Abstract
Background
Research on calcium intake as well as variants in the calcium sensor receptor (CaSR) gene and their interaction in relation to CRC survival is still limited.
Methods
Data from 18,952 CRC patients, were included. Associations between primarily pre-diagnostic dietary (n = 13.085), supplemental (n = 11,837), total calcium intake (n = 5970) as well as 325 single nucleotide polymorphisms (SNPs) of the CaSR gene (n = 15,734) in relation to CRC-specific and all-cause mortality were assessed using Cox proportional hazard models. Also interactions between calcium intake and variants in the CaSR gene were assessed.
Results
During a median follow-up of 4.8 years (IQR 2.4–8.4), 6801 deaths occurred, of which 4194 related to CRC. For all-cause mortality, no associations were observed for the highest compared to the lowest sex- and study-specific quartile of dietary (HR 1.00, 95%CI 0.92–1.09), supplemental (HR 0.97, 95%CI 0.89–1.06) and total calcium intake (HR 0.99, 95%CI 0.88–1.11). No associations with CRC-specific mortality were observed either. Interactions were observed between supplemental calcium intake and several SNPs of the CaSR gene.
Conclusion
Calcium intake was not associated with all-cause or CRC-specific mortality in CRC patients. The association between supplemental calcium intake and all-cause and CRC-specific mortality may be modified by genetic variants in the CaSR gene.
Introduction
Epidemiologic studies provide considerable evidence for a protective association between calcium intake and the risk of colorectal cancer (CRC) [1–4]. In a dose-response meta-analyses including 15 studies and 12,305 CRC patients, each 300 mg/day increase in total calcium intake was associated with an approximately 8% reduced risk of CRC (RR 0.92 95%CI 0.89–0.95) [1]. A similar association was observed for dietary calcium intake (RR 0.90 95%CI 0.85–0.96) as well as supplementary calcium intake (RR 0.91 95%CI 0.86–0.98) [1].
In contrast to CRC risk [5], limited evidence is available for the association between calcium intake and survival in persons already diagnosed with CRC. The association between calcium intake and survival in CRC patients was examined in a total of 6 observational studies, involving between 148 and 3859 CRC survivors, with conflicting results [6–11]. No associations were observed for pre-diagnostic calcium intake in relation to all-cause and CRC-specific mortality in CRC patients [6–9]. An inverse association of post-diagnostic calcium intake with all-cause and CRC-specific mortality was observed in three cohort studies [6, 8, 10], but this was only statistically significant in one study for all-cause mortality [6] and in another study for CRC-specific mortality [8]. Thus, the relationship between calcium intake and mortality in CRC patients remains inconclusive.
Moreover, the underlying mechanisms by which calcium exerts its potential effect on CRC outcomes are still unknown. The inverse association between calcium intake and CRC risk is suggested to be mediated by the calcium-sensing receptor (CaSR) [12, 13], which is primarily activated by extracellular calcium. The CaSR plays a critical role in sensing of extracellular calcium to maintain serum calcium concentrations in a narrow physiological range. In the intestine, the CaSR is responsible for calcium absorption from the diet. Besides its primary function in the control of calcium homeostasis, the CaSR also has tumor suppressor functions as it can regulate inflammation, cell proliferation, cell differentiation and apoptosis [14, 15]. A lower expression of CaSR is associated with more aggressive tumors [15]. In addition, a higher expression of the CaSR in CRC tumor tissue was associated with a decreased CRC-specific mortality, but not all-cause mortality [14]. In addition, some indications for a gene-environment interaction between calcium intake and genetic variance of the CaSR gene were observed in CRC patients [16], where a specific haplotype of the CaSR gene seems to be associated with a decreased overall survival only in patients with a dietary calcium intake below the median. However, research on genetic variants of the CaSR gene and on the interaction between calcium intake and genetic variants in relation to CRC mortality is still limited.
Therefore, in this analysis, we examined the hypothesis that dietary and supplemental calcium intake is associated with all-cause and CRC-specific mortality with possible effect modification by genetic variants in the CaSR gene in a large population of 18,952 CRC patients.
Methods
Study design and participants
The study population for analyses consists of participants of studies included in the International Survival Analyses in Colorectal Cancer Consortium (ISACC), which is part of the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO). Data from 9 observational cohort studies, 3 clinical trials with a long-term follow-up and 2 case-control studies was used. Characteristics of the studies are listed in Table S1 and described in detail elsewhere [17–38].
In brief, for this study the participants from the mentioned studies who developed CRC during follow-up or were cases in the two case-control studies were selected. Only those with available dietary or supplemental calcium intake data were included for data-analyses. All participants gave written informed consent and studies were approved by the Institutional Review Boards.
Study population
In Table S1, the characteristics of the 14 included studies are summarized. Studies are conducted in the USA, Europe and Australia. The number of CRC patients included per study varied widely between 280 and 3654. Thirteen out of fourteen studies had data about dietary calcium intake available. Nine studies had data about supplemental intake available and eight studies had data about both dietary and supplemental intake available. All studies had data about all-cause mortality available, while thirteen studied had data about CRC-specific mortality available.
Epidemiological data collection
Data about demographics, lifestyle and clinical factors was collected by self-report using structured questionnaires or in-person interviews. Information about how data was collected in each study can be found in Table S1.
Data from each included study was harmonized for ISACC. The methods of data-harmonization are described in detail elsewhere [20]. Data about study characteristics including country in which the study was conducted, study acronym and methods of exposure and outcome assessments was harmonized. In addition, information about the study population was harmonized: race, education level, sex, age at diagnosis, CRC stage, body mass index (BMI), physical activity level, dietary intake, calcium supplement use, follow-up time and clinical outcomes. Data on clinical outcomes were collected via regular follow up with confirmation using medical chart review, and or linkage with death and cancer registries (Table S1).
Calcium intake assessment
Dietary intake, including dietary calcium intake, was measured using a food frequency questionnaire or diet history questionnaire in all studies. Dietary intake was measured before diagnosis (n = 14,792; median 3 years IQR 1–7), around diagnosis i.e., in the same year as the cancer diagnosis (n = 4153) or after diagnosis (n = 6). Length of the dietary questionnaires ranged from very short (19 items) to extended (178 items) (Table S1). Sex- and study- specific quartiles of calcium intake were used for analysis, because absolute values between studies may differ due to differences in the dietary assessment methods. Calcium from supplements (including single, multivitamins, and antacids) was measured in tablets per day. When actual quantities were unavailable, it was assumed that regular use of supplements was 500 mg/day or 500 mg/tablet for single calcium and antacids, and 130 mg/day or 130 mg/tablet for multivitamins. For the analyses, supplemental intake was defined as <1 pill (<500 mg) and ≥1 pill (≥500 mg). Total calcium intake was calculated for persons with both dietary and supplemental calcium intake data available and defined as sex- and cohort specific quartiles.
Genotyping and SNP selection
From the included populations, blood samples have been sent for genotyping. In total, 15,734 blood samples could be successfully genotyped. Details on genotyping and quality control have been previously published [39] and genotyping platforms used are summarized in Table S1. DNA samples were validated with quality controls, and genotypic data that passed initial control were analysed by the analysis team of University of Washington Genetic Analysis Center. A call rate of >95% was applied and individuals from whom more than 95% of the typed SNPs was missing, were excluded. All SNPs of all studies were imputed to the Haplotype Reference Consortium r1.1 (2016) reference panel via the Michigan Imputation Server [40]. A candidate gene approach was used to investigate the interaction between calcium intake and genetic variance in the Calcium-Sensing Receptor (CaSR). The molecular location of the CaSR gene is base pairs 121,902,530–122,005,342 on chromosome 3 (GRCh37). In total, 1412 SNPs located in the CaSR region were selected for further analysis. After exclusion of 1087 SNPs as a result of MAF < 0.05, 325 SNPs were retained in the analysis (Table S3). All SNPs had an imputation accuracy of R2 > 0.85. For the genetic data analyses part, all participants were of Caucasian ancestry.
Data analyses
Patient characteristics were described as medians with interquartile range (IQR) for the total population and by high versus low calcium intake (quartile 1 and 2 versus 3 and 4 of sex- and study-specific quartiles of intake). In addition, patients’ characteristics were described for each individual study.
The association between calcium intake (sex- and study- specific quartiles) and all-cause as well as CRC-specific mortality was assessed using two methods. First, a one stage model was applied, where individual data of participants of all studies were harmonized. The association was investigated using a Cox proportional hazards regression model.
Age, sex and cohort were included in the models a priori. Additionally, other potential confounders (education, family history of CRC, BMI, intake of total energy intake, folate, red meat, processed meat, fiber, vegetable, fruit and alcohol, physical activity, smoking status, regular aspirin/NSAID use, diabetes, and cancer site) were tested and included in the model when the HR changed by more than 10%. None of the mentioned potential confounders did change the HR with >10% and therefore only age, sex and cohort were included in the final models. To future explore timing of calcium intake, time between assessment of calcium intake and diagnosis in years was added to the model.
Subgroup analyses were done for sex (male, female), tumor location (proximal, distal, rectum), stage of disease (local, regional, distant), age at diagnosis (Early onset ≤50 years, late onset >50 years), family history of CRC (no, yes), timing of calcium intake (before diagnosis, around diagnosis) and study design (cohort, trial with follow-up, case-control), since associations between calcium intake and mortality could potentially be different for before mentioned subgroups [2, 3, 6, 8]. In a sensitivity analysis, data from two studies (DACHs and PHS) with very low calcium intake (median <440 mg/day), which was probably due to the restricted dietary assessment method, was excluded.
As a secondary analysis, a meta-analysis was conducted, where associations between calcium intake and mortality were first assessed for each study separately using Cox proportional hazards analyses. Models were adjusted for age and sex. Subsequently, obtained statistics were used to calculate a weighted average over all included studies. The DerSimonian and Laird (DL) random-effects model was used to account for heterogeneity of study populations and designs [41, 42]. The heterogeneity among the included studies was investigated using the I2 index and Cochran’s Q test, with significant heterogeneity assumed for I2 > 50% or a Q-test p < 0.05. Forest plots were made to visualize the data.
The associations between SNPs in the CaSR gene and mortality were assessed, assuming an additive model in which SNPs were encoded as 0,1,2, by using Cox proportional hazards regression analyses. In addition, SNPs were entered categorical as three groups (i.e., AA, Aa, aa). Models were adjusted for age, sex, study center and the first 3 principal components of genetic ancestry. These associations were investigated using a harmonized dataset of individual participant data.
Interaction between calcium and genetic variants in the CaSR gene was also investigated. For the interaction analyses only the additive model in which SNPs were encoded as 0,1,2 was used. We tested multiplicative interaction using SNP x calcium product terms, adjusting for age, sex, study center, first 3 principal components of genetic ancestry, and SNP and calcium main effects. Additive interaction was assessed by calculating the relative excess risk due to interaction (RERI) based on the estimates extracted from the multiplicative model (e^((βCalcium + βSNP+βCalcium*SNP))-e^(βCalcium)-e^(βSNP) + 1). The delta method was used to estimate the variance and 95% confidence intervals (CI) of RERI [43]. A RERI of zero means no additive interaction, a RERI < 0 a negative additive interaction and a RERI > 0 a positive additive interaction. Sex- and study- specific quartiles as well as SNPs were entered as continuous variables for both the multiplicative as well as the additive interaction models.
To provide more insights into the nature of multiplicative and additive interactions between calcium intake and genetic variants in the CaSR gene, the 2 SNPs for which a multiplicative as well as an additive interaction for all-cause or CRC-specific mortality was observed were further investigated by A: joint effects of genotype (3 categories) and calcium intake (2 categories, median-split), where the reference group was a low calcium intake and the presence of the homozygous reference allele; and B: stratified analyses, examining the association of the SNP in relation to mortality in strata of calcium intake and the association of calcium intake in relation to mortality in strata of SNP genotypes.
All analyses were performed using R statistical software, version 4.0.3. The simple M method was used to calculate the number of independent tests for 325 SNPs [44]. To provide the number of effective test, additive SNP coding was used (0,1,2). The number of independent tests was 35, meaning that a p-value of 0.05/35 < 0.001 was considered statistically significant. Correlations between SNPs, SNPs in LD, were assessed using plink. SNPs with r2 > 0.6 were considered dependent and were called clusters.
Results
Characteristics of the study population
The median age at diagnosis was 67 (IQR 60–73) years, half of the population was male and the median BMI was 27 (IQR 24–30) kg/m2. Proximal colon tumors were most prevalent (38%), followed by distal colon (30%) and rectal cancers (22%) and unknown location (11%). The median dietary calcium intake in the total population was 694 g/day (IQR 467–995), 676 g/day (IQR 444; 993) for men and 706 (IQR 483; 997) for women. Almost 11% of the population used calcium supplements (>500 mg/day). Almost 40% of the population died, of which 4,914 (22%) were related to CRC, during follow-up time (median 4.8 years, IQR 2.4–8.4).
When comparing characteristics of participants with a low dietary calcium intake (quartile 1 and 2 of sex- and study-specific quartiles) with characteristics of participants with a high dietary calcium intake (quartile 3 and 4 of sex- and study-specific quartiles) the most striking differences observed were differences in the dietary intake. In general intake of energy, fiber, folate, fruit and vegetables was markedly lower in the low dietary calcium intake group compared to the high dietary calcium group. Detailed information can be found in Table 1. In addition, patients’ characteristics for each individual study can be found in Supplementary Table S2 and distribution of total and dietary calcium intake per study can be found in Supplementary Fig. S1.
Table 1.
Characteristics of Colorectal cancer survivors for the total population and stratified by calcium intake.
| Total population | Low dietary calcium intakea | High dietary calcium intakea | |
|---|---|---|---|
| N = 18952 | N = 6755 | N = 6330 | |
| Age at diagnosis (years) | 67.0 [60.0, 73.0] | 69.0 [63.0, 75.0] | 69.0 [62.0, 75.0] |
| Sex (male) | 9516 (50) | 3334 (49) | 2905 (46) |
| BMI (kg/m2) | 26.7 [24.1, 29.8] | 26.6 [24.0, 29.4] | 26.4 [24.0, 29.5] |
| Unknown | 248 | 114 | 87 |
| Self-reported race | |||
| Caucasian | 17885 (94) | 6553 (97) | 6186 (98) |
| Other | 161 (1) | 50 (1) | 30 (0) |
| Unknown | 906 (5) | 152 (2) | 114 (2) |
| Smoking status | |||
| Current smoker | 2351 (12) | 859 (13) | 693 (11) |
| Former smoker | 7913 (42) | 2917 (43) | 2757 (44) |
| Never smoker | 8064 (43) | 2843 (42) | 2764 (44) |
| Unknown | 624 (3) | 136 (2) | 116 (2) |
| Education | |||
| Very low | 3458 (18) | 1486 (22) | 1076 (17) |
| Low | 4289 (23) | 1872 (28) | 1548 (25) |
| Medium | 4715 (25) | 1494 (22) | 1554 (25) |
| High | 5608 (30) | 1835 (27) | 2080 (33) |
| Unknown | 882 (5) | 68 (1) | 72 (1) |
| Dietary calcium intake (mg/day) | 694 [467, 995] | 488 [334, 653] | 1001 [752, 1267] |
| Unknown | 5867 | 0 | 0 |
| Calcium supplement use | |||
| <1 pill (<500 mg/day) | 9755 (52) | 2133 (32) | 2168 (34) |
| ≥1 pill (≥500 mg/day) | 2082 (11) | 758 (11) | 911 (14) |
| Unknown | 7115 (38) | 3864 (57) | 3251 (51) |
| Total calcium intakec (mg/day) | 991 [658, 1457] | 658 [484, 990] | 1294 [978, 1719] |
| Unknown | 12982 | 3864 | 3251 |
| Energy intake (kcal/day) | 1843 [1430, 2347] | 1590 [1 (232, 2003] | 2123 [1701, 2702] |
| Unknown | 9109 | 1822 | 1420 |
| Total folate intake (µg/day) | 485 [236, 895] | 353 [245, 848] | 554 [361, 1095] |
| Unknown | 2878 | 1627 | 1251 |
| Fiber intake (g/day) | 20 [14, 26] | 17 [13, 22] | 22 [17, 30] |
| Unknown | 9057 | 1790 | 1400 |
| Red meat intake (portion/day) | 0.6 [0.3, 0.9] | 0.7 [0.4, 1.0] | 0.7 [0.4, 1.1] |
| Unknown | 415 | 93 | 73 |
| Processed meat intake (portion/day) | 0.2 [0.1, 0.6] | 0.3 [0.1, 0.6] | 0.3 [0.1, 0.6] |
| Unknown | 3037 | 96 | 68 |
| Vegetable intake (portion/day) | 1.5 [1.0, 3.0] | 1.7 [1.1, 3.0] | 2.2 [1.1, 3.9] |
| Unknown | 411 | 109 | 87 |
| Fruit intake (portion/day) | 1.4 [0.9, 2.5] | 1.0 [0.6, 2.0] | 1.8 [1.0, 2.8] |
| Unknown | 477 | 122 | 98 |
| Alcohol (g/day) | 3.8 [0.0, 16.0] | 3.9 [0.0, 16.5] | 2.9 [0.0, 13.5] |
| Unknown | 338 | 46 | 45 |
| Family history | |||
| No | 11297 (60) | 4410 (65) | 4161 (66) |
| Yes | 2624 (14) | 844 (13) | 855 (14) |
| Unknown | 5031 (27) | 1501 (22) | 1314 (21) |
| Stage of disease | |||
| Stage 1 or local | 3654 (19) | 1580 (23) | 1586 (25) |
| Stage 2/3 or regional | 7325 (39) | 3170 (47) | 2959 (47) |
| Stage 4 or distant | 1625 (9) | 748 (11) | 653 (10) |
| Unknown | 6348 (34) | 1257 (19) | 1132 (18) |
| Tumor location | |||
| Distal colon | 5649 (30) | 2051 (30) | 1881 (30) |
| Proximal colon | 7116 (38) | 2632 (39) | 2700 (43) |
| Rectum | 4192 (22) | 1441 (21) | 1177 (19) |
| Unknown | 1995 (11) | 631 (9) | 572 (9) |
| Aspirine use | |||
| No | 6655 (35) | 2650 (39) | 2192 (35) |
| Yes | 3551 (19) | 1375 (20) | 1253 (20) |
| Unknown | 8746 (46) | 2730 (40) | 2885 (46) |
| NSAID use | |||
| No | 7957 (42) | 3099 (46) | 2620 (41) |
| Yes | 1274 (7) | 389 (6) | 377 (6) |
| Unknown | 9721 (51) | 3267 (48) | 3333 (53) |
| Diabetes | |||
| No | 15699 (83) | 5442 (81) | 5071 (80) |
| Yes | 1707 (9) | 581 (9) | 563 (9) |
| Unknown | 1546 (8) | 732 (11) | 696 (11) |
| CRC-specific deaths | |||
| Yes | 4194 (22) | 1490 (22) | 1398 (22) |
| Unknown | 372 (2) | 112 (2) | 95 (2) |
| Deaths | 6801 (36) | 2404 (36) | 2249 (36) |
| Cohort (Acronym) | |||
| CCFR | 3564 (19) | 402 (6) | 431 (7) |
| CPSII | 1453 (8) | 781 (12) | 672 (11) |
| DACHS | 2878 (15) | 1627 (24) | 1251 (20) |
| DALS | 1115 (6) | 568 (8) | 547 (9) |
| EPIC | 2025 (11) | 1108 (16) | 917 (15) |
| HPFS | 358 (2) | 197 (3) | 161 (3) |
| MCCS | 784 (4) | 397 (6) | 387 (6) |
| NHS | 594 (3) | 328 (5) | 266 (4) |
| NSHDS | 305 (2) | 104 (2) | 92 (2) |
| PHS | 312 (2) | 163 (2) | 149 (2) |
| PLCO | 913 (5) | 449 (7) | 464 (7) |
| UKB | 2994 (16) | 0 (0) | 0 (0) |
| VITAL | 280 (2) | 134 (2) | 116 (2) |
| WHI | 1377 (7) | 497 (7) | 877 (14) |
Values presented are median [quartile 1 – quartile 3] or number (percentage).
aLow dietary calcium intake was defined as quartile 1 and 2 of sex- and cohort- specific quartiles and a high dietary calcium intake was defined as quartile 3 and 4 of sex- and cohort-specific quartiles.
bVery low: less than high school graduate; low: high school graduate or completed GED; medium: some college or technical school; high: college graduate or graduate degree.
ctotal calcium intake is only calculated when data of both dietary as well as supplemental calcium intake was available.
Associations between dietary, supplemental and total calcium intake in relation to CRC-specific and all-cause mortality
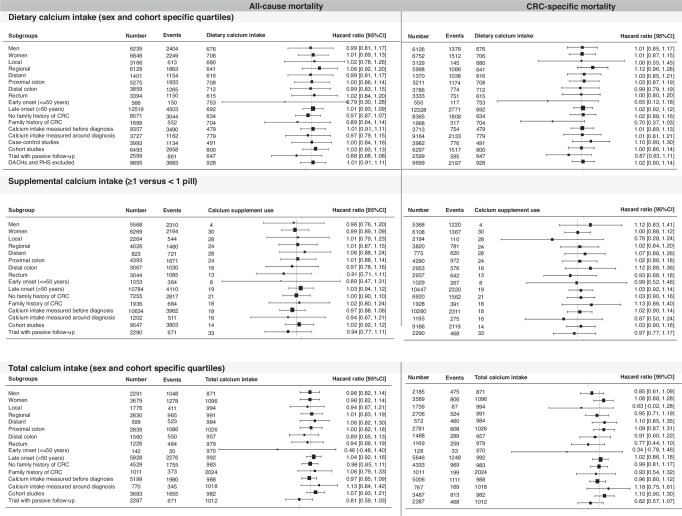
For all-cause mortality, no associations were observed for the highest compared to the lowest sex- and study-specific quartile of dietary (HR 1.00, 95%CI 0.92–1.09), supplemental (HR 0.97, 95%CI 0.89–1.06) and total calcium intake (HR 0.99, 95%CI 0.88–1.11) (Table 2); similar patterns were noted for CRC-specific mortality. In addition, no associations were observed in the subgroup analyses, based on sex, tumor location, stage of disease, age at diagnosis, timing of calcium intake or study design (Fig. 1). Dietary calcium intake seems to be associated with CRC-specific mortality in persons with a family history of CRC (HR 0.70 95%CI 0.37–1.03). Timing of assessment of calcium intake did not influence the association between calcium intake and mortality (HR Q4 versus Q1 of dietary calcium intake 1.00 95%CI 0.91–1.10 for all-cause mortality and HR 1.01 95%CI 0.89–1.14 for CRC-specific mortality). For dietary and total calcium intake no heterogeneity between studies was observed (I2 0–7%). For supplemental calcium intake in relation to all-cause mortality, moderate heterogeneity between included studies was observed (I2 30%) (Supplementary data Figure S2).
Table 2.
Associations between dietary calcium intake, supplemental calcium intake and total calcium intake in relation to CRC-specific and all-cause mortality in CRC survivors.
| Dietary calcium intake | |||||
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Sex- and cohort- specific quartiles | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Number/events | 3368/1216 | 3387/1188 | 3157/1124 | 3173/1125 | |
| HR (95%CI) | 1.0 (ref) | 0.97 (0.89–1.05) | 0.99 (0.91–1.07) | 1.00 (0.92–1.09) | 0.862 |
| CRC-specific mortality | |||||
| Number/events | 3298/745 | 3345/745 | 3106/689 | 3129/709 | 0.824 |
| HR (95%CI) | 1.00 (Ref) | 0.98 (0.88–1.08) | 1.00 (0.90–1.10) | 1.01 (0.91–1.12) | |
| Supplemental calcium intake | |||||
| All-cause mortality | |||||
| Supplement use | <1 pill | ≥1 pill | |||
| Number/events | 9755/3708 | 2082/766 | |||
| HR (95%CI) | 1.00 (Ref) | 0.97 (0.89–1.06) | |||
| CRC-specific mortality | |||||
| Supplement use | <1 pill | ≥1 pill | |||
| Number/events | 9399/2111 | 2077/476 | |||
| HR (95%CI) | 1.00 (Ref) | 1.01 (0.90–1.13) | |||
| Total calcium intake (dietary and supplemental intake) | |||||
| All-cause mortality | |||||
| Sex- and cohort- specific quartiles | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Number/events | 1501/602 | 1487/573 | 1436/535 | 1546/616 | |
| HR (95%CI) | 1 (Ref) | 0.98 (0.88–1.10) | 0.95 (0.85–1.07) | 0.99 (0.88–1.11) | 0.735 |
| CRC-specific mortality | |||||
| Number/events | 1450/338 | 1440/313 | 1388/287 | 1496/434 | |
| HR (95%CI) | 1.0 (ref) | 0.93 (0.80–1.08) | 0.88 (0.75–1.03) | 0.99 (0.85–1.15) | 0.707 |
Fig. 1. Associations between calcium intake and mortality in CRC survivors stratified by sex, stage of disease, tumor location, age at diagnosis, family history, timing of calcium intake and study design.
The left sight of the figure shows the associations with all-cause mortality as the outcome, the right side of the figure for CRC-specific mortality. Analyses were done for dietary calcium (top 2 panels), supplemental calcium (middle 2 panels) and total calcium intake (bottom 2 panels).
Associations between genetic variants in the Calcium Sensing Receptor gene in relation to CRC-specific and all-cause mortality
Two related SNPs in the CaSR gene (rs62269066 and rs17282015) were statistically significantly associated with all-cause mortality after correction for multiple testing (Table S4). For both SNPs, a homozygous genotype for the alternative allele was associated with a lower risk of all-cause mortality (HR 0.86 95%CI 0.79–0.94). No statistically significant associations between SNPs and CRC-specific mortality were observed (Table S5). The 10 SNPs most significantly associated with CRC outcomes are depicted in Tables S4 and S5.
Interaction between calcium intake and CaSR genetic variants in relation to CRC-specific and all-cause mortality
No statistically significant interactions were observed for dietary or total calcium intake and genetic variants in the CaSR gene in relation to all-cause or CRC-specific mortality (Table 3). On the contrary, multiplicative interaction between supplemental calcium intake and genetic variants in the CaSR gene was observed in relation to both all-cause mortality (independent SNP rs11713280 and correlated SNPs: rs62269066, rs11708053, rs11711698 and rs17282015) as well as CRC-specific mortality (rs11713280) (Table 3). Also, additive interaction between supplemental calcium intake and genetic variants in the CaSR gene was observed in relation to all-cause mortality (independent SNPs rs11713280 and rs62269066 and correlated SNPs: rs6780443, rs1782008 and rs7637874).
Table 3.
Top 5 SNPS for which interaction between calcium intake and that specific genetic variant in relation to CRC outcomes was observed.
| MULTIPLICATIVE INTERACTIONS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interaction GxE (calcium and CaSR) | |||||||||||
| All-cause mortality | CRC-specific mortality | ||||||||||
| Dietary calcium sex- and cohort- specific quartiles | Dietary calcium sex- and cohort- specific quartiles | ||||||||||
| SNP | N/events | RERIa | 95%CI | P-valueb | MAF | SNP | N/events | RERIa | 95%CI | P-valueb | MAF |
| 3:121910706_A/G rs1553309 | 11173/3841 | 1.04 | 1.00–1.08 | 0.075 | 0.42 | 3:121903842_C/A rs6780443d | 11173/2499 | 1.08 | 1.02–1.14 | 0.012 | 0.24 |
| 3:121914667_T/C rs6764205c | 11173/3841 | 1.04 | 1.00–1.09 | 0.049 | 0.30 | 3:121909830_C/G rs17282008d | 11173/2499 | 1.07 | 1.01–1.14 | 0.018 | 0.24 |
| 3:121921700_T/C rs46780431219c | 11173/3841 | 1.05 | 1.00–1.09 | 0.048 | 0.30 | 3:121932422_C/T rs62269066e | 11173/2499 | 1.06 | 1.00–1.11 | 0.038 | 0.36 |
| 3:121922863_A/G rs4678045c | 11173/3841 | 1.05 | 1.00–1.09 | 0.048 | 0.30 | 3:121936453_G/A rs11713280 | 11173/2499 | 1.12 | 1.01–1.23 | 0.026 | 0.07 |
| 3:121936453_G/A rs11713280 | 11173/3841 | 1.09 | 1.01–1.18 | 0.023 | 0.07 | 3:121966952_C/T rs7637874d | 11173/2499 | 1.07 | 1.01–1.13 | 0.031 | 0.24 |
| Supplement ≥ 1 versus < 1 pill | Supplement ≥ 1 versus < 1 pill | ||||||||||
| 3:121913708_C/T rs17282015e | 9207/3210 | 1.24 | 1.09–1.42 | 0.0009 | 0.36 | 3:121913708_C/T rs17282015e | 9207/1999 | 1.25 | 1.07–1.47 | 0.006 | 0.36 |
| 3:121917298_T/C rs11708053e | 9207/3210 | 1.25 | 1.10–1.42 | 0.0007 | 0.36 | 3:121917298_T/C rs11708053e | 9207/1999 | 1.26 | 1.07–1.47 | 0.005 | 0.36 |
| 3:121917387_A/G rs11711698e | 9207/3210 | 1.25 | 1.10–1.42 | 0.0007 | 0.36 | 3:121917387_A/G rs11711698e | 9207/1999 | 1.25 | 1.07–1.47 | 0.005 | 0.36 |
| 3:121932422_C/T rs62269066e | 9207/3210 | 1.25 | 1.10–1.43 | 0.0006 | 0.36 | 3:121932422_C/T rs62269066e | 9207/1999 | 1.24 | 1.06–1.46 | 0.008 | 0.36 |
| 3:121936453_G/A rs11713280 | 9207/3210 | 1.49 | 1.19–1.87 | 0.0006 | 0.07 | 3:121936453_G/A rs11713280 | 9207/1999 | 1.6 | 1.21–2.11 | 0.0009 | 0.07 |
| Total calcium intake sex- and cohort- specific quartiles | Total calcium intake sex- and cohort- specific quartiles | ||||||||||
| 3:121992825_C/T rs3804594f | 4646/1695 | 0.87 | 0.78–0.97 | 0.011 | 0.08 | 3:121994941_G/A rs2279802f | 4646/1005 | 0.81 | 0.70–0.94 | 0.005 | 0.08 |
| 3:121995415_C/T rs13093602f | 4646/1695 | 0.87 | 0.78–0.97 | 0.013 | 0.08 | 3:121995415_C/T rs13093602f | 4646/1005 | 0.81 | 0.70–0.94 | 0.005 | 0.08 |
| 3:121996810_T/A rs1827381f | 4646/1695 | 0.87 | 0.78–0.97 | 0.013 | 0.08 | 3:121996810_T/A rs1827381f | 4646/1005 | 0.81 | 0.70–0.94 | 0.005 | 0.08 |
| 3:121997268_T/C rs3792291f | 4646/1695 | 0.87 | 0.78–0.97 | 0.013 | 0.08 | 3:121997268_T/C rs3792291f | 4646/1005 | 0.81 | 0.70–0.94 | 0.005 | 0.08 |
| 3:121999059_G/A rs2055427f | 4646/1695 | 0.87 | 0.78–0.97 | 0.012 | 0.08 | 3:121999059_G/A rs2055427f | 4646/1005 | 0.81 | 0.70–0.94 | 0.005 | 0.08 |
| ADDITIVE INTERACTIONS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary calcium sex- and cohort- specific quartiles | Dietary calcium sex- and cohort- specific quartiles | ||||||||||
| 3:121921700_T/C rs4678043c | 11173/3841 | 0.04 | −0.00–0.08 | 0.068 | 0.30 | 3:121903842_C/A rs6780443d | 11173/2499 | 0.06 | 0.02–0.11 | 0.013 | 0.24 |
| 3:121922863_A/G rs4678045c | 11173/3841 | 0.04 | −0.00–0.09 | 0.070 | 0.30 | 3:121909830_C/G rs17282008d | 11173/2499 | 0.06 | 0.01–0.11 | 0.018 | 0.24 |
| 3:121936453_G/A rs11713280 | 11173/3841 | 0.07 | 0.02–0.13 | 0.015 | 0.07 | 3:121936453_G/A rs11713280 | 11173/2499 | 0.09 | 0.03–0.15 | 0.012 | 0.07 |
| 3:121996235_G/A rs77663377 | 11173/3841 | 0.07 | −0.01–0.14 | 0.080 | 0.15 | 3:121966952_C/T rs7637874d | 11173/2499 | 0.06 | 0.01–0.10 | 0.037 | 0.24 |
| 3:122005273_A/G rs34042920 | 11173/3841 | 0.04 | −0.01–0.10 | 0.107 | 0.10 | 3:121996235_G/A rs77663377 | 11173/2499 | 0.09 | 0.003–0.17 | 0.051 | 0.15 |
| Supplement ≥ 1 versus < 1 pill | Supplement ≥ 1 versus < 1 pill | ||||||||||
| 3:121903842_C/A rs6780443d | 9207/3210 | 0.22 | 0.09–0.36 | 0.0009 | 0.24 | 3:121903842_C/A rs6780443d | 9207/1999 | 0.23 | 0.05–0.41 | 0.010 | 0.24 |
| 3:121909830_C/G rs17282008d | 9207/3210 | 0.22 | 0.09–0.36 | 0.0009 | 0.24 | 3:121909830_C/G rs17282008d | 9207/1999 | 0.23 | 0.05–0.41 | 0.009 | 0.24 |
| 3:121932422_C/T rs62269066e | 9207/3210 | 0.21 | 0.10–0.32 | 0.00006 | 0.36 | 3:121917298_T/C rs11708053e | 9207/1999 | 0.22 | 0.07–0.36 | 0.003 | 0.36 |
| 3:121936453_G/A rs11713280 | 9207/3210 | 0.40 | 0.15–0.64 | 0.0009 | 0.07 | 3:121936453_G/A rs11713280 | 9207/1999 | 0.51 | 0.18–0.84 | 0.002 | 0.07 |
| 3:121966952_C/T rs7637874d | 9207/3210 | 0.22 | 0.08–0.36 | 0.0009 | 0.24 | 3:121966952_C/T rs76377874d | 9207/1999 | 0.22 | 0.04–0.09 | 0.015 | 0.24 |
| Total calcium intake sex- and cohort- specific quartiles | Total calcium intake sex- and cohort- specific quartiles | ||||||||||
| 3:121984792_G/A rs17197671g | 4646/1695 | 0.07 | 0.004–0.13 | 0.015 | 0.14 | 3:121930454_C/G rs75473459h | 4646/1005 | 0.10 | 0.02–0.18 | 0.021 | 0.11 |
| 3:121988851_G/C rs6783855g | 4646/1695 | 0.07 | 0.004–0.13 | 0.015 | 0.14 | 3:121917298_T/C rs11708053e | 4646/1005 | 0.08 | 0.01–0.15 | 0.030 | 0.36 |
| 3:121990800_T/C rs1604446g | 4646/1695 | 0.07 | 0.004–0.13 | 0.015 | 0.14 | 3:121917387_A/G rs11711698e | 4646/1005 | 0.08 | 0.01–0.15 | 0.032 | 0.36 |
| 3:122002178_T/C rs2134224g | 4646/1695 | 0.07 | 0.003–0.13 | 0.014 | 0.14 | 3:121939743_C/T rs36004543h | 4646/1005 | 0.11 | 0.03–0.19 | 0.004 | 0.12 |
| 3:122005273_A/G rs34042920g | 4646/1695 | 0.09 | 0.03–0.16 | 0.005 | 0.10 | 3:121958475_G/A rs62269089h | 4646/1005 | 0.10 | 0.01–0.18 | 0.020 | 0.11 |
aModels were adjusted for age at diagnosis, sex, cohort, and the first three principal components of genetic ancestry as well as main effects of calcium intake (dietary, supplemental or total) and SNPs. Sex- and cohort- specific quartiles as well as SNPs were entered as continuous variables for both the multiplicative as the additive interaction models.
bThe estimated effective number of independent tests among 325 SNPS was 35 based on the simple M approach. Therefore, p < 0.001 were considered statistically significant (n = 35; 0.05/35 = 0.001).
c,d,e,f,g,hCorrelated SNPS (r2 > 0.6).
We further explored the two independent SNPs (rs11713280 and rs62269066) for which both additive and multiplicative interaction with supplemental calcium intake were observed in relation to all-cause mortality (p multiplicative interaction = 0.0006 and 0.0006; p additive interaction = 0.0009 and 0.0006 and CRC-specific mortality (p multiplicative interaction = 0.0009 and 0.008; p additive interaction = 0.002 and 0.004, respectively (Table 4). For rs11713280, the association between supplemental calcium intake and all-cause mortality differed between persons who were homozygous for the reference allele (GG) (HR 0.93 95%CI 0.84–1.04) compared to persons who were heterozygous (GA) (HR 1.35 95%CI 1.07–1.70) or for the homozygous alternative allele (AA) (HR 3.83 95%CI 1.23–11.96). A similar trend was observed for CRC-specific mortality (GG: HR 0.97 95%CI 0.85–1.11; GA 1.50 95%CI 1.13–1.99; AA: HR 3.92 95%CI 1.05–14.72). Also, different associations for supplemental calcium intake in relation to mortality were observed depending on polymorphism of rs62269066. Persons who were homozygous for the reference allele (CC) had a lower risk of all-cause mortality when taking supplements (HR 0.80 95%CI 0.69-0.93), while persons with a CT or TT genotype had a non-significant higher risk of all-cause mortality (HR 1.12 95%CI 0.98–1.28 and HR 1.19 95%CI 0.92–1.53, respectively). A similar trend was observed for CRC-specific mortality (CC: HR 0.85 95%CI 0.71–1.03; CT 1.20 95%CI 1.02–1.43; TT: HR 1.23 95%CI 0.90–1.67).
Table 4.
Elaborate example Multiplicative and Additive interactions of two SNPs in the CaSR gene and CRC outcomes
| ALL-CAUSE MORTALITY | COLORECTAL CANCER SPECIFIC MORTALITY | ||||||
|---|---|---|---|---|---|---|---|
| SUPPLEMENTAL CALCIUM INTAKE*3:121936453_G/A (rs11713280) | SUPPLEMENTAL CALCIUM INTAKE**3:121936453_G/A (rs11713280) | ||||||
| <1 pill | ≥1 pill | Effect of supplement intake within the strata of ‘3:121936453_G/A‘ | <1 pill | ≥1 pill | Effect of supplement intake within the strata of ‘3:121936453_G/A‘ | ||
| 3:121936453_G/A |
Number/events HR [95% CI] |
Number/events HR [95% CI] |
Number/events HR [95% CI] |
3:121936453_G/A |
Number/events HR [95% CI] |
Number/events HR [95% CI] |
Number/events HR [95% CI] |
| GG |
6493/2276 Ref |
1436/496 0.93 (0.84–1.04) |
7929/2772 0.93 (0.84–1.04) |
GG |
6493/1410 Ref |
1436/316 0.97 (0.85–1.11) |
7929/1726 0.97 (0.85–1.11) |
| GA |
1003/326 0.86 (0.76–0.96) |
237/98 1.15 (0.93–1.41) |
1240/424 1.35 (1.07–1.70) |
GA |
1003/196 0.88 (0.76–1.02) |
237/68 1.31 (1.02–1.69) |
1240/264 1.50 (1.13–1.99) |
| AA |
31/9 0.64 (0.33–1.24) |
7/5 2.13 (0.88–5.13) |
38/14 3.83 (1.23–11.96) |
AA |
31/5 0.67 (0.28–1.62) |
7/4 2.57 (0.96–6.89) |
38/9 3.92 (1.05–14.72) |
| Effect of ‘3:121936453_G/A‘ within the strata of supplement intake |
7527/2611 0.85 (0.76–0.95) |
1680/599 1.27 (1.04–1.55) |
Effect of ‘3:121936453_G/A‘ within the strata of supplement intake |
7527/1611 0.87 (0.76–1.00) |
1680/388 1.39 (1.09–1.77) |
||
| Multiplicative scale | 1.49 (1.19–1.87) P = 0.0006 | Multiplicative scale |
1.60 (1.21–2.11) P = 0.0009 |
||||
| RERI |
0.40 (0.15–0.64) P = 0.0009 |
RERI |
0.51 (0.18–0.84) P = 0.002 |
||||
| SUPPLEMENTAL CALCIUM INTAKE*3:121932422_C/T (rs62269066) | SUPPLEMENTAL CALCIUM INTAKE*3:121932422_C/T (rs62269066) | ||||||
|---|---|---|---|---|---|---|---|
| <1 pill | ≥1 pill | Effect of supplement intake within the strata of ‘3:121932422_C/T‘ | <1 pill | ≥1 pill | Effect of supplement intake within the strata of ‘3:121932422_C/T ‘ | ||
| 3:121932422_C/T |
Number/events HR [95% CI] |
Number/events HR [95% CI] |
Number/events HR [95% CI] |
3:121932422_C/T |
Number/events HR [95% CI] |
Number/events HR [95% CI] |
Number/events HR [95% CI] |
| CC |
3113/1130 Ref |
706/223 0.81 (0.69–0.94) |
3819/1353 0.80 (0.69–0.93) |
CC |
3113/707 Ref |
706/145 0.85 (0.71–1.03) |
3819/852 0.85 (0.71–1.03) |
| CT |
3476/1193 0.90 (0.83–0.98) |
747/296 1.01 (0.88–1.16) |
4223/1486 1.12 (0.98–1.28) |
CT |
3476/724 0.90 (0.81–0.99) |
747/190 1.08 (0.91–1.28) |
4223/911 1.20 (1.02–1.43) |
| TT |
938/288 0.78 (0.68–0.89) |
227/80 0.93 (0.74–1.17) |
1165/368 1.19 (0.92–1.53) |
TT |
938/180 0.81 (0.69–0.96) |
227/53 0.99 (0.75–1.32) |
1165/233 1.23 (0.90–1.67) |
| Effect of ‘3:121932422_C/T‘ within the strata of supplement intake |
7527/2611 0.89 (0.84–0.94) |
1680/599 1.11 (0.99–1.25) |
Effect of ‘3:121932422_C/T ‘ within the strata of supplement intake |
7527/1611 0.90 (0.84–0.97) |
1680/388 1.11 (0.97–1.29) |
||
| Multiplicative scale | 1.25 (1.10–1.43) P = 0.0006 | Multiplicative scale |
1.24 (1.06–1.46) P = 0.008 |
||||
| RERI |
0.21 (0.10–0.32) P = 0.0006 |
RERI |
0.21 (0.06–0.35) p = 0.004 |
||||
Models were adjusted for age at diagnosis, sex, cohort, and the first three principal components of genetic ancestry and for the interaction models also for the main effects of calcium intake (dietary, supplemental or total) and SNP.
The estimated effective number of independent tests among 325 SNPS was 35 based on the simple M approach. Therefore, p < 0.001 were considered statistically significant (n = 35; 0.05/35 = 0.001).
A RERI of zero should be interpret as no additive interaction, a RERI < 0 a negative additive interaction and a RERI > 0 a positive additive interaction.
Total calcium intake is not included in this table because of the small sample sizes in the subgroups (n = 4646 for total population with total calcium intake available).
*Median split sex- and cohort-specific dietary calcium intake.
Discussion
In this large consortium of CRC patients, no association between dietary, supplemental or total calcium intake in relation to all- cause mortality nor CRC-specific mortality was observed. Two SNPs in the CaSR gene were associated with all-cause mortality after correction for multiple testing, while no associations between SNPs in the CaSR gene and CRC-specific mortality were observed. In addition, no interactions between dietary calcium intake or total calcium intake and SNPs in the CaSR gene in relation to mortality were observed. However, multiplicative interactions were observed between supplemental calcium intake and 5 SNPs, of which 2 independent clusters, in relation to all-cause mortality and 1 SNP in relation to CRC-specific mortality. In addition, additive interactions were observed between supplemental calcium intake and 5 SNPs, of which 3 independent clusters, in relation to all-cause mortality.
In our study we did not observe an association between dietary, supplemental or total calcium intake and all-cause or CRC-specific mortality in CRC patients. This is against our initial hypothesis, since we expected a better survival rate with a higher calcium intake given the associations of calcium intake with CRC risk and mortality [1, 45]. However, our results are consistent with previous studies investigating pre-diagnostic calcium intake and CRC survival, which also did not observe associations in CRC patients (n = 148–3859), with HRs ranging from 0.63–1.35 [6–9, 11]. Three of the previously mentioned analyses [6–8] included study populations that also participated in the ISACC consortium (NHS/HPFS; EPIC; CPSII), and part of their data is used in these analyses. We now hypothesize that the timing of calcium intake is of importance. For the data available for the present analyses, calcium intake was assessed before or around diagnosis (median 2.0 years before diagnosis IQR 1–6 years before diagnosis); however, it is possible that post-diagnostic intake may be most relevant to outcomes. Previous studies have noted an inverse association between post-diagnostic calcium intake and CRC-specific and all-cause mortality [6, 8, 10]. This was only statistically significant in NHS/HPFS for CRC-specific mortality (total calcium intake HR quartile 4 vs quartile 1 0.56 95%CI 0.32–0.96) [6] and in CPSII for all-cause mortality (total calcium intake HR quartile 4 vs quartile 1 0.72 95%CI 0.53–0.98) [8]. In addition, previous studies investigating the effect of dietary calcium intake in normal colonic mucosa, found a direct upregulation of several genes, including FOXJ2 and C3aR1, indicating a rather short term effect of calcium intake [46]. As no associations were observed in the subgroup analyses either, calcium intake before or around diagnosis does not appear to be associated with survival after a CRC diagnosis. Thus, although calcium intake before diagnosis does not improve CRC outcomes, it also does not hamper prognosis and thus can be consumed safely. Further studies should focus on post-diagnostic calcium intake and/or more long-term calcium exposure, for example by measuring calcium intake multiple times during follow-up. Also, sources of calcium intake, e.g., dairy or vegetables, as well as nutrients known to interact with calcium homeostasis such as vitamin D and magnesium [47] should be considered in these further studies. Different sources of calcium may have different effects on the gut microbiome [48] and likely subsequently the tumor microenvironment and immune-response [49] and could thus differentially influence survival.
Two correlated SNPs (rs62269066 and rs17282015) were associated with all-cause mortality, where a genotype of two minor alleles was associated with a lower risk of all-cause mortality for both SNPs. The function of these two SNPs is unknown and both SNPs are intron variants. No SNPs in the CaSR gene were associated with CRC-specific mortality after correction for multiple testing. To our knowledge, only four relatively small studies (ranging from 531 to 1,202 participants) have previously investigated the association between CaSR polymorphism and CRC outcomes [16, 50–52]. The CaSR rs1801725 SNP was associated with overall survival in a case-control study in China [52], but was not associated with survival in the an European cohort [51]. In addition, this SNP seems to be associated with CRC recurrence in a Hungarian population [50]. This specific SNP was not associated with mortality in the current study. Thus, although there are some indications that certain SNPs in the CaSR gene are associated with CRC prognosis, these SNPS are not confirmed in multiple studies.
In line with our results, a study in a Canadian population (n = 531 CRC patients) did not observe an interaction between dietary calcium intake and CaSR polymorphisms in relation to overall or disease-free survival after correction for multiple testing [16]. While no interaction was shown for dietary intake of calcium, we did observe an interaction with supplemental intake of calcium. To our knowledge, no previous studies investigated multiplicative and additive interactions between supplemental calcium intake and genetic variants of the CaSR in relation to CRC outcomes. Based on our results, there seems to be an interaction between supplemental calcium intake (>500 mg/day) and genetic variants in the CaSR gene in relation to all-cause mortality and CRC-specific mortality. Although we expected these interactive effects of CaSR also for dietary calcium intake, it could be that higher calcium levels, as with supplemental intake, are needed to exert these effects. In our population the median dietary calcium intake was 694 [IQR 467–995] mg/day, while the recommended daily intake is 1000–1200 mg/day (depending on age and sex) [53]. With supplemental intake of calcium (1 pill was assumed to be 500 mg, although there is some variation) on top of dietary intake, this amount is easily reached. In our analyses, an interaction between 7 SNPs in the CaSR gene and supplemental calcium intake in relation to mortality was observed. Whether these 7 SNPs, or correlated SNPs, change function and activity of the CaSR is unknown, all SNPs were intron variants. To conclude, our findings need to be confirmed in other studies and underlying mechanisms as well as functions of these SNPs should be further investigated before we can either discourage or encourage calcium supplement use in CRC patients based on their genotype.
Besides the CaSR gene, which encodes for the CaSR that plays a critical role in calcium homeostasis and has several tumor suppressing functions [12, 15], many more genes could potentially influence regulation and functioning of enzymes involved in calcium homeostasis and metabolism as well as influence effects of calcium on cancer progression [54]. In this study, we used a candidate-gene approach to investigate whether calcium intake in relation to all-cause and CRC-specific mortality was modified by genetic variants of the CaSR gene, which could potentially affect the functioning of the CaSR. As a complementary method, further studies should consider a genome wide approach when investigating interactions between calcium intake and genetic variants on mortality. Besides, in addition to investigating more variants across the genome, it would be interesting to evaluate the intake of the nutrients closely related to or interacting with calcium homeostasis, such as magnesium and vitamin D, at the same time.
This study has several important strengths. First of all, this is the largest consortium of CRC patients to date, consisting of almost 19,000 persons. Second, both dietary and supplemental sources of calcium intake were investigated. Third, we had detailed information about demographic and clinical characteristics, thus we were able to investigate several subgroups and adjust for potential confounders. However, this study is not without limitations. Given the nature of this large consortium, dietary and supplemental intake of calcium as well as genetic variants were assessed using different methods. This could hamper the ability to detect true associations for example due to misclassification of calcium intake. To prevent this as much as possible we used sex- and study specific quartiles of calcium intake. Furthermore, we imputed all SNPs of all studies using the same reference panel and imputation server. In addition, we had no information available about dietary sources of calcium intake, e.g., dairy, nor about nutrients closely related to calcium homeostasis, such as magnesium and vitamin D. Although we observed that total calcium intake is not associated with survival, we do not know whether specific dietary sources of calcium intake or the relative contribution of calcium compared to other nutrients in the diet, for example, the calcium to magnesium ratio, influence CRC survival [55, 56]. Also, data on treatment received e.g., chemotherapy, radiotherapy, was lacking. Adding stage of disease and tumor location, which together are closely linked to treatment provided, did not change the results. Finally, our study only included individuals of European ancestry, limiting the generalizability of our findings to other racial/ethnic groups.
To conclude, calcium intake before and around diagnosis was not associated with all-cause or CRC-specific mortality. However, multiplicative as well as additive interactions between supplemental calcium intake and genetic variants in the CaSR in relation to all-cause mortality and CRC-specific mortality were observed. Further studies should focus on post-diagnostic calcium intake, and include sources of calcium as well as closely related nutrients, and should investigate interactions between calcium intake and genetic variants in relation to mortality using a genome-wide approach.
Supplementary information
Acknowledgements
CCFR: The Colon CFR graciously thanks the generous contributions of their study participants, dedication of study staff, and the financial support from the U.S. National Cancer Institute, without which this important registry would not exist. The authors would like to thank the study participants and staff of the Seattle Colon Cancer Family Registry and the Hormones and Colon Cancer study (CORE Studies). CPS-II: The authors express sincere appreciation to all Cancer Prevention Study-II participants, and to each member of the study and biospecimen management group. The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention's National Program of Cancer Registries and cancer registries supported by the National Cancer Institute's Surveillance Epidemiology and End Results Program. The authors assume full responsibility for all analyses and interpretation of results. The views expressed here are those of the authors and do not necessarily represent the American Cancer Society or the American Cancer Society – Cancer Action Network. DACHS: We thank all participants and cooperating clinicians, and everyone who provided excellent technical assistance. EPIC: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization. Harvard cohorts: The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. We acknowledge Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital as home of the NHS. The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming. The authors assume full responsibility for analyses and interpretation of these data. NSHDS investigators thank the Västerbotten Intervention Program, the Northern Sweden MONICA study, the Biobank Research Unit at Umeå University and Biobanken Norr at Region Västerbotten for providing data and samples and acknowledge the contribution from Biobank Sweden, supported by the Swedish Research Council. PLCO: The authors thank the PLCO Cancer Screening Trial screening center investigators and the staff from Information Management Services Inc and Westat Inc. Most importantly, we thank the study participants for their contributions that made this study possible. Cancer incidence data have been provided by the District of Columbia Cancer Registry, Georgia Cancer Registry, Hawaii Cancer Registry, Minnesota Cancer Surveillance System, Missouri Cancer Registry, Nevada Central Cancer Registry, Pennsylvania Cancer Registry, Texas Cancer Registry, Virginia Cancer Registry, and Wisconsin Cancer Reporting System. All are supported in part by funds from the Center for Disease Control and Prevention, National Program for Central Registries, local states or by the National Cancer Institute, Surveillance, Epidemiology, and End Results program. The results reported here and the conclusions derived are the sole responsibility of the authors. WHI: The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Investigator-Long-List.pdf.
Author contributions
EW, FJBvD, and UP designed research; EW, WG, SIB, HB, DDB, PTC, ATC, JCC, MC, MJG, MH, ADJ, CCN, RKP, AJP, AIP, MS, CYU, BG, EW, UP, FJbvD conducted research; WG, SIB, HB, DDB, PTC, ATC, JCC, MC, MJG, MH, ADJ, CCN, RKP, AJP, AIP, MS, CYU, BG, EW, UP contributed to recruitment of participants or provided essential materials; EW performed statistical analysis; EW, FJBvD, WG, UP interpreted data; EW and FJBvD wrote paper; FJBvD has primary responsibility for final content; All authors have edited and reviewed versions of the manuscripts and have read and approved the final manuscript.
Funding
Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO): National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (U01 CA137088, R01 CA059045, U01 CA164930, R21 CA191312, R01 CA201407, R01 CA488857). Genotyping/Sequencing services were provided by the Center for Inherited Disease Research (CIDR) contract number HHSN268201700006I and HHSN268201200008I. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA015704. Scientific Computing Infrastructure at Fred Hutch funded by ORIP grant S10OD028685. The Colon Cancer Family Registry (CCFR, www.coloncfr.org) is supported in part by funding from the National Cancer Institute (NCI), National Institutes of Health (NIH) (award U01 CA167551). Support for case ascertainment was provided in part from the Surveillance, Epidemiology, and End Results (SEER) Program and the following U.S. state cancer registries: AZ, CO, MN, NC, NH; and by the Victoria Cancer Registry (Australia) and Ontario Cancer Registry (Canada). The CCFR Set-1 (Illumina 1 M/1M-Duo) and Set-2 (Illumina Omni1-Quad) scans were supported by NIH awards U01 CA122839 and R01 CA143237 (to GC). The CCFR Set-3 (Affymetrix Axiom CORECT Set array) was supported by NIH award U19 CA148107 and R01 CA81488 (to SBG). The CCFR Set-4 (Illumina OncoArray 600 K SNP array) was supported by NIH award U19 CA148107 (to SBG) and by the Center for Inherited Disease Research (CIDR), which is funded by the NIH to the Johns Hopkins University, contract number HHSN268201200008I. The content of this manuscript does not necessarily reflect the views or policies of the NCI, NIH or any of the collaborating centers in the Colon Cancer Family Registry (CCFR), nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government, any cancer registry, or the CCFR. CPS-II: The American Cancer Society funds the creation, maintenance, and updating of the Cancer Prevention Study-II (CPS-II) cohort. The study protocol was approved by the institutional review boards of Emory University, and those of participating registries as required. DACHS: This work was supported by the German Research Council (BR 1704/6-1, BR 1704/6-3, BR 1704/6-4, CH 117/1-1, HO 5117/2-1, HE 5998/2-1, KL 2354/3-1, RO 2270/8-1 and BR 1704/17-1), the Interdisciplinary Research Program of the National Center for Tumor Diseases (NCT), Germany, and the German Federal Ministry of Education and Research (01KH0404, 01ER0814, 01ER0815, 01ER1505A and 01ER1505B). DALS: National Institutes of Health (R01 CA048998 to M. L. Slattery). EPIC: The coordination of EPIC is financially supported by International Agency for Research on Cancer (IARC) and also by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Center (BRC). The national cohorts are supported by: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition Potsdam- Rehbruecke (DIfE), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Health Research Fund (FIS) - Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology - ICO (Spain); Swedish Cancer Society, Swedish Research Council and and Region Skåne and Region Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford). (United Kingdom). Harvard cohorts: HPFS is supported by the National Institutes of Health (P01 CA055075, UM1 CA167552, U01 CA167552, R01 CA137178, R01 CA151993, and R35 CA197735), NHS by the National Institutes of Health (P01 CA087969, UM1 CA186107, R01 CA137178, R01 CA151993, and R35 CA197735), and NHS2 (NHSII) by the National Institutes of Health (U01 CA176726 and R35 CA197735). Harvard cohorts: HPFS is supported by the National Institutes of Health (P01 CA055075, UM1 CA167552, U01 CA167552, R01 CA137178, R01 CA151993, and R35 CA197735), NHS by the National Institutes of Health (P01 CA087969, UM1 CA186107, R01 CA137178, R01 CA151993, and R35 CA197735). Harvard cohorts: HPFS is supported by the National Institutes of Health (P01 CA055075, UM1 CA167552, U01 CA167552, R01 CA137178, R01 CA151993, and R35 CA197735), NHS by the National Institutes of Health (P01 CA087969, UM1 CA186107, R01 CA137178, R01 CA151993, and R35 CA197735), and PHS by the National Institutes of Health (R01 CA042182). MCCS cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further supported by Australian NHMRC grants 509348, 209057, 251553 and 504711 and by infrastructure provided by Cancer Council Victoria. Cases and their vital status were ascertained through the Victorian Cancer Registry (VCR) and the Australian Institute of Health and Welfare (AIHW), including the National Death Index and the Australian Cancer Database. NSHDS: The research was supported by the Swedish Research Council (VR 2017-01737 and, through funding to Biobank Sweden, VR 2017-00650), the Swedish Cancer Society (21 0467 FE 01 H, 20 1154 PjF), Region Västerbotten, Knut and Alice Wallenberg Foundation, and the Cancer Research Foundation in Northern Sweden and Lion’s Cancer Research Foundation, both at Umeå University. PLCO: Intramural Research Program of the Division of Cancer Epidemiology and Genetics and supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. Funding was provided by National Institutes of Health (NIH), Genes, Environment and Health Initiative (GEI) Z01 CP 010200, NIH U01 HG004446, and NIH GEI U01 HG 004438. UK Biobank: This research has been conducted using the UK Biobank Resource under Application Number 8614. VITAL: National Institutes of Health (K05 CA154337). WHI: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.”
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All participants gave written informed consent and studies were approved by the Institutional Review Boards. The ISACC consortium was approved by Fred Hutchinson Cancer Center (IRB File #8017).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s44276-024-00077-3.
References
- 1.Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose–response meta-analysis of prospective observational studies. Int J Cancer. 2014;135:1940–8. [DOI] [PubMed] [Google Scholar]
- 2.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer. 2008;61:47–69. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Keum N, Wu K, Smith-Warner SA, Ogino S, Chan AT, et al. Calcium intake and colorectal cancer risk: Results from the nurses’ health study and health professionals follow-up study. Int J Cancer. 2016;139:2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. [DOI] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and colorectal cancer. Continuous Update Project Expert Report 2018. 2018. p. Available at dietandcancerreport.org.
- 6.Yang W, Ma Y, Smith-Warner S, Song M, Wu K, Wang M, et al. Calcium intake and survival after colorectal cancer diagnosis. Clin Cancer Res. 2019;25:1980 LP–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dik VK, Murphy N, Siersema PD, Fedirko V, Jenab M, Kong SY, et al. Prediagnostic intake of dairy products and dietary calcium and colorectal cancer survival—results from the EPIC cohort study. Cancer Epidemiol Biomarkers Prev. 2014;23:1813 LP–1823. [DOI] [PubMed] [Google Scholar]
- 8.Yang B, McCullough ML, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, et al. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: the cancer prevention study-II nutrition cohort. J Clin Oncol. 2014;32:2335–43. [DOI] [PubMed] [Google Scholar]
- 9.Dray X, Boutron-Ruault M-C, Bertrais S, Sapinho D, Benhamiche-Bouvier A-M, Faivre J. Influence of dietary factors on colorectal cancer survival. Gut. 2003;52:868 LP–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng K, Meyerhardt JA, Chan JA, Niedzwiecki D, Hollis DR, Saltz LB, et al. Multivitamin use is not associated with cancer recurrence or survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2010;28:4354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesselink, Kok DE E, Bours MJL, de Wilt JHW, van Baar H, van Zutphen M, et al. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am J Clin Nutr. 2020;111:1007–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal A, Prinz-Wohlgenannt M, Tennakoon S, Höbaus J, Boudot C, Mentaverri R, et al. The calcium-sensing receptor: a promising target for prevention of colorectal cancer. Biochim Biophys Acta. 2015;1853:2158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araghi SO, Jayakkumaran A, Mulder M, Stricker BH, Ruiter R, Kiefte-de Jong JC. Calcium intake, levels and supplementation and effect modification by genetic variation of calcium homeostasis on the risk of colorectal cancer: the Rotterdam study. Eur J Cancer Prev. 2021;30:364–72. [DOI] [PubMed] [Google Scholar]
- 14.Momen-Heravi F, Masugi Y, Qian ZR, Nishihara R, Liu L, Smith-Warner SA, et al. Tumor expression of calcium sensing receptor and colorectal cancer survival: Results from the nurses’ health study and health professionals follow-up study. Int J Cancer. 2017;141:2471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iamartino L, Elajnaf T, Kallay E, Schepelmann M. Calcium-sensing receptor in colorectal inflammation and cancer: current insights and future perspectives. World J Gastroenterol. 2018;24:4119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Wang PP, Zhai G, Bapat B, Savas S, Woodrow JR, et al. Vitamin D receptor and calcium-sensing receptor polymorphisms and colorectal cancer survival in the Newfoundland population. Br J Cancer. 2017;117:898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–8. [DOI] [PubMed] [Google Scholar]
- 18.Ganna A, Ingelsson E. 5 year mortality predictors in 498 103 UK Biobank participants: a prospective population-based study. Lancet. 2015;386:533–40. [DOI] [PubMed] [Google Scholar]
- 19.Gaziano, JM and Hennekens, CH (2014). Physicians’ Health Study (PHS). In Wiley StatsRef: Statistics Reference Online (eds Balakrishnan, N, Colton, T, Everitt, B, Piegorsch, W, Ruggeri, F, and Teugels, JL). 10.1002/9781118445112.stat06831
- 20.The Women’s Health Initiative Study. Design of the Women’s Health Initiative Clinical Trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 21.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 22.White E, Patterson RE, Kristal AR, Thornquist M, King I, Shattuck AL, et al. VITamins And Lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol. 2004;159:83–93. [DOI] [PubMed] [Google Scholar]
- 23.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11:462–8. [DOI] [PubMed] [Google Scholar]
- 24.Slattery ML, Berry TD, Potter J, Caan B. Diet diversity, diet composition, and risk of colon cancer (United States). Cancer Causes Control. 1997;8:872–82. [DOI] [PubMed] [Google Scholar]
- 25.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort. Cancer. 2002;94:2490–501. [DOI] [PubMed] [Google Scholar]
- 26.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy. Ann Intern Med. 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Women’s Heal. 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 28.Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26:S6–S14. [DOI] [PubMed] [Google Scholar]
- 29.Giles GG, English DR. The Melbourne Collaborative Cohort Study. In: Nutrition and lifestyle: opportunities for cancer prevention European Conference on Nutrition and Cancer held in Lyon, France on 21–24 June, 2003. International Agency for Research on Cancer (IARC); 2002. p. 69–70.
- 30.Mozaffarian D, Ascherio A, Hu FB, Stampfer MJ, Willett WC, Siscovick DS, et al. Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation. 2005;111:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haile RW, Siegmund KD, Gauderman WJ, Thomas DC. Study-design issues in the development of the University of Southern California Consortium’s Colorectal Cancer Family Registry. JNCI Monogr. 1999;26:89–93. [DOI] [PubMed]
- 32.Hutter CM, Chang-Claude J, Slattery ML, Pflugeisen BM, Lin Y, Duggan D, et al. Characterization of gene–environment interactions for colorectal cancer susceptibility loci. Cancer Res. 2012;72:2036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr PR, Weigl K, Edelmann D, Jansen L, Chang-Claude J, Brenner H, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a population-based study. Gastroenterology. 2020;159:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milne RL, Fletcher AS, MacInnis RJ, Hodge AM, Hopkins AH, Bassett JK, et al. Cohort profile: the Melbourne collaborative cohort study (Health 2020). Int J Epidemiol. 2017;46:1757–1757i. [DOI] [PubMed] [Google Scholar]
- 35.Johansson I, Hallmans G, Wikman A, Biessy C, Riboli E, Kaaks R. Validation and calibration of food-frequency questionnaire measurements in the Northern Sweden Health and Disease cohort. Public Health Nutr. 2002;5:487–96. [DOI] [PubMed] [Google Scholar]
- 36.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, David Crawford E, et al. Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21:273S–309S. [DOI] [PubMed] [Google Scholar]
- 37.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodén S, Wennberg M, Van Guelpen B, Johansson I, Lindahl B, Andersson J, et al. Dietary inflammatory index and risk of first myocardial infarction; a prospective population-based study. Nutr J. 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huyghe JR, Bien SA, Harrison TA, Kang HM, Chen S, Schmit SL, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet. 2019;51:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 42.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–6. [DOI] [PubMed] [Google Scholar]
- 44.Gao X. Multiple testing corrections for imputed SNPs. Genet Epidemiol. 2011;35:154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naghshi S, Naemi M, Sadeghi O, Darooghegi Mofrad M, Moezrad M, Azadbakht L. Total, dietary, and supplemental calcium intake and risk of all-cause cardiovascular, and cancer mortality: a systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2022;62:5733–43. [DOI] [PubMed] [Google Scholar]
- 46.Pellatt AJ, Slattery ML, Mullany LE, Wolff RK, Pellatt DF. Dietary intake alters gene expression in colon tissue: possible underlying mechanism for the influence of diet on disease. Pharmacogenet Genomics. 2016;26:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosanoff A, Dai Q, Shapses SA. Essential nutrient interactions: does low or suboptimal magnesium status interact with vitamin D and/or calcium status? Adv Nutr. 2016;7:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bielik V, Kolisek M. Bioaccessibility and bioavailability of minerals in relation to a healthy gut microbiome. Int J Mol Sci. 2021;22:6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xing C, Du Y, Duan T, Nim K, Chu J, Wang HY, et al. Interaction between microbiota and immunity and its implication in colorectal cancer. Front Immunol. 2022;13:963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bácsi K, Hitre E, Kósa JP, Horváth H, Lazáry A, Lakatos PL, et al. Effects of the lactase 13910 C/T and calcium-sensor receptor A986S G/T gene polymorphisms on the incidence and recurrence of colorectal cancer in Hungarian population. BMC Cancer. 2008;8:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fedirko V, Riboli E, Tjønneland A, Ferrari P, Olsen A, Bueno-de-Mesquita HB. et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European populations. Cancer Epidemiol Biomarkers Prev. 2012;21:582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diao Y-E, Xu Q. CASR rs1801725 polymorphism is associated with the risk and prognosis of colorectal cancer: a case-control study. J Clin Lab Anal. 2020;34:e23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and CalciumRoss AC, Taylor CL, Yaktine AL, Del Valle HB Dietary Reference Intakes for Calcium and Vitamin Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC); 2011.
- 54.Wu L, Lian W, Zhao L. Calcium signaling in cancer progression and therapy. FEBS J. 2021;288:6187–205. [DOI] [PubMed] [Google Scholar]
- 55.Costello RB, Rosanoff A, Dai Q, Saldanha LG, Potischman NA. Perspective: characterization of dietary supplements containing calcium and magnesium and their respective ratio—is a rising ratio a cause for concern? Adv Nutr. 2021;12:291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai Q, Shu X-O, Deng X, Xiang Y-B, Li H, Yang G, et al. Modifying effect of calcium/magnesium intake ratio and mortality: a population-based cohort study. BMJ Open. 2013;3:e002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.