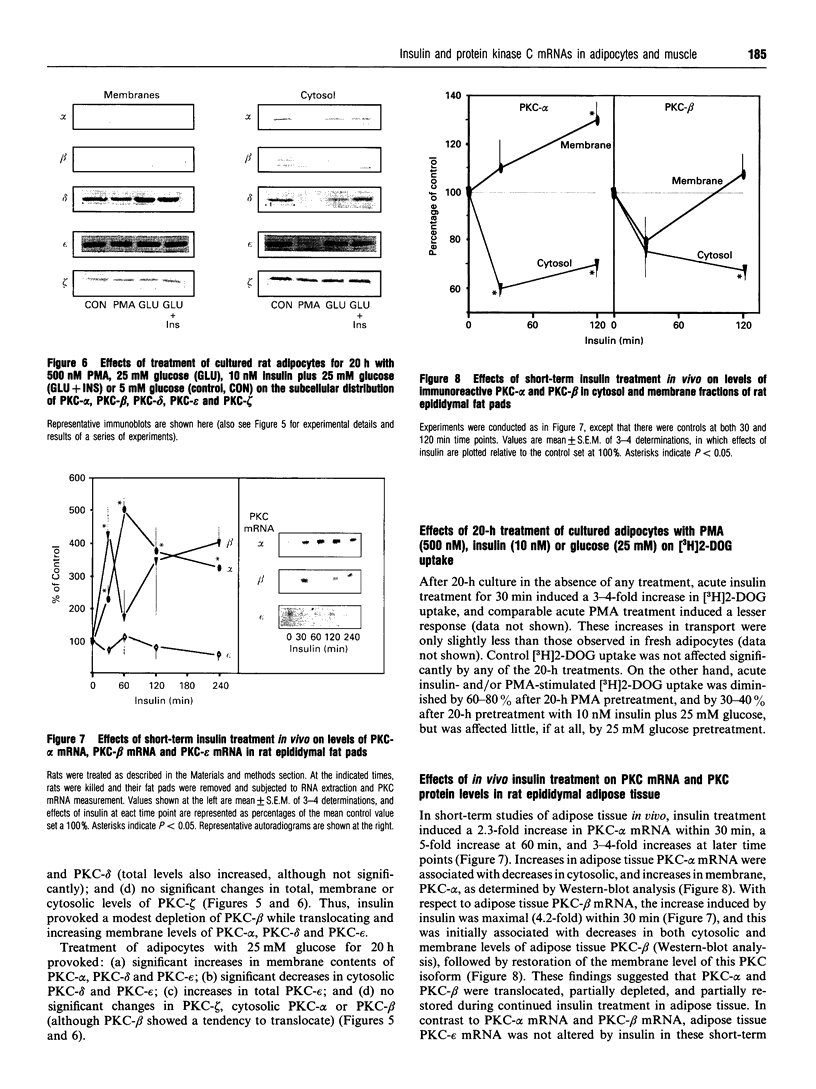
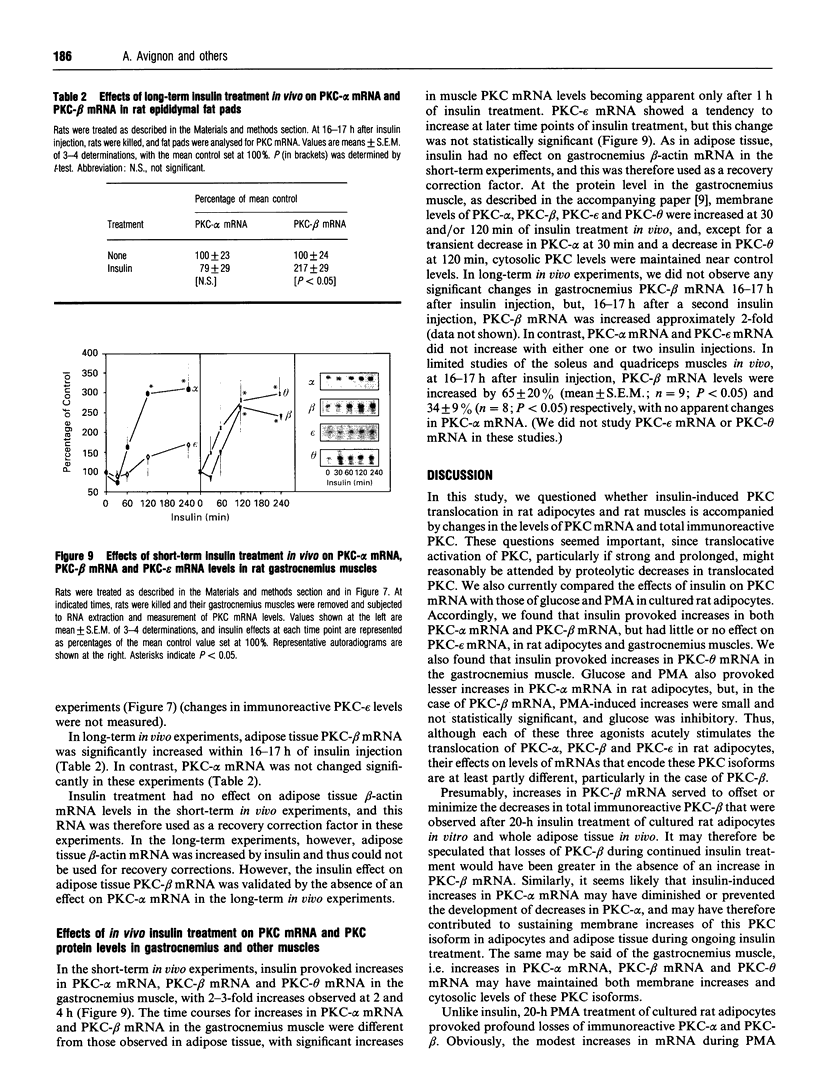
Effects of insulin of levels of mRNA encoding protein kinase C (PKC)-alpha, PKC-beta, PKC-epsilon and PKC-theta were examined by ribonuclease protection assay in primary cultures of rat adipocytes in vitro, and in rat adipose tissue and gastrocnemius muscle in vivo. In all cases, insulin increased the levels of PKC-alpha mRNA and PKC-beta mRNA, and, in muscle, insulin also increased the level of PKC-theta mRNA. PKC-epsilon mRNA levels, on the other hand, were not altered significantly. Insulin also stimulated the apparent translocation of PKC-alpha, -beta, -epsilon and -theta, to the membrane fractions of adipocytes, adipose tissue and gastrocnemius muscles, and, in some instances, total PKC levels were diminished, e.g. PKC-alpha and PKC-beta in cultured adipocytes in vitro and/or whole adipose tissue in vivo, and PKC-alpha and PKC-theta in the gastrocnemius muscle. Thus, insulin-induced increases in PKC mRNA may have been partly compensatory in nature to restore PKC levels following translocation and proteolytic losses. However, much more severe depletion of PKC-alpha and PKC-beta by phorbol ester treatment in cultured rat adipocytes in vitro resulted in, if anything, smaller increases in PKC-alpha mRNA and PKC-beta mRNA, and it therefore appears that insulin effects on PKC mRNA levels were not simply due to decreases in respective PKC levels. In addition, effects of insulin, particularly on PKC-beta mRNA, could not be attributed to increased glucose metabolism, which alone decreased PKC-beta mRNA in cultured adipocytes in vitro. We conclude that insulin-induced translocation and degradation of PKC-alpha, PKC-beta and PKC-theta are attended by selective increases in their mRNAs. This mechanism of increasing mRNA may be important in maintaining PKC levels during the continued action of insulin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold T. P., Standaert M. L., Hernandez H., Watson J., Mischak H., Kazanietz M. G., Zhao L., Cooper D. R., Farese R. V. Effects of insulin and phorbol esters on MARCKS (myristoylated alanine-rich C-kinase substrate) phosphorylation (and other parameters of protein kinase C activation) in rat adipocytes, rat soleus muscle and BC3H-1 myocytes. Biochem J. 1993 Oct 1;295(Pt 1):155–164. doi: 10.1042/bj2950155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G., Goss M. W., East J. A., Hart I. R. Growth of melanocytic cells is associated with down-regulation of protein kinase C alpha, delta, and epsilon isoforms. Possible role of diacylglycerol. J Biol Chem. 1993 Nov 15;268(32):23868–23875. [PubMed] [Google Scholar]
- Chen K. S., Heydrick S., Kurowski T., Ruderman N. B. Diacylglycerol-protein kinase C signalling in skeletal muscle: a possible link to insulin resistance. Trans Assoc Am Physicians. 1991;104:206–212. [PubMed] [Google Scholar]
- Chin J. E., Liu F., Roth R. A. Activation of protein kinase C alpha inhibits insulin-stimulated tyrosine phosphorylation of insulin receptor substrate-1. Mol Endocrinol. 1994 Jan;8(1):51–58. doi: 10.1210/mend.8.1.7512195. [DOI] [PubMed] [Google Scholar]
- Draznin B., Leitner J. W., Sussman K. E., Sherman N. A. Insulin and glucose modulate protein kinase C activity in rat adipocytes. Biochem Biophys Res Commun. 1988 Oct 14;156(1):570–575. doi: 10.1016/s0006-291x(88)80880-5. [DOI] [PubMed] [Google Scholar]
- Egan J. J., Saltis J., Wek S. A., Simpson I. A., Londos C. Insulin, oxytocin, and vasopressin stimulate protein kinase C activity in adipocyte plasma membranes. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1052–1056. doi: 10.1073/pnas.87.3.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Standaert M. L., Arnold T. P., Yamada K., Musunuru K., Hernandez H., Mischak H., Cooper D. R. Preferential activation of microsomal diacylglycerol/protein kinase C signaling during glucose treatment (De Novo phospholipid synthesis) of rat adipocytes. J Clin Invest. 1994 May;93(5):1894–1899. doi: 10.1172/JCI117180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R. V., Standaert M. L., Francois A. J., Ways K., Arnold T. P., Hernandez H., Cooper D. R. Effects of insulin and phorbol esters on subcellular distribution of protein kinase C isoforms in rat adipocytes. Biochem J. 1992 Nov 15;288(Pt 1):319–323. doi: 10.1042/bj2880319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Azhar S., Hoffman B. B. Prolonged activation of alpha 1 adrenoceptors induces down-regulation of protein kinase C in vascular smooth muscle. J Cardiovasc Pharmacol. 1992 Dec;20(6):982–989. doi: 10.1097/00005344-199212000-00020. [DOI] [PubMed] [Google Scholar]
- Häring H., Kirsch D., Obermaier B., Ermel B., Machicao F. Tumor-promoting phorbol esters increase the Km of the ATP-binding site of the insulin receptor kinase from rat adipocytes. J Biol Chem. 1986 Mar 15;261(8):3869–3875. [PubMed] [Google Scholar]
- Ishizuka T., Cooper D. R., Arnold T., Hernandez H., Farese R. V. Downregulation of protein kinase C and insulin-stimulated 2-deoxyglucose uptake in rat adipocytes by phorbol esters, glucose, and insulin. Diabetes. 1991 Oct;40(10):1274–1281. doi: 10.2337/diab.40.10.1274. [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Cooper D. R., Farese R. V. Insulin stimulates the translocation of protein kinase C in rat adipocytes. FEBS Lett. 1989 Nov 6;257(2):337–340. doi: 10.1016/0014-5793(89)81565-0. [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Cooper D. R., Hernandez H., Buckley D., Standaert M., Farese R. V. Effects of insulin on diacylglycerol-protein kinase C signaling in rat diaphragm and soleus muscles and relationship to glucose transport. Diabetes. 1990 Feb;39(2):181–190. doi: 10.2337/diab.39.2.181. [DOI] [PubMed] [Google Scholar]
- Ishizuka T., Hoffman J., Cooper D. R., Watson J. E., Pushkin D. B., Farese R. V. Glucose-induced synthesis of diacylglycerol de novo is associated with translocation (activation) of protein kinase C in rat adipocytes. FEBS Lett. 1989 Jun 5;249(2):234–238. doi: 10.1016/0014-5793(89)80630-1. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto A., Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Mischak H., Goodnight J., Henderson D. W., Osada S., Ohno S., Mushinski J. F. Unique expression pattern of protein kinase C-theta: high mRNA levels in normal mouse testes and in T-lymphocytic cells and neoplasms. FEBS Lett. 1993 Jul 12;326(1-3):51–55. doi: 10.1016/0014-5793(93)81759-s. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J Biol Chem. 1994 Jan 28;269(4):2758–2763. [PubMed] [Google Scholar]
- Ono Y., Fujii T., Igarashi K., Kikkawa U., Ogita K., Nishizuka Y. Nucleotide sequences of cDNAs for alpha and gamma subspecies of rat brain protein kinase C. Nucleic Acids Res. 1988 Jun 10;16(11):5199–5200. doi: 10.1093/nar/16.11.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–6932. [PubMed] [Google Scholar]
- Ono Y., Kikkawa U., Ogita K., Fujii T., Kurokawa T., Asaoka Y., Sekiguchi K., Ase K., Igarashi K., Nishizuka Y. Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science. 1987 May 29;236(4805):1116–1120. doi: 10.1126/science.3576226. [DOI] [PubMed] [Google Scholar]
- Roth B. L., Mehegan J. P., Jacobowitz D. M., Robey F., Iadarola M. J. Rat brain protein kinase C: purification, antibody production, and quantification in discrete regions of hippocampus. J Neurochem. 1989 Jan;52(1):215–221. doi: 10.1111/j.1471-4159.1989.tb10919.x. [DOI] [PubMed] [Google Scholar]
- Standaert M. L., Cooper D. R., Hernandez H., Arnold T. P., Farese R. V. Differential down-regulation of insulin-sensitive protein kinase-C isoforms by 12-O-tetradecanoylphorbol-13-acetate in rat adipocytes and BC3H-1 myocytes. Endocrinology. 1993 Feb;132(2):689–692. doi: 10.1210/endo.132.2.8425487. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Horn R. S., Adler A., Albert K. A., Walaas O. Insulin increases membrane protein kinase C activity in rat diaphragm. FEBS Lett. 1987 Aug 17;220(2):311–318. doi: 10.1016/0014-5793(87)80837-2. [DOI] [PubMed] [Google Scholar]
- Yamada K., Avignon A., Standaert M. L., Cooper D. R., Spencer B., Farese R. V. Effects of insulin on the translocation of protein kinase C-theta and other protein kinase C isoforms in rat skeletal muscles. Biochem J. 1995 May 15;308(Pt 1):177–180. doi: 10.1042/bj3080177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Standaert M., Arnold T., Hernandez H., Watson J., Ways K., Cooper D. R., Farese R. V. Effects of insulin on diacylglycerol/protein kinase-C signalling and glucose transport in rat skeletal muscles in vivo and in vitro. Endocrinology. 1992 Jun;130(6):3345–3355. doi: 10.1210/endo.130.6.1597146. [DOI] [PubMed] [Google Scholar]