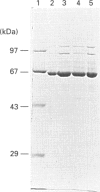
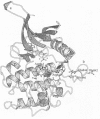
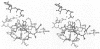Abstract
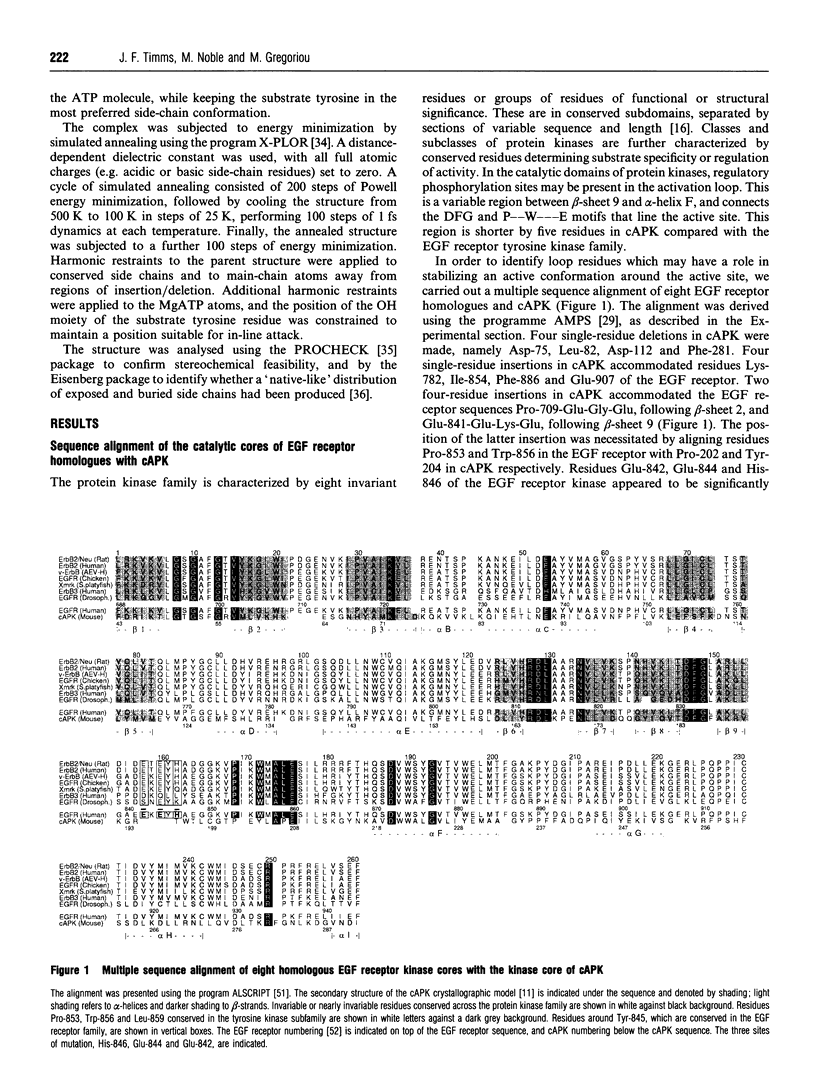
Activation of several protein kinases is mediated, at least in part, by phosphorylation of conserved Thr or Tyr residues located in a variable loop region, near the active site. In certain kinases, this activation loop also controls access of peptide substrates to the active site. In the corresponding region of the epidermal growth factor (EGF) receptor, a potential phosphorylation site, Tyr-845, does not appear to have a major regulatory role. In order to find out whether this variable loop can modulate the peptide phosphorylation and self-phosphorylation activities of the EGF receptor kinase, we investigated the role of residues around Tyr-845, using site-directed mutagenesis. Multiple sequence alignment showed that residues Glu-842, Glu-844 and His-846 are conserved or nearly conserved in eight members of the EGF receptor family. Mutants Glu-842-->Ser, Glu-844-->Gln and His-846-->Ala were expressed in the baculovirus/insect cell system, purified to near-homogeneity and characterized with respect to their peptide phosphorylation and self-phosphorylation activities. All three mutants were active, and these changes did not affect ATP binding directly. However, all mutations increased the Km(app.) for peptide substrates and MnATP in peptide phosphorylation reactions. The Vmax. for the phosphorylation of peptide RREELQDDYEDD was unaltered, but the Vmax. for self-phosphorylation (with variable [MnATP]) decreased 4-, 2- and 7-fold for mutants Glu-842-->Ser, Glu-844-->Gln and His-846-->Ala respectively, compared with the wild-type. These results suggest that binding of this peptide restored an optimal conformation at the active site that might be impaired by the mutations. A study of the dependence of initial rates of self-phosphorylation on cytoplasmic domain concentration showed that the order of reaction increased with the progress of self-phosphorylation. Both pre-phosphorylation and high concentrations of ammonium sulphate restored maximal or near-maximal levels of self-phosphorylation in the mutants, possibly through compensating conformational changes. A plausible homology model, based on the cyclic AMP-dependent protein kinase catalytic subunit, accommodated the sequence Glu-841-Glu-Lys-Glu as an insertion in the peptide binding loop at the edge of the active site cleft. The model suggests that Glu-844 and His-846 may participate in H-bonding interactions, thus stabilizing the active site region, while Glu-842 does not appear to interact with regions of the catalytic core.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bairoch A., Boeckmann B. The SWISS-PROT protein sequence data bank. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2247–2249. doi: 10.1093/nar/19.suppl.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton G. J. ALSCRIPT: a tool to format multiple sequence alignments. Protein Eng. 1993 Jan;6(1):37–40. doi: 10.1093/protein/6.1.37. [DOI] [PubMed] [Google Scholar]
- Barton G. J., Sternberg M. J. Flexible protein sequence patterns. A sensitive method to detect weak structural similarities. J Mol Biol. 1990 Mar 20;212(2):389–402. doi: 10.1016/0022-2836(90)90133-7. [DOI] [PubMed] [Google Scholar]
- Bertics P. J., Gill G. N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J Biol Chem. 1985 Nov 25;260(27):14642–14647. [PubMed] [Google Scholar]
- Bossemeyer D., Engh R. A., Kinzel V., Ponstingl H., Huber R. Phosphotransferase and substrate binding mechanism of the cAMP-dependent protein kinase catalytic subunit from porcine heart as deduced from the 2.0 A structure of the complex with Mn2+ adenylyl imidodiphosphate and inhibitor peptide PKI(5-24). EMBO J. 1993 Mar;12(3):849–859. doi: 10.1002/j.1460-2075.1993.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun Y. V., Breton R., Lapointe J. Large scale sequencing projects using rapidly prepared double-stranded plasmid DNA. DNA Seq. 1991;1(5):285–289. doi: 10.3109/10425179109020784. [DOI] [PubMed] [Google Scholar]
- Böni-Schnetzler M., Pilch P. F. Mechanism of epidermal growth factor receptor autophosphorylation and high-affinity binding. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7832–7836. doi: 10.1073/pnas.84.22.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadena D. L., Chan C. L., Gill G. N. The intracellular tyrosine kinase domain of the epidermal growth factor receptor undergoes a conformational change upon autophosphorylation. J Biol Chem. 1994 Jan 7;269(1):260–265. [PubMed] [Google Scholar]
- Canals F. Signal transmission by epidermal growth factor receptor: coincidence of activation and dimerization. Biochemistry. 1992 May 12;31(18):4493–4501. doi: 10.1021/bi00133a016. [DOI] [PubMed] [Google Scholar]
- Connell-Crowley L., Solomon M. J., Wei N., Harper J. W. Phosphorylation independent activation of human cyclin-dependent kinase 2 by cyclin A in vitro. Mol Biol Cell. 1993 Jan;4(1):79–92. doi: 10.1091/mbc.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bondt H. L., Rosenblatt J., Jancarik J., Jones H. D., Morgan D. O., Kim S. H. Crystal structure of cyclin-dependent kinase 2. Nature. 1993 Jun 17;363(6430):595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Waterfield M. D., Parker P. J. Autophosphorylation and protein kinase C phosphorylation of the epidermal growth factor receptor. Effect on tyrosine kinase activity and ligand binding affinity. J Biol Chem. 1985 Nov 25;260(27):14538–14546. [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fung M. C., Chiu K. Y., Weber T., Chang T. W., Chang N. T. Detection and purification of a recombinant human B lymphotropic virus (HHV-6) in the baculovirus expression system by limiting dilution and DNA dot-blot hybridization. J Virol Methods. 1988 Jan;19(1):33–42. doi: 10.1016/0166-0934(88)90005-5. [DOI] [PubMed] [Google Scholar]
- Garger S. J., Griffith O. M., Grill L. K. Rapid purification of plasmid DNA by a single centrifugation in a two-step cesium chloride-ethidium bromide gradient. Biochem Biophys Res Commun. 1983 Dec 28;117(3):835–842. doi: 10.1016/0006-291x(83)91672-8. [DOI] [PubMed] [Google Scholar]
- Gotoh N., Tojo A., Hino M., Yazaki Y., Shibuya M. A highly conserved tyrosine residue at codon 845 within the kinase domain is not required for the transforming activity of human epidermal growth factor receptor. Biochem Biophys Res Commun. 1992 Jul 31;186(2):768–774. doi: 10.1016/0006-291x(92)90812-y. [DOI] [PubMed] [Google Scholar]
- Gregoriou M., Jones P. F., Timms J. F., Yang J. J., Radford S. E., Rees A. R. Physicochemical characterization of the cytoplasmic domain of the epidermal growth factor receptor and evidence for conformational changes associated with its activation by ammonium sulphate. Biochem J. 1995 Mar 15;306(Pt 3):667–678. doi: 10.1042/bj3060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Kris R. M., Ullrich A., Schlessinger J. Evidence that autophosphorylation of solubilized receptors for epidermal growth factor is mediated by intermolecular cross-phosphorylation. Proc Natl Acad Sci U S A. 1989 Feb;86(3):925–929. doi: 10.1073/pnas.86.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger A. M., Schmidt A., Ullrich A., Schlessinger J. Evidence for epidermal growth factor (EGF)-induced intermolecular autophosphorylation of the EGF receptors in living cells. Mol Cell Biol. 1990 Aug;10(8):4035–4044. doi: 10.1128/mcb.10.8.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. H., Parker M. W., Lei J. Y., Wilce M. C., Benian G. M., Kemp B. E. Insights into autoregulation from the crystal structure of twitchin kinase. Nature. 1994 Jun 16;369(6481):581–584. doi: 10.1038/369581a0. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Zheng J., Xuong N., Taylor S. S., Sowadski J. M. Structure of the mammalian catalytic subunit of cAMP-dependent protein kinase and an inhibitor peptide displays an open conformation. Acta Crystallogr D Biol Crystallogr. 1993 Jul 1;49(Pt 4):381–388. doi: 10.1107/S0907444993002306. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Design and use of peptide substrates for protein kinases. Methods Enzymol. 1991;200:121–134. doi: 10.1016/0076-6879(91)00134-i. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Cadena D. L., Zheng J., Ten Eyck L. F., Taylor S. S., Sowadski J. M., Gill G. N. Structural features that specify tyrosine kinase activity deduced from homology modeling of the epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5001–5005. doi: 10.1073/pnas.90.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livingstone C., Jones I. Baculovirus expression vectors with single strand capability. Nucleic Acids Res. 1989 Mar 25;17(6):2366–2366. doi: 10.1093/nar/17.6.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthy R., McLachlan A. D., Eisenberg D. Secondary structure-based profiles: use of structure-conserving scoring tables in searching protein sequence databases for structural similarities. Proteins. 1991;10(3):229–239. doi: 10.1002/prot.340100307. [DOI] [PubMed] [Google Scholar]
- Morris A. L., MacArthur M. W., Hutchinson E. G., Thornton J. M. Stereochemical quality of protein structure coordinates. Proteins. 1992 Apr;12(4):345–364. doi: 10.1002/prot.340120407. [DOI] [PubMed] [Google Scholar]
- Olah G. A., Mitchell R. D., Sosnick T. R., Walsh D. A., Trewhella J. Solution structure of the cAMP-dependent protein kinase catalytic subunit and its contraction upon binding the protein kinase inhibitor peptide. Biochemistry. 1993 Apr 13;32(14):3649–3657. doi: 10.1021/bi00065a018. [DOI] [PubMed] [Google Scholar]
- Orellana S. A., McKnight G. S. Mutations in the catalytic subunit of cAMP-dependent protein kinase result in unregulated biological activity. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4726–4730. doi: 10.1073/pnas.89.10.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington J., Donnelly D., Johnson M. S., Sali A., Blundell T. L. Environment-specific amino acid substitution tables: tertiary templates and prediction of protein folds. Protein Sci. 1992 Feb;1(2):216–226. doi: 10.1002/pro.5560010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton H. A., Fujii Y., Price I. R., Jones I. M. The protease and gag gene products of the human immunodeficiency virus: authentic cleavage and post-translational modification in an insect cell expression system. Virology. 1989 May;170(1):107–116. doi: 10.1016/0042-6822(89)90357-7. [DOI] [PubMed] [Google Scholar]
- Pike L. J. Assay of growth factor-stimulated tyrosine kinases using synthetic peptide substrates. Methods Enzymol. 1987;146:353–362. doi: 10.1016/s0076-6879(87)46036-9. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987 Apr 10;49(1):75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- Reed J., Kinzel V., Kemp B. E., Cheng H. C., Walsh D. A. Circular dichroic evidence for an ordered sequence of ligand/binding site interactions in the catalytic reaction of the cAMP-dependent protein kinase. Biochemistry. 1985 Jun 4;24(12):2967–2973. doi: 10.1021/bi00333a024. [DOI] [PubMed] [Google Scholar]
- Singh J. Comparison of conservation within and between the Ser/Thr and Tyr protein kinase family: proposed model for the catalytic domain of the epidermal growth factor receptor. Protein Eng. 1994 Jul;7(7):849–858. doi: 10.1093/protein/7.7.849. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Lee T., Kirschner M. W. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992 Jan;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. A., Cauthron R. D., Symcox M. M., Shuntoh H. Autoactivation of catalytic (C alpha) subunit of cyclic AMP-dependent protein kinase by phosphorylation of threonine 197. Mol Cell Biol. 1993 Apr;13(4):2332–2341. doi: 10.1128/mcb.13.4.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. S., Radzio-Andzelm E. Three protein kinase structures define a common motif. Structure. 1994 May 15;2(5):345–355. doi: 10.1016/s0969-2126(00)00036-8. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P. B., Gill G. N. Activation of the purified protein tyrosine kinase domain of the epidermal growth factor receptor. J Biol Chem. 1989 Jul 5;264(19):11346–11353. [PubMed] [Google Scholar]
- Weyer U., Knight S., Possee R. D. Analysis of very late gene expression by Autographa californica nuclear polyhedrosis virus and the further development of multiple expression vectors. J Gen Virol. 1990 Jul;71(Pt 7):1525–1534. doi: 10.1099/0022-1317-71-7-1525. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987 Mar 10;26(5):1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]
- Zhang F., Strand A., Robbins D., Cobb M. H., Goldsmith E. J. Atomic structure of the MAP kinase ERK2 at 2.3 A resolution. Nature. 1994 Feb 24;367(6465):704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]
- Zheng J., Knighton D. R., Xuong N. H., Taylor S. S., Sowadski J. M., Ten Eyck L. F. Crystal structures of the myristylated catalytic subunit of cAMP-dependent protein kinase reveal open and closed conformations. Protein Sci. 1993 Oct;2(10):1559–1573. doi: 10.1002/pro.5560021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Knighton D. R., ten Eyck L. F., Karlsson R., Xuong N., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide inhibitor. Biochemistry. 1993 Mar 9;32(9):2154–2161. doi: 10.1021/bi00060a005. [DOI] [PubMed] [Google Scholar]