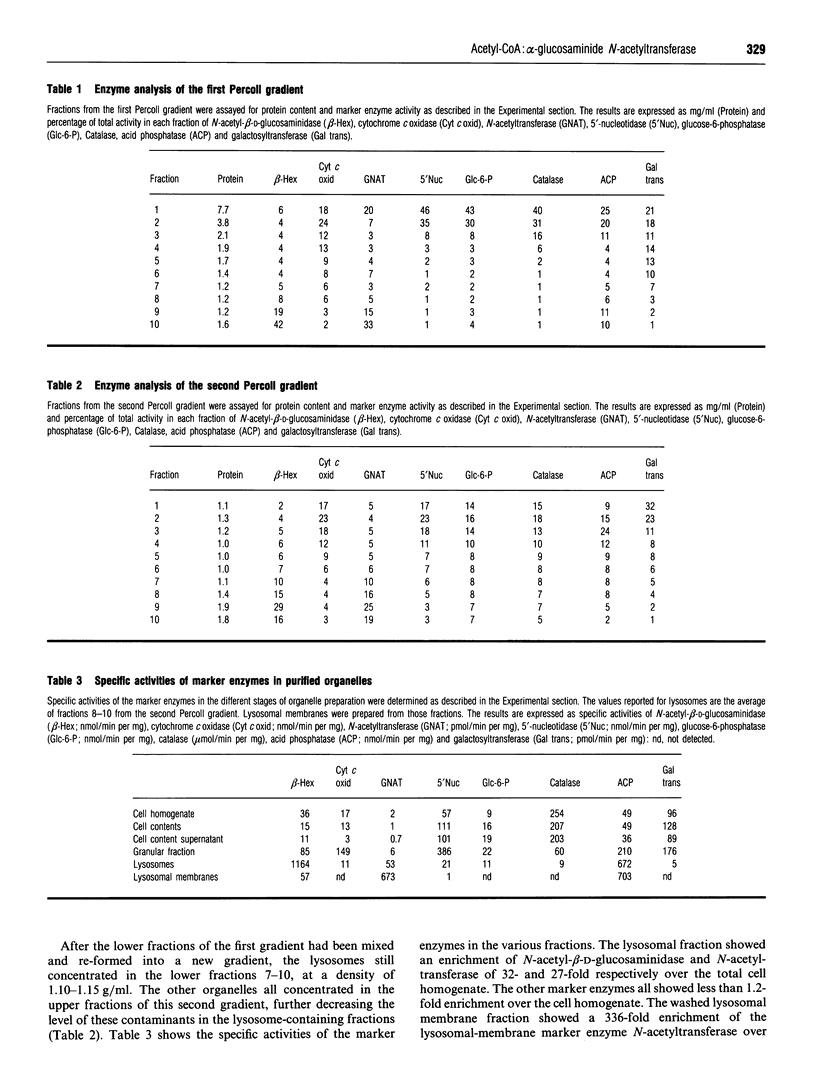
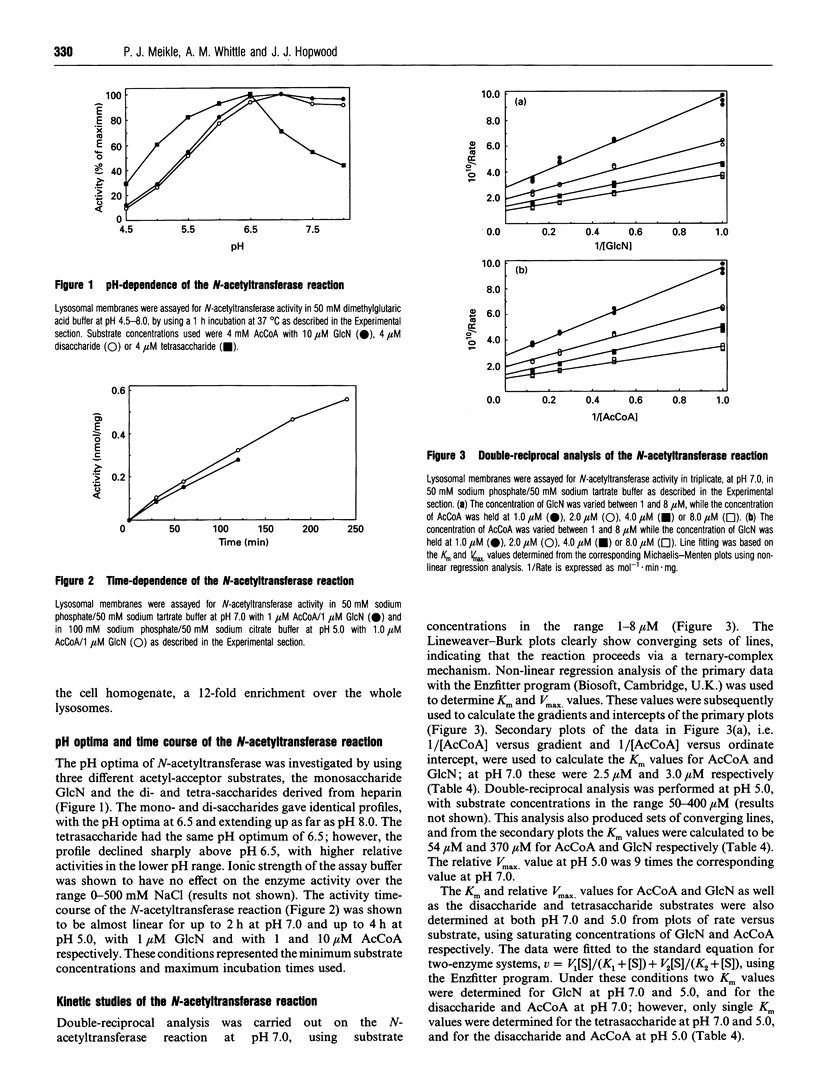
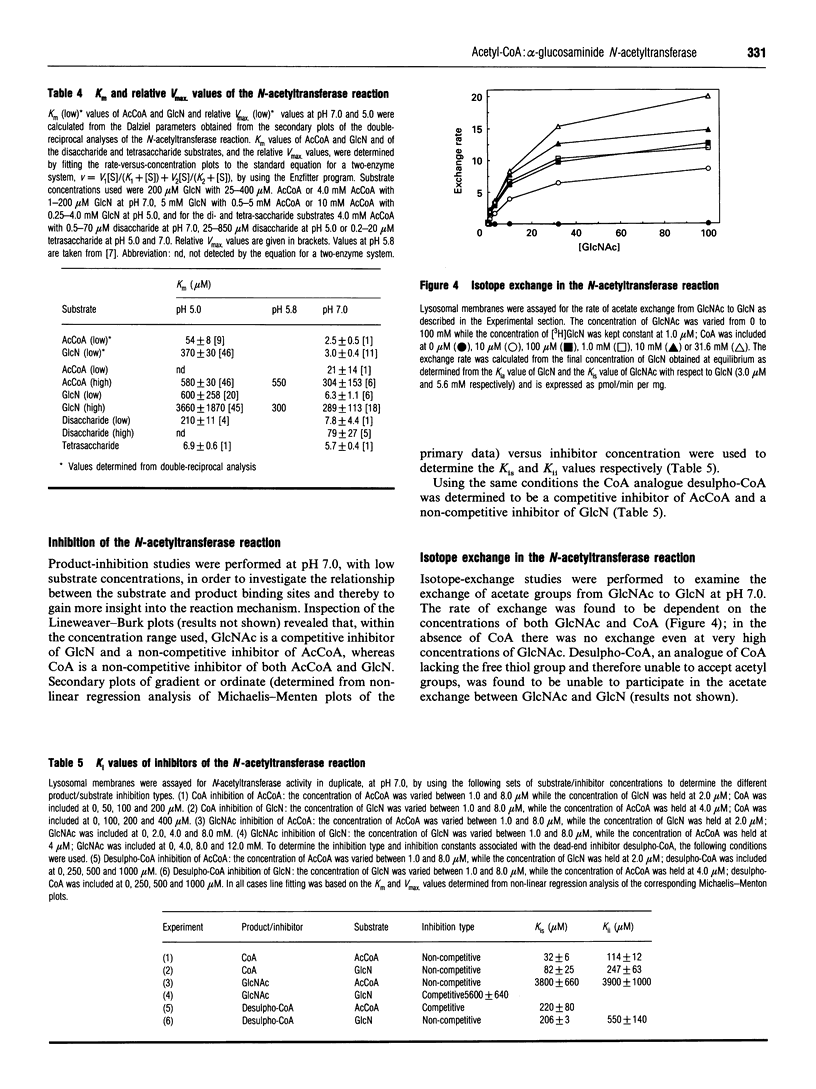
Abstract
Acetyl-CoA: alpha-glucosaminide N-acetyltransferase (N-acetyltransferase) is an integral lysosomal membrane protein which catalyses the transfer of acetyl groups from acetyl-CoA on to the terminal glucosamine in heparin and heparan sulphate chains within the lysosome. In vitro, the enzyme is capable of acetylating a number of mono- and oligo-saccharides derived from heparin, provided that a non-reducing terminal glucosamine is present. We have prepared highly enriched lysosomal membrane fractions from human placenta by a combination of differential centrifugation and density-gradient centrifugation in Percoll. This preparation was used to investigate the kinetics of the enzyme with three acetyl-acceptor substrates, i.e. glucosamine and a disaccharide and a tetrasaccharide derived from heparin, each containing a terminal glucosamine residue. The enzyme showed a pH optimum at 6.5, extending to 8.0 for the mono- and di-saccharide substrates but falling off sharply above pH 6.5 for the tetrasaccharide substrate. We identified two distinct Km values for the glucosamine substrate at both pH 7.0 and pH 5.0, whereas the tetrasaccharide substrate displayed only a single Km value at each pH. The Km values were found to be highly pH-dependent, and at pH 5.0 the values for the acetyl-acceptor substrates showed a decreasing trend as the size of the substrate increased, suggesting that the enzyme recognizes an extended region of the non-reducing terminus of the heparin or heparan sulphate polysaccharides. Double-reciprocal analysis, isotope exchange between N-acetylglucosamine and glucosamine, and inhibition studies with desulpho-CoA indicate that the enzyme operates by a random-order ternary-complex mechanism. Product inhibition studies display a complex pattern of dead-end inhibition. Taken in context with what is known about lysosomal utilization and physiological levels of acetyl-CoA, these results suggest that in vivo the enzyme operates via a random-order ternary-complex mechanism which involves the utilization of cytosolic acetyl-CoA to transfer acetyl groups on to the terminal glucosamine residues of heparin within the lysosome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bame K. J., Rome L. H. Acetyl coenzyme A: alpha-glucosaminide N-acetyltransferase. Evidence for a transmembrane acetylation mechanism. J Biol Chem. 1985 Sep 15;260(20):11293–11299. [PubMed] [Google Scholar]
- Brew K., Shaper J. H., Olsen K. W., Trayer I. P., Hill R. L. Cross-linking of the components of lactose synthetase with dimethylpimelimidate. J Biol Chem. 1975 Feb 25;250(4):1434–1444. [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Cummings J., Graham A. B., Wood G. C. Kinetic studies of latent microsomal UDP-glucuronyltransferases. Kinetics of glucuronidation in intact and perturbant-treated membranes. Biochim Biophys Acta. 1984 Apr 11;771(2):127–141. doi: 10.1016/0005-2736(84)90525-x. [DOI] [PubMed] [Google Scholar]
- Dobrota M., Hinton R. H. Large-scale preparation of highly purified lysosomes from normal rat liver. Anal Biochem. 1980 Feb;102(1):97–102. doi: 10.1016/0003-2697(80)90323-1. [DOI] [PubMed] [Google Scholar]
- Freeman C., Hopwood J. Lysosomal degradation of heparin and heparan sulphate. Adv Exp Med Biol. 1992;313:121–134. doi: 10.1007/978-1-4899-2444-5_13. [DOI] [PubMed] [Google Scholar]
- Hanson R. L., Rudolph F. B., Lardy H. A. Rabbit muscle phosphofructokinase. The kinetic mechanism of action and the equilibrium constant. J Biol Chem. 1973 Nov 25;248(22):7852–7859. [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Radiolabelled oligosaccharides as substrates for the estimation of sulfamidase and the detection of the Sanfilippo type A syndrome. Clin Chim Acta. 1981 Apr 27;112(1):55–66. doi: 10.1016/0009-8981(81)90268-0. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Morris C. P. The mucopolysaccharidoses. Diagnosis, molecular genetics and treatment. Mol Biol Med. 1990 Oct;7(5):381–404. [PubMed] [Google Scholar]
- Jonas A. J., Jobe H. Sulfate transport by rat liver lysosomes. J Biol Chem. 1990 Oct 15;265(29):17545–17549. [PubMed] [Google Scholar]
- Kamrath F. J., Dodt G., Debuch H., Uhlenbruck G. The isolation of lysosomes from normal rat liver by affinity chromatography. Hoppe Seylers Z Physiol Chem. 1984 May;365(5):539–547. doi: 10.1515/bchm2.1984.365.1.539. [DOI] [PubMed] [Google Scholar]
- Klein U., Kresse H., von Figura K. Sanfilippo syndrome type C: deficiency of acetyl-CoA:alpha-glucosaminide N-acetyltransferase in skin fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5185–5189. doi: 10.1073/pnas.75.10.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny E. H., Mumford R. A. Human leukocyte acid hydrolases: characterization of eleven lysosomal enzymes and study of reaction conditions for their automated analysis. Clin Chim Acta. 1976 Jul 15;70(2):247–257. doi: 10.1016/0009-8981(76)90426-5. [DOI] [PubMed] [Google Scholar]
- LEABACK D. H., WALKER P. G. Studies on glucosaminidase. 4. The fluorimetric assay of N-acetyl-beta-glucosaminidase. Biochem J. 1961 Jan;78:151–156. doi: 10.1042/bj0780151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G. M., de Jonge H. R., Galjaard H., Verheijen F. W. Characterization of a proton-driven carrier for sialic acid in the lysosomal membrane. Evidence for a group-specific transport system for acidic monosaccharides. J Biol Chem. 1989 Sep 15;264(26):15247–15254. [PubMed] [Google Scholar]
- Ohsumi Y., Ishikawa T., Kato K. A rapid and simplified method for the preparation of lysosomal membranes from rat liver. J Biochem. 1983 Feb;93(2):547–556. [PubMed] [Google Scholar]
- Prospero T. D., Burge M. L., Norris K. A., Hinton R. H., Reid E. Alkaline ribonuclease and phosphodiesterase activity in rat liver plasma membranes. Biochem J. 1973 Mar;132(3):449–458. doi: 10.1042/bj1320449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. H., Crain L. R. Degradation of mucopolysaccharide in intact isolated lysosomes. J Biol Chem. 1981 Nov 10;256(21):10763–10768. [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Hill D. F., Bame K. J., Crain L. R. Utilization of exogenously added acetyl coenzyme A by intact isolated lysosomes. J Biol Chem. 1983 Mar 10;258(5):3006–3011. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Symons L. J., Jonas A. J. Isolation of highly purified rat liver lysosomal membranes using two Percoll gradients. Anal Biochem. 1987 Aug 1;164(2):382–390. doi: 10.1016/0003-2697(87)90508-2. [DOI] [PubMed] [Google Scholar]
- Taylor J. A., Gibson G. J., Brooks D. A., Hopwood J. J. Human N-acetylgalactosamine-4-sulphatase biosynthesis and maturation in normal, Maroteaux-Lamy and multiple-sulphatase-deficient fibroblasts. Biochem J. 1990 Jun 1;268(2):379–386. doi: 10.1042/bj2680379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. A., Gibson G. J., Brooks D. A., Hopwood J. J. alpha-L-iduronidase in normal and mucopolysaccharidosis-type-I human skin fibroblasts. Biochem J. 1991 Feb 15;274(Pt 1):263–268. doi: 10.1042/bj2740263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Hayashi H., Natori Y. A simple procedure for the isolation of highly purified lysosomes from normal rat liver. J Biochem. 1984 Apr;95(4):1155–1160. doi: 10.1093/oxfordjournals.jbchem.a134704. [DOI] [PubMed] [Google Scholar]
- el-Aaser A. A., Reid E. Phosphatase activities in rat liver before and after birth. Histochem J. 1969 Aug;1(5):439–458. doi: 10.1007/BF01086984. [DOI] [PubMed] [Google Scholar]


