Abstract
Nonhuman primates (NHPs), which are closely related to humans, are useful in biomedical research, and an increasing number of NHP disease models have been reported using gene editing. However, many disease-related genes cause perinatal death when manipulated homozygously by gene editing. In addition, NHP resources, which are limited, should be efficiently used. Here, to address these issues, we developed a method of introducing heterozygous genetic modifications into common marmosets by combining Platinum transcription activator-like effector nuclease (TALEN) and a gene-editing strategy in oocytes. We succeeded in introducing the heterozygous exon 9 deletion mutation in the presenilin 1 gene, which causes familial Alzheimer’s disease in humans, using this technology. As a result, we obtained animals with the expected genotypes and confirmed several Alzheimer’s disease-related biochemical changes. This study suggests that highly efficient heterozygosity-oriented gene editing is possible using TALEN and oocytes and is an effective method for producing genetically modified animals.
Subject terms: Genetic engineering, Disease model
This study shows that using gene-editing technology in oocytes using Platinum TALEN is an effective method for producing genetically modified marmosets by exon skipping.
Main
Common marmosets, belonging to the New World primates, exhibit similarities to humans in terms of genetics, physiological functions, brain structure and complex cognitive/social behaviors; therefore, they are used as model animals in the biomedical sciences1,2. The fecundity characteristics of marmosets, including their short period to reach sexual maturity, multiple births and short gestation intervals, are suitable for producing genetically modified animal models of human diseases. The production of genetically modified animals using gene-editing techniques has become commonplace, and the generation of disease models of nonhuman primates (NHPs) has been reported2–4. However, the use of NHPs not only requires more time for breeding compared with rodents but also necessitates more careful ethical considerations. Therefore, the most desirable strategy in gene editing using NHPs is the expression of the intended genetic modification and disease phenotypes in the founder animal.
Gene-editing technology is an ideal tool for disrupting the responsible genes for diseases, leading to loss of function and the establishment of animal models of disease. Zinc finger nuclease5, transcription activator-like effector nuclease (TALEN)6,7, clustered regularly interspaced short palindromic repeats (CRISPR)–Cas98–10, and its derivative, base editor11–13, are representative gene-editing techniques. Among them, TALEN has a higher specificity of sequence recognition than CRISPR–Cas9, which minimizes the insertion of off-target mutations into the genome9,14. In addition, Platinum TALENs with periodic pattern repeat mutations with non-repeat variable di-residues mutations, which differ from conventional TALENs without non-repeat variable di-residues mutations, have been developed, enabling the production of highly efficient genetically modified animals15. In our previous report on the production of target gene knockout NHPs, all animals obtained from Platinum TALEN-injected embryos were complete knockouts of the target gene without mosaic mutations, confirming that it is a very efficient gene-editing tool2. Although gene-editing technologies are useful methods for producing target gene knockout animals, some issues need to be improved. For example, gene-editing techniques can produce unexpected indels, and mosaic mutations are often observed, especially with CRISPR–Cas9 methods16. Furthermore, previous studies in mice have shown that, for 23.4% of genes, homozygous loss of function causes the animals to die perinatally17. Few technologies are available to directly generate a genetically engineered heterozygote with high efficiency and target selectivity in NHPs. In this study, we succeeded in artificially reproducing a heterozygous genetic modification by combining gene editing in oocytes and Platinum TALEN.
For the validation of oocyte gene editing, we selected presenilin (PSEN) 1, a gene associated with Alzheimer’s disease (AD), as the target gene. AD is the most common neurodegenerative disease, with a globally increasing incidence18. Typically, AD cases are sporadic and develop with aging; however, a small proportion of patients exhibit familial AD (FAD), which is inherited in an autosomal-dominant manner19. The genes encoding amyloid-β (Aβ) precursor proteins (APP), PSEN1 and PSEN2 have been identified as etiological genes associated with FAD, and mutations in these genes increase the production of Aβ42, which is highly aggregative and readily accumulates in the brain20–22. In particular, more than 300 mutations, most of which are pathogenic, are found in the PSEN1 gene23. Among them, PSEN1 exon 9 skipping (PSEN1-ΔE9) mutations cause early-onset FAD; the mutations include large deletion mutations including exon 9 and point mutations at the 3′ splice site in intron 8, which cause exon 9 skipping during splicing24. Currently, no method has been established to precisely delete only specific exons of target genes in NHPs. In this study, we applied gene-editing technology in oocytes using Platinum TALEN and accurately introduced the heterozygous PSEN1-ΔE9 mutation in marmosets, which showed molecular biochemical signatures specific to AD in initial evaluations. While pathognomonic neuropathological changes will be examined in aged animals in the future, the developed technology in this study may be highly useful in producing NHP models of human diseases, especially for hemi-allelic diseases.
Results
Evaluation of TALEN activity
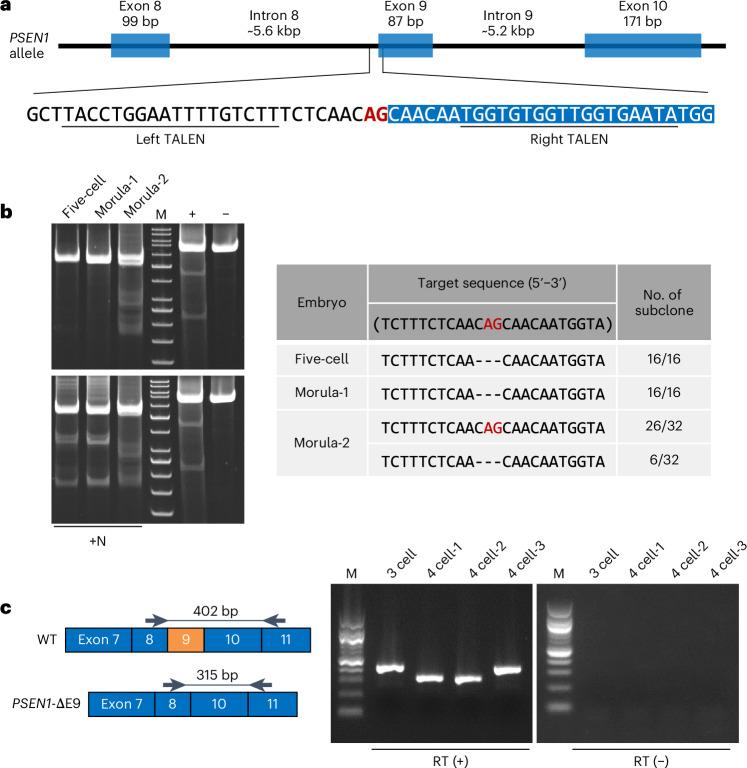
In this study, we implemented two strategies to skip exon 9 of PSEN1 using eight pairs of Platinum TALENs. One was to simultaneously cut introns 8 and 9 with two pairs of TALENs to excise the exon 9 from the genome (TALEN pair numbers 1, 2, 7 and 8), and the other was to disrupt the splice site related to exon 9 (TALEN pair numbers 3, 4, 5 and 6) (Supplementary Fig. 1a). Eight sets of Platinum TALEN plasmids were transfected into wild-type (WT) marmoset fibroblasts and evaluated by the Surveyor mutation detection assay, which revealed that the two pairs of TALENs that targeted the 3′ splice site in intron 8 showed gene modification activities (TALENs pair numbers 3 and 4; Supplementary Fig. 1b). Sequencing analysis of the genome of TALEN-transfected fibroblasts revealed that the 3′ splice site in intron 8 was disrupted when TALEN pair number 4 was used and showed gene modification at the target nucleotides ‘AG’ (Supplementary Table 1). Therefore, TALEN pair number 4 was selected for further analyses (Fig. 1a). Next, the TALEN pair number 4 was tested in marmoset embryos, which confirmed the successful genetic modification. After 4–5 days, the cultured TALEN mRNA-injected embryos were subjected to Surveyor assay and sequencing analysis, which confirmed that the 3′ splice site in intron 8 was involved in the splicing of PSEN1 exon 9 and was disrupted as intended (Fig. 1b). To confirm gene sequence modification, after 3 days the cultured TALEN mRNA-injected embryos were subjected to reverse transcription polymerase chain reaction (RT–PCR), followed by sequence analyses of the subcloned RT–PCR products. The results indicated that exon 9 was eliminated and predicted that the 290th amino acid serine was substituted with cysteine (S290C) at the junction site of exons 8 and 10 in two out of four embryos (Fig. 1c and Supplementary Fig. 2).
Fig. 1. Validation of candidate TALEN using marmoset embryos.
a, Schematic representation of PSEN1 and TALEN used in the present study. The underlines indicate TALEN binding sites, the blue boxes indicate exons of the marmoset PSEN1 gene, and the red letters (AG) represent the 3′ splice site of intron 8. b, Left: the Surveyor assay of marmoset embryos after TALEN mRNA injection. +N: equal amount of embryo-derived PCR products and WT genomic DNA-derived PCR products were mixed for detecting the biallelic mutations of the PSEN1 gene; +/−: reagent control (the right two columns denote experimental controls included in the Surveyor mutation detection kit); M: GeneRuler 50 bp DNA Ladder (Thermo Fisher Scientific, SM0371). Right: the results of the sequencing analysis of subclones obtained from PCR products. c, Right: evaluation of exon skipping using TALEN mRNA-injected marmoset embryos. Left: illustration of the analysis. The black arrows represent the design positions of the primers used for RT–PCR. Samples with PCR product sizes smaller than 402 bp suggested exclusion of exon 9. M: 100 bp DNA ladder (New England Biolabs; N3231); RT (+): reverse transcriptase-treated samples; RT (−): samples with water added instead of reverse transcriptase.
Arbitrary control of the monoallelic genetic modification
To produce PSEN1-ΔE9 marmosets, 63 zygotes were injected with TALEN mRNA, and 19 of the embryos that reached the 6-cell stage or later were transferred into the uteri of 14 recipient surrogate mothers; however, none of them was pregnant (Supplementary Table 2). Thus, we speculated that biallelic PSEN1 mutations may result in poor pregnancy outcomes. To test our hypothesis and elucidate whether it is possible to control or increase the monoallelic genetic modification through gene editing, TALEN mRNA was injected into the germinal vesicle (GV) stage ovum before oocyte maturation and in vitro fertilization (IVF) (Fig. 2a). When TALEN was injected into zygotes, genetic modification of PSEN1 was biallelic in 49% of cases and monoallelic in 22% of cases, whereas when TALEN was injected into oocytes, biallelic modification occurred in 12% of cases and monoallelic modification in 43% of cases. This result indicates a significant increase in monoallelic modification (P < 0.05) and a significant decrease in biallelic modification (P < 0.01) with oocyte injections compared with zygote injections (Table 1).
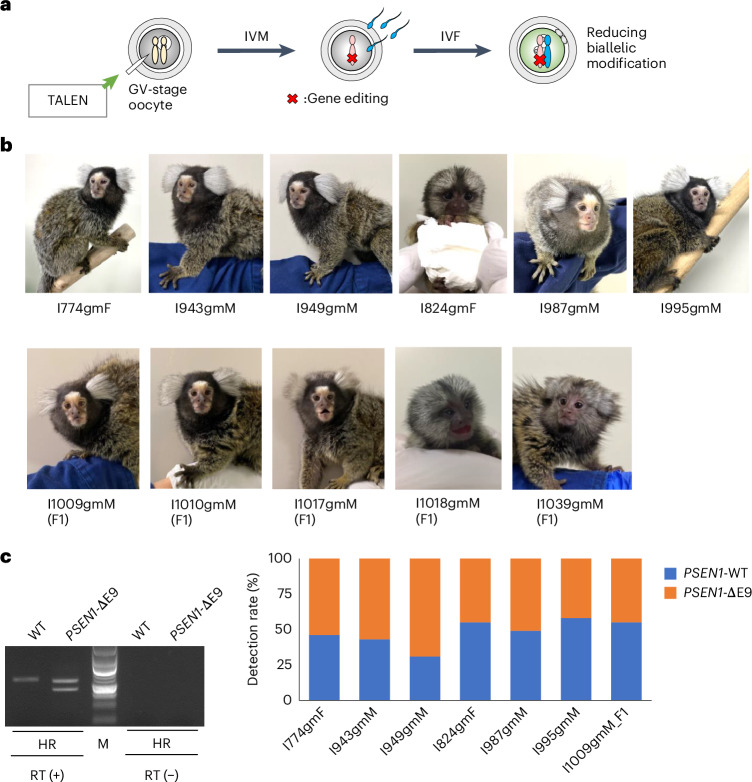
Fig. 2. PSEN1-modified animals and genotypic analyses.
a, Schematic representation of animal production using marmoset oocytes. b, Photos of obtained PSEN1-ΔE9 marmosets. c, Left: an example of RNA obtained from hair roots of PSEN1-ΔE9 marmoset analyzed by RT–PCR. HR: hair root genome; M: 100 bp DNA ladder (New England Biolabs; N3231); RT (+): reverse transcriptase-treated samples; RT (−): samples with water added instead of reverse transcriptase. Right: results of sequencing analysis of amplified products by RT–PCR obtained from seven mutant animals.
Table 1.
Comparison of gene modification activity of TALEN
| Use | Number of samples | Number of analyzed blastomeres | PSEN1 modification (%) | ||
|---|---|---|---|---|---|
| Biallelic | Monoallelic | Intact | |||
| Zygote | 21 | 121 | 59 (49%) | 27 (22%) | 35 (29%) |
| Oocyte | 17 | 145 | 17 (12%)* | 62 (43%)** | 66 (45%) |
*P < 0.01, **P < 0.05 (chi-squared test and residual analysis).
Production of PSEN1-∆E9 marmosets using TALEN injection into oocytes
To produce PSEN1-ΔE9 founder marmosets, TALEN mRNA was injected into 1,895 GV-stage oocytes collected from a total of 146 female marmosets. Subsequent IVF yielded 566 zygotes; 444 (78.4%) developed to the 6-cell stage or later stage, and 370 were transferred into the uterus of 191 surrogate mothers (Table 2). Fourteen marmoset neonates were obtained via normal delivery or cesarean section on days 141–146 from the day of ovulation of the surrogate mother. Genetic analysis was performed using genomic DNA and total RNA purified from hair root samples obtained from all animals. The Surveyor assay using genomic DNA suggested that 7 of 14 founder neonates were genetically modified (Fig. 2b). RT–PCR analyses and sequencing of subclones of the PCR products indicated exon 9 skipping in six founder neonates, with a carrier rate of approximately 50% (Fig. 2c). Genomic analyses of the six marmosets in which exon 9 skipping was detected confirmed the disruption of the 3′ splice site in intron 8 of PSEN1 as intended. However, a gene insertion occurred in I1008gmM, the seventh neonate, that reproduced the 3′ splice site (Supplementary Table 3), preventing exon skipping. The genotyping process was further refined through high-throughput amplicon sequencing for five PSEN1-ΔE9 founder animals (I774gmF, I943gmM, I949gmM, I987gmM and I995gmM), along with the F1 animal I1009gmM, which originated from I943gmM. The results indicated that the PSEN1 gene showed heterozygous mutations in two founder animals (I774gmF and I943gmM), while three animals (I949gmM, I987gmM and I995gmM) carried two mutations. The second mutation (−6 bp) in I949gmM was only detected at the trace level (1.9%). Furthermore, the mutated PSEN1 gene observed in I943gmM was accurately passed down to the F1 animal, I1009gmM. (Table 3 and Supplementary Table 4). From the six founder animals, I824gmF died of circulatory failure due to lung dysfunction. Further genetic analysis of the other five surviving PSEN1 mutant marmosets revealed the absence of unintended indels in exons 8 or 10 of PSEN1, thus confirming the successful production of PSEN1-ΔE9 marmosets (Supplementary Fig. 2). In older PSEN1-∆E9 marmosets (I774gmF, I943gmM and I949gmM), the established fibroblasts were genetically analyzed on a single-cell basis to confirm mosaicism, suggesting that I774gmF and I949gmM cells were heterozygous for PSEN1-∆E9 mutation and that I943gmM was a mosaic modification containing WT and null PSEN1 (Supplementary Fig. 3). To confirm the presence of genetic modifications in off-targets, an online tool named paired target finder was used to identify ten candidate sites (Supplementary Table 5). Genomic DNA extracted from the hair follicles of all PSEN1-ΔE9 founder marmosets was analyzed, and none showed mutations at the candidate sites. In addition, whole-genome sequencing (WGS) performed on the first PSEN1-ΔE9 marmoset (I774gmF) had an average coverage of 42.1, while the genetic father and genetic mother had an average coverage of 57.4 and 62.2, respectively (Supplementary Table 6). WGS data were processed using the Illumina Dynamic Read Analysis for Genomics (DRAGEN) pipeline (version 3.5.7) for mapping and variant calling process at a default parameter with the ‘CJA1912RKC’ marmoset reference genome (GCA_013373975.1). We used this highly permissive parameter in variant calling and did not filter out variants to retain as many variants in off-target candidate sites as possible. By comparing all variants from the PSEN1-ΔE9 marmoset (I774gmF) with those from its parents, 306,492 variants were found only in the PSEN1-ΔE9 marmoset (I774gmF) (Supplementary Fig. 4). However, none of these variants was present within the ten off-target candidate sites, further confirming the absence of off-target mutations. We also searched for de novo variants in I774gmF that may be randomly incurred by TALEN using the WGS data. The raw variants were filtered by multiple quality filtering steps (Methods), and only two single-nucleotide variants and one short indel remained on exons (including untranslated regions (UTRs)) when calculated genome-wide. Although one heterozygous single-nucleotide variant chr9: 67061285C>G (calJac4) was a missense variant (p. Gln358His in ASIC1, XM_017976046.3) with predicted deleterious effect by CADD v1.7 (Supplementary Table 7), no monoallelic hereditary disease has been reported for human ASIC1. The other two variants were in 3′ UTRs of SPATA6 and LONRF2, respectively. In PSEN1, there were no de novo variants. The results of the Illumina DRAGEN pipeline on WGS for large structural variants were filtered, but a large structural variant in PSEN1 was not detected.
Table 2.
Summary of PSEN1-ΔE9 founder marmoset generation
| Donor female marmosets | 146 |
| TALEN-injected oocytes | 1,895 |
| Matured ova |
1,382 (72.9%) |
| Fertilized ova |
566 (41.0%) |
| Developed embryos |
444 (78.4%) |
| Transferred embryos | 370 |
| Recipient animals | 191 |
| Pregnant animals |
27 (14.1%) |
| Aborted case |
16 (59.3%) |
| Obtained neonates | 14 (male: 10, female: 4) |
| Intact animals | 7 (male: 5, female: 2) |
| Mutant animals | 7 (male: 5, female: 2) |
Table 3.
Percentage of top 10 sequences obtained from high-throughput sequencing analysis
| Sequence | Genotype | Percentage of read counts (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| WT | I774gmM | I943gmM | I949gmM | I987gmM | I995gmM | I1009gmM _F1 | ||
| TGTCTTTCTCAACAGCAACAATGGTRTG | WT | 99.691 | 47.362 | 47.379 | 54.726 | 50.689 | 19.348 | 47.252 |
| TGTCTTTCT------CAACAATGGTRTG | −6 bp | 0.000 | 52.509 | 52.477 | 1.932 | 0.733 | 39.945 | 52.622 |
| TGTCTTTCT---------CAATGGTRTG | −9 bp | 0.000 | 0.001 | 0.001 | 41.401 | 16.010 | 40.637 | 0.001 |
| TGTCTTTCTCAACA-CAACAATGGTRTG | −1 bp | 0.000 | 0.000 | 0.000 | 1.785 | 32.449 | 0.001 | 0.000 |
| TGTCTTTCTCAACAGTAACAATGGTRTG | 0.040 | 0.037 | 0.041 | 0.043 | 0.027 | 0.019 | 0.035 | |
| TTTCTTTCTCAACAGCAACAATGGTRTG | 0.066 | 0.028 | 0.029 | 0.030 | 0.027 | 0.009 | 0.027 | |
| TGTCTTTCTCAACAACAACAATGGTRTG | 0.040 | 0.028 | 0.027 | 0.020 | 0.018 | 0.009 | 0.026 | |
| TGTCTTTCTCAATAGCAACAATGGTRTG | 0.036 | 0.012 | 0.016 | 0.041 | 0.025 | 0.015 | 0.014 | |
| TGTCTTTCTCAACAGCAACAATGTTRTG | 0.034 | 0.019 | 0.024 | 0.018 | 0.017 | 0.007 | 0.018 | |
| TGTCTTTCTCAACAGCAACAATGGTRTT | 0.092 | 0.005 | 0.006 | 0.004 | 0.005 | 0.010 | 0.004 | |
Biochemical analyses of PSEN1-∆E9 marmoset fibroblast cells and plasma
PSEN1 protein, the product of PSEN1, is the catalytic subunit of the γ-secretase complex whose substrates are type 1 transmembrane proteins including amyloid precursor protein. PSEN1 is cleaved into N-terminal fragments (NTFs) and C-terminal fragments (CTFs) to form mature γ-secretase complex by its endoproteolytic activity. However, this cleavage will not occur in the PSEN1 protein lacking the exon 9-coding region because this region resides at the auto-endoproteolytic site25. Cell membrane fractions were extracted from fibroblasts established from WT and PSEN1-ΔE9 marmosets (I774gmF and I949gmM) and subjected to western blotting using antibodies that recognize the NTFs and CTFs of PSEN1. The exon 9 deficiency in the two independent mutants, that is, I774gmF and I943gmM, resulted in the presence of full-length PSEN1 in the cell membrane fraction (Fig. 3a). Consistently, the quantities of the NTFs and CTFs in the mutants were reduced to approximately 20% in the NTFs and 40% in the CTFs of those in the WT (Supplementary Fig. 5). Furthermore, Aβ42 and Aβ40 were quantified using plasma samples collected from 17 WT and 8 PSEN1-ΔE9 marmosets (I774gmF, I943gmM, I949gmM, I987gmM, I995gmM, I1009gmM, I1010gmM and I1017gmM) by ultrasensitive enzyme-linked immunosorbent assays. The results indicated that the concentration of Aβ40 significantly decreased in the plasma of PSEN1-ΔE9 marmosets (P < 0.01), and the ratio of Aβ42/Aβ40 significantly increased (Fig. 3b, Supplementary Table 8 and Supplementary Data 1).
Fig. 3. Biochemical analyses of PSEN1-ΔE9 marmosets.
a, Biochemical analysis of PSEN1ΔE9 marmoset fibroblasts. Western blot analysis of membrane fractions obtained from four WT and two mutant (ΔE9) marmoset fibroblasts using antibodies to NTFs (left) and to CTFs (right) of PSEN1. The arrowheads indicate NTF and CTF produced by endoproteolysis of PSEN1; arrows indicate uncleaved, full-length PSEN1 protein. Na–K ATPase was used as a loading control of the membrane fraction. b, Aβ40 and Aβ42 in the plasma of 13 WT and 8 PSEN1-ΔE9 marmosets were quantified using Simoa. Aβ43 levels were below the detection limit. The data represent mean ± standard error of the mean (WT: n = 17, PSEN1-ΔE9: n = 8). Statistical analyses were performed using the Student’s two-tailed t-test for the Aβ40 and Aβ42 concentration (**P < 0.01, ****P < 0.0001), and Mann–Whitney U test for the Aβ42/Aβ40 ratio (****P < 0.0001).
Breeding of second-generation animals
Animals (I774gmF, I943gmM and I949gmM) reached sexual maturity and their gametes were used to produce a subsequent generation of animals. From these animals, of 93 embryos produced, 12 embryos were naturally fertilized, 9 were artificially inseminated and obtained by uterine flushing, and 72 were obtained using IVF (Supplementary Table 9). Biopsies of the blastomere were performed for all embryos, followed by the Surveyor assays. Among these 93 embryos, 38 (40.9%) carried the PSEN1-ΔE9 mutation and were transferred into the uteri of 22 surrogate mothers, resulting in 6 F1 animals, 5 of which were determined to have the PSEN1-ΔE9 mutation, and 4 animals survived so far (Fig. 2b and Supplementary Table 9). Moreover, a total of three embryos—two from IVF and one from artificial insemination (AI)—were vitrified during the Surveyor assay. All embryos that were confirmed to carry the mutant genotype were subsequently thawed and embryo transfer experiments were performed. As a result, one neonate was successfully obtained from the AI-derived embryo (Supplementary Table 9). The overall results of the second-generation animals showed that five of the six (83.3%) were mutants (Supplementary Table 9).
Discussion
In this study, two elements were used to produce PSEN1-ΔE9 marmosets. The first critical step was the introduction of a heterozygous mutation into the target gene using TALEN mRNA injection into oocytes. The GV-stage oocyte injection resulted in significantly increased and decreased monoallelic and biallelic mutations respectively, contributing to the obtention of live neonates (Table 1). In this study, after injection of Platinum TALEN mRNA into GV-stage oocytes, in vitro maturation (IVM) was performed for 22–24 h, followed by IVF at 28–45 h after the injection. A previous study reported that TALEN mRNA expresses TALEN protein after 4–24 h and declines to an undetectable level at 48 h in primary human T cells26. Although species and cell types differ from those in this study, the previous study strongly suggests that the TALEN protein is expressed during IVM and induces target gene mutation. Subsequently, when IVF was initiated 28 h after TALEN mRNA injection, TALEN protein expression was reduced or lost, resulting in a decreased modification rate of sperm-derived target genes and, thus, an increase in heterozygous mutations of the target gene.
Furthermore, genomic analysis showed that all five PSEN1-ΔE9 founder marmosets exhibited three or fewer sequence patterns, suggesting that the relatively short period of TALEN activity contributes to a reduced mosaic mutation in TALEN-mediated gene editing (Table 3 and Supplementary Tables 3 and 4). This strategy—producing heterozygous mutations by injection of GV-stage oocytes—might be specific to TALEN. In a previous study, when Cas9 protein was introduced into cultured human cells, genome cleavage began 24 h after the introduction and plateaued at 48–72 h (ref. 27). Moreover, a study using CRISPR–Cas9 demonstrated that when WT Cas9 mRNA was injected into cynomolgus monkey zygotes, Cas9 protein remained in the nucleus even after 40 h (ref. 28). Another study reported that injecting CRISPR–Cas9 or base editor into porcine GV-stage oocytes followed by IVF resulted in 61.9% of embryos carrying the target gene mutation, but with up to five patterns29,30. This phenomenon may be due to longer CRISPR–Cas9 or base editor activity, compared with TALENs. Therefore, considering the kinetics of TALEN and CRISPR–Cas9, methods for producing heterozygous mutant animals by oocyte injection may be suitable only for TALEN. We also compared the effect of TALEN injection on oocyte development with our previous data and found that the embryonic development rates were similar to those of untreated oocytes, suggesting no major limitations of this technique2. A previous study reported the production of heterozygous mutant animals in cynomolgus monkeys, targeting a single allele using allele-specific polymorphisms for gene editing3. This method reported by Tsukiyama et al. is efficient; however, our method is simpler and more flexible because it can be used when polymorphism does not exist in the target gene.
Although three out of five animals did not show mosaic mutation by single-cell analysis, one animal exhibited a mosaic of WT/WT, −6 bp/WT and −6 bp/−6 bp genotypes. The −6 bp/−6 bp biallelic mutant cells may have lost their intrinsic function, but these cells could survive with complementation by WT/WT and −6 bp/WT cells. However, the detailed mechanisms of this phenomenon are unknown. In mice, introducing various pathogenic point mutations in PSEN1 has shown that, except in a few cases, the resulting phenotype is nonlethal, even in the biallelic state31,32. Furthermore, PSEN1 and PSEN2 are extensively involved in embryonic development and show functional redundancy in mice33. Given the differences in the function of PSEN1 (and PSEN2) between mice and primates, our PSEN1-ΔE9 marmosets may be a good model for understanding the function of PSEN1 in primates, including humans.
The second critical step was to delete the specific exon completely and precisely by disrupting the splice site on the intron of the target gene. In this study, TALEN was used to disrupt the 3′ splice site in intron 8 of the PSEN1 gene to skip only exon 9, without indels or other effects on exons 8 or 10. In addition, the S290C mutation at the junction of exons 8 and 10 of the PSEN1 gene is the same mutation observed in patients with PSEN1-ΔE9 FAD. (Supplementary Fig. 2). The strategy used here to induce mutations in the splice site of the target gene is an ideal method because it precisely deletes the entire target exon of the target gene without allowing mutations in neighboring exons. Although several methodologies of exon skipping by gene editing in somatic cells have been reported34–38, this study is the first to produce model animals by exon skipping using gene editing in NHPs.
In the second generation of animal models, biopsies were performed on all embryos before embryo transfer and only embryos with the mutant genotype were transferred in recipient animals, resulting in five mutant animals out of six neonates. At the beginning of the study, during the second-generation animal production experiment, embryos were transferred directly to surrogate mothers based on the results of a simple genetic analysis. Unfortunately, this approach resulted in the unintentional creation of WT animals. While the Surveyor assay was used as a convenient analysis tool, subsequent evaluation revealed a single-nucleotide polymorphism (SNP) near the gene-editing target site in one of the WT oocyte donor animals (data not shown). This SNP discrepancy caused mismatches between the SNPs in the female and male genomes, leading to false positives in the Surveyor assay and subsequent erroneous embryo transfers. Therefore, it is critical to validate findings through both a Surveyor assay and sequencing analysis to prevent false positives. Currently, vitrification freezing of all embryos is performed after embryo biopsy to guarantee enough time for genetic analysis, and sequencing analysis ensures the detection of mutant genes. Therefore, the acquisition rate of the next generation of genetically modified individuals is expected to increase in the future. Preimplantation analysis for second-generation animal production is effective in reducing the number of births of unintended and non-genetically modified animals.
In this study, exon skipping of PSEN1 was selected as the target of gene editing because it is the only exon deletion mutation among more than 300 mutations in the PSEN1 gene, and we speculated that it could be reproduced by exon excision or splice site disruption using Platinum TALEN. TALEN is expected to suppress mosaicism as much as possible because it takes a very short time for protein expression from mRNA and rapidly disintegrates after a gene-editing event. In addition, gene modification using Platinum TALEN was confirmed to occur homogeneously within a narrow range of one to several dozen bases near the target site2. Since this study included a plan to pinpoint and remove a splice site consisting of only two bases, we concluded that the use of Platinum TALEN would be optimal to prevent unintended genetic modification in a wide gene area.
Our initial experiments suggest that biallelic PSEN1-ΔE9 was embryonically lethal in marmosets, and this mutation may be suitable to validate the usefulness of our new controllable gene-editing technology to introduce heterozygous mutations. Although further studies are needed to determine whether PSEN1-ΔE9 marmosets exhibit AD-like symptoms and brain pathology, the presence of the full-length PSEN1 protein due to the inhibition of PSEN1 endoproteolysis observed in the cell membrane fraction of fibroblasts obtained from PSEN1-ΔE9 marmosets suggests that PSEN1 is functionally abnormal in these mutant animals (Fig. 3a). The PSEN1-ΔE9 marmoset also showed a significantly higher plasma Aβ42/Aβ40 ratio than the WT marmoset (Fig. 3b), similar to what has been observed in patients (age 9–17) carrying the PSEN1 E280A mutation39. The results of the biochemical analyses indicate that the dysfunction of the PSEN1 gene occurred due to the skipping of exon 9, which was caused by the disruption of the 3′ splice site in intron 8. Furthermore, the relationship between the S290C mutation created at the junction of exon 8 and 10 and the increase in Aβ42 supports previous findings that suggest that the overexpression of a PSEN1 complementary DNA with a same point mutation in Caenorhabditis elegans increased production of Aβ42 (ref. 40). It should be noted that attempts to perform exon 9 skipping of PSEN1 in mice, similarly to what has been done in this study, would be difficult because it involves the generation of stop codons (unpublished observations by K.N.). Therefore, NHP PSEN1-ΔE9 models can have a critical role in understanding the relation between PSEN1 and AD.
In human AD, Aβ deposition in the brain occurs approximately 20 years before the onset of the disease41, and various reports suggest that the age of onset in PSEN1-ΔE9 humans is 40–50 years23,42. Previous studies on WT marmosets reported that Aβ deposition in the brain occurs at 7 years of age or even earlier43. Therefore, the PSEN1-ΔE9 marmosets established in this study are potentially useful as disease models that mimic human FAD under normal breeding conditions.
Because the NHPs are generally bred until the end of their lifespan, ethical considerations must be made, such as providing sufficient breeding space based on NHPs’ intrinsic social behavior and other characteristics. This can also be said for animals that have not been successfully gene edited. Usually, these animals will not be killed because of ethical concerns, requiring space for maintenance and increasing the workload of their keepers. Therefore, it is essential to optimize the experiments so that the intended genetic modifications and phenotypes can be reproduced in the founder generation of gene editing in genetically modified animals. This study successfully achieved the desired genetic modification in founder individuals and showed that the pattern of genetic modification (homozygous or heterozygous) can be controlled by combining Platinum TALENs with fertilized eggs or oocytes. Our study demonstrates that Platinum TALEN is a useful gene-editing tool for producing a variety of animal models, not only using NHPs but also animals of other taxa.
Methods
Animals
All animal experiments were performed in accordance with the guidelines for animal experimentation of the Central Institute for Experimental Medicine and Life Science (CIEM: 18031A, 19033A). The CIEM standard guidelines are in accordance with the guidelines for the proper conduct of animal experiments, as determined by the Science Council of Japan. In addition, the implementation of the gene recombination experiment in this study was approved by the Gene Recombination Experiment Safety Committee of CIEM (Kawasaki, Japan; 200021). The marmosets used in this study were purchased from CLEA Japan. The animals were 2.0–4.5 years of age, with body weights between 300 and 450 g. All animals were housed in cages in accordance with the following two international standards for laboratory animal care: National Research Council of the National Academies, Guide for the Care and Use of Laboratory Animals and the EU Commission recommendation of 18 June 2007, on guidelines for the accommodation and care of animals used for experimental and other scientific purposes. The cage consisted of one main body (900 mm length, 750 mm width, and 2,050 mm height) and two ceiling cages (300 mm length, 425 mm width, and 375 mm height), and marmosets were bred in family units. The room temperature was maintained at a constant 28 °C by an air conditioning system. The light–dark cycle was 12 h, and CMS-1M (CLEA Japan), a complete diet, was used as feed.
In vitro transcription of TALEN mRNA
For preparing Platinum TALEN plasmids containing the homodimer-type FokI nuclease domain, a two-step Golden Gate assembly method using the Platinum Gate TALEN Kit (#1000000043, Addgene) was used15,44. TALEN mRNA was synthesized using the mMESSAGE mMACHINE T7 Ultra Transcription Kit (AM1345, Thermo Fisher Scientific). The transcribed mRNA was purified using the MEGAclear Transcription Clean-Up Kit (AM1908, Thermo Fisher Scientific), and nuclease-free water (AM9937, Thermo Fisher Scientific) was used for the elution step and subsequent dilution.
Procedures for oocyte collection and IVF
Oocyte collection and IVF were performed as described previously45,46. Briefly, female marmosets with plasma progesterone levels of < 10 ng/ml were used for follicular stimulation. To collect oocytes, the selected marmosets were intramuscularly injected with 25 IU of follicle-stimulating hormone (hFSH; FOLYRMON-P injections, Fuji Pharma) five times every other day. The day after the last hFSH administration, 75 IU of human chorionic gonadotropin (hCG; gonadotropin for animals, ASKA Pharmaceutical) was intramuscularly administered. The hormone-treated female marmosets were pre-anesthetized with 0.04 mg/kg medetomidine (Domitor, Nippon Zenyaku Kogyo), 0.40 mg/kg midazolam (Dormicam, Astellas Pharma) and 0.40 mg/kg butorphanol (Vetorphale, Meiji Seika Pharma). Oocyte pickup was performed 17–20 h after hCG injection. During the procedure, the marmosets were anesthetized by isoflurane (Isoflurane inhalation solution, Pfizer) inhalation. After oocyte pickup, the abdominal wall and skin were sutured, and the marmoset was kept warm in the intensive care unit until awakened from anesthesia. For the experimental group, microinjection of TALEN mRNA into GV-stage oocytes was performed at this point. The oocytes were incubated in POM (basic medium for porcine oocyte maturation) medium (335-00661, Fujifilm Wako Pure Chemical) for 28–32 h at 37 °C, 5% CO2 and 5% O2, and 90% N2 for IVM47. The semen was collected from male marmosets using the Marmoset Holder (CL-4532, Japan CLEA) and an electronic vibrator and suspended in 500 µl of TYH medium (248238, LSI Medience). After centrifugation at 100g for 5 min and discarding the supernatant, 500 μl of new TYH medium was gently added and incubated for 30 min at 37 °C, 5% CO2 and 5% O2, and 90% N2. The supernatant containing motile sperm was collected and the sperm count was calculated using the Sperm Motility Analysis System (SMAS, DITECT). Droplets containing motile sperm at a rate of 3.2 × 106/ml were prepared using TYH medium. The matured ova were transferred to droplets and co-cultured for 10–16 h at 37 °C, 5% CO2, 5% O2 and 90% N2, followed by fertilization determination. For the experimental group, microinjection of TALEN mRNA into zygotes was performed at this point. The zygotes were cultured in Sequential Cleav (83040010A, CooperSurgical) at 37 °C, 5% CO2, 5% O2 and 90% N2, and the incubation period was modified according to the purpose of the experiment.
Microinjection
For TALEN injection, an aqueous solution was used in which the left and right TALEN mRNAs were mixed in equal quantities to a final concentration of 8 ng/μl. Approximately 4–8 pl of the mRNA mixture was microinjected into the oocyte or zygote using a glass syringe and air-pressure injector (FemtoJet, Eppendorf).
Blastomere analysis for TALEN validation
TALEN mRNA-injected embryos that had reached the six-cell stage or above were directed to blastomere splitting as previously described2,16. Briefly, embryo specimens were treated with an acidified Tyrode’s solution (10605000, CooperSurgical) to lyse the zona pellucida. Naked embryos were transferred to an embryo biopsy medium (10620010, CooperSurgical). After several minutes, the embryos that exhibited weak adhesion between blastomeres were split into single blastomeres using a glass capillary and washed immediately in Ca–Mg-free phosphate-buffered saline (PBS) droplets. The blastomere was then transferred to a test tube containing a small quantity of PBS and used for subsequent analysis.
Animal production
Embryos grown to a six-cell stage or above were transferred to the uteri of surrogate mothers, as described previously45,46. If a breech infant was diagnosed by ultrasound just before the due date and delivery was considered difficult, a cesarean section was performed to procure the neonate2. After cesarean section, the foster mother’s wounds on the uterus, abdominal muscular tunic and skin were sutured, and the animal was placed in the intensive care unit overnight. The newborns acquired by a cesarean section were placed in a newborn intensive care unit overnight. The next day, cesarean-sectioned foster mothers and newborn babies and foster fathers were placed in the same cage, and newborn babies were raised by foster parents without mother–infant separation.
Collection of hair root cells
Body hair was collected only once from the backs of marmoset neonates within 3 days of birth. The hairs were collected from several sites using clean tweezers to obtain a total of approximately 50 hairs.
PCR for genotyping
Genomic DNA was extracted from marmoset hair roots and tissues using the QIAamp DNA Micro Kit (56304, Qiagen) according to the manufacturer’s instructions. Embryos and blastomeres were used as templates without genome extraction. PCR was performed using specific primers (Supplementary Table 10), followed by sequencing or the Surveyor assay. Each PCR mixture contained 1× PCR buffer, 0.2 mM dNTPs, 0.5 mM of each primer, 20 ng of template DNA and 2.5 U of KOD-Plus-Neo (KOD-401, Toyobo) in a total volume of 20 μl. Amplification was performed using a thermal cycler (C1000, Bio-Rad Hercules) as described in Supplementary Table 9.
RT–PCR
Total RNA was extracted from TALEN-injected marmoset embryos, blastomeres, and hair roots using the NucleoSpin RNA Plus XS kit (U0990B, Takara Bio) according to the manufacturer’s instructions. Complementary DNA was synthesized using the ReverTra Ace-α (FSK-101F, Toyobo). For the reaction, the sample was divided into two groups: one containing reverse transcriptase and the other containing the same quantity of water as the control to evaluate genomic DNA contamination. PCR was performed using primers designed for exons (Fig. 1c, Supplementary Table 10). Each PCR mixture contained 1× PCR buffer, 0.2 mM of dNTPs, 0.5 mM of each primer, 20 ng of template DNA and 2.5 U of KOD-Plus-Neo (KOD-401, Toyobo) in a total volume of 20 μl. Amplification was performed using a thermal cycler (Supplementary Table 11).
Surveyor assay
The Surveyor mutation detection kit (706025, Integrated DNA Technologies), a mutation analysis method that identifies mismatches in double-stranded DNA, was used to detect genetic modifications at the TALEN target site. Briefly, 8 µl of the PCR product described above was used for genotyping. For the analysis of biallelic mutations in PSEN1, a sample was prepared in which 4 µl of the PCR product was mixed with an equal quantity of the PCR product prepared using WT PSEN1 as a template. The sample was digested with nuclease, electrophoresed on Novex TBE gels (EC62752BOX, Thermo Fisher Scientific) and then subjected to nucleic acid staining with GelRed (518-24031, Fujifilm Wako Pure Chemicals).
Sanger sequencing analysis
To confirm the sequences of the TALEN target sites, PCR products were subcloned using the Zero Blunt PCR Cloning Kit (44-0302, Thermo Fisher Scientific) according to the manufacturer’s instructions. The obtained plasmids were used to transform ECOS competent Escherichia coli DH5α cells (316-06233, NIPPON GENE) and cultured on Luria–Bertani agar containing kanamycin. After amplifying the plasmid using an emerged single colony, its presence was confirmed with an M13 primer and the BigDye Terminator v3.1 cycle sequence kit (4337455, Thermo Fisher Scientific), and gene sequences were analyzed using a 3130 Genetic Analyzer (Thermo Fisher Scientific).
High-throughput sequencing analysis
To ensure accurate genotyping of the founder animals, deep sequencing of genomic PCR products near TALEN target sites was conducted using a next-generation sequencer. Amplicon libraries were prepared following the ‘16S Metagenomic Sequencing Library Preparation Protocol’ (Illumina). Sequencing was performed using an Illumina MiSeq sequencer with 2 × 300 cycle MiSeq Reagent Kit v3 (MS-102-3001, Illumina). Adapter sequences were trimmed using Cutadapt, and low-quality regions were removed using Trimmomatic. After preprocessing, paired-end sequence reads were assembled using the script fastq-join. Further data processing was performed using in-house scripts. In brief, sequence reads were merged to generate clusters of homologous sequences. Sequences of each cluster were counted to calculate the relative frequency for each sample. To filter off any potential PCR and sequencing error-associated reads, sequence variants composed of <10 individual reads per sample were removed.
Off-target analysis with the paired target finder tool
A paired target finder (https://tale-nt.cac.cornell.edu/node/add/talef-off-paired) was used to search for potential off-target sites. As a reference, CJA1912RKC (GenBank assembly accession GCA_013373975.1), which is the whole-genome sequence of the marmoset, was set in the search database, and the RVD sequence of TALEN was provided as follows: RVD sequence 1, NI HD HD NG NN NN NI NI NG NG NG NG NN NG HD NG NG; RVD sequence 2, NI NG NG HD NI HD HD NI NI HD HD NI NG NI HD HD NI. The minimum and maximum spacer lengths were set to 10 and 30, respectively. The other parameters were set as recommended. To identify genome regions with predicted off-target sequences from the genome reference sequence in the WGS data, a ‘GGGenome’ ultrafast sequence search site was performed, and off-target bed files were produced (https://gggenome.dbcls.jp/en/calJacRKC1912_chrM_d3g2003/). A total of 428 paired-oriented off-target sequence sets were obtained from 10 TALEN off-target sequence sets. After filtering (spacer size of <100 bp) with 428 off-target sequencing sets, 10 paired-oriented off-target sequence sets were predicted as off-target effect candidates (Supplementary Table 12). Genomic DNA extracted from marmoset hair roots using the QIAamp DNA Micro Kit was subjected to PCR using specific primers (Supplementary Table 10), followed by direct sequencing. Each PCR mixture contained 1× KOD One PCR Master Mix (KMM-101, Toyobo), 0.5 mM of each primer and 10 ng template DNA in a total volume of 20 μl. PCR was performed using the following cycling conditions: 30 cycles of 98 °C for 10 s, 60 °C for 5 s and 68 °C for 1 s using a thermal cycler (Supplementary Table 11). The amplicon was purified using NucleoSpin Gel and PCR Clean-up (740609, Takara Bio), confirmed with one of the PCR primers and the BigDye Terminator v3.1 cycle sequence kit (4337455, Thermo Fisher Scientific). The gene sequence was identified using a Genetic Analyzer (3130, Thermo Fisher Scientific).
Establishment of fibroblast cells
Earlobe sampling was performed after the F1 offspring reached 6 months of age when the earlobe tissue was of a sufficient size. Approximately 3 mm2 of the earlobe was cut only once with sterilized sharp scissors; the dehaired and shredded tissue pieces were then affixed to the Primaria Cell Culture Dish (353801, Corning) and cultured with Dulbecco’s modified Eagle medium + GlutaMAX (10566-024, Thermo Fisher Scientific), containing 10% fetal bovine serum (10270106, Thermo Fisher Scientific) and penicillin–streptomycin cocktail (15240-062, Thermo Fisher Scientific) at 37 °C and 5% CO2 until the extension of fibroblast cells.
Single-cell PCR using fibroblasts
The cultured fibroblast cells obtained from PSEN1-modified marmosets were harvested and washed with PBS and then picked up one by one with a glass capillary into sampling tubes as a template and used for direct PCR and sequencing.
WGS
Marmoset earlobes collected by the previously described method were used for WGS. Genomic DNA was extracted from the ear tissue using the QIAamp Micro DNA kit (56304, Qiagen) according to the manufacturer’s instructions. Briefly, the tissue was incubated in lysis buffer and proteinase K overnight at 56 °C, and then genomic DNA was collected by column purification with low-TE (Tris–HCl, 10 mM, EDTA 0.1 mM and pH 8.0). WGS was performed using Illumina NovaSeq. FASTQ files for all WGS runs were deposited under accession numbers DRR239155, DRR239156 and DRR239157 in the DNA Data Bank of Japan Sequence Read Archive. For processing WGS datasets, Illumina DRAGEN pipeline (version 3.5.7) was performed for mapping and the variant calling process was used as a default parameter with the ‘CJA1912RKC’ marmoset genome reference sequence (GCA_013373975.1; Supplementary Table 7). We used this highly permissive parameter in variant calling and did not filter out variants in an effort to retain as many variants in off-target regions as possible. By comparing all variants from the mutant PSEN1 (I774gmF) marmoset with those from her parents, 306,492 variants were found without any filtering. We also analyzed the trio WGS data for de novo variants. We used the Illumina DRAGEN de novo variant calling pipeline for single-nucleotide variants and short indels. Subsequently, the resulting vcf file was filtered using the following steps: (1) biallelic SNPs; (2) each of the trios was ‘PASS’; (3) parents were both homozygous for WT and the offspring was heterozygous; (4) the offspring had coverage ≥16; (5) each of the trios had genotyping quality of 20 or more; (5) fraction of the ALT allele in the offspring >0.30; (6) fraction of the ALT allele in both the parents did not exceed 0.04; (7) the variant was on autosomes; and (8) the variant was marked ‘de novo’ as a result of the DRAGEN pipeline. For analysis of structural variants including large deletions, we used the result of the DRAGEN pipeline for structural variant calling with additional filtering with the following criteria: (1) either of the parents did not have an alternative allele; (2) the offspring had the ‘PASS’ mark and was heterozygous; (3) the genotyping quality for the offspring was ≥20; and (4) the variant was in the PSEN1 region defined by ncbiRefSeq All.
Western blot analysis
Primary fibroblast lines were established from ear skin tissues from WT and PSEN1-ΔE9 founder marmosets as described above. Cells were subjected to subcellular fractionation using the ProteoExtract Subcellular Proteome Extraction Kit (539790, Merck) according to the manufacturer’s instructions. Each fraction was loaded onto polyacrylamide gels and detected using primary antibodies against the PS1 N-terminus (G1Nr5) and C-terminus (G1L3) (obtained from Taisuke Tomita of Tokyo University, Tokyo, Japan). An antibody against sodium–potassium ATPase (ab76020, Clone: EP1845Y, Abcam) was used as the loading control.
Ultrasensitive enzyme-linked immunosorbent assay
After the WT control and mutant animals were past 6 months of age, approximately 0.5 ml of peripheral blood was collected from the femoral vein of the marmosets in BD Microtainer Blood Collection Tubes EDTA-2K (365974, Becton, Dickinson and Company). The blood was then centrifuged at 1,400g for 15 min, and the plasma was collected. Subsequently, plasma Aβ40 and Aβ42 were measured by Simoa, a bead-based immunoenzymatic technology, and a dedicated assay kit Neurology 3-Plex A Advantage Kit (Quanterix, 101995) according to the manufacturer’s instructions48.
AI and embryo collection
AI was performed as described previously with some modifications49. Briefly, the semen was collected from PSEN1-ΔE9 founder marmosets using the previously described method and diluted with 70 μl of TYH medium. Several WT female marmosets whose sexual cycles were known through periodic measurements of plasma progesterone levels were prepared, and AI was performed at the time expected to be just before the day of ovulation. For the AI procedure, the female marmoset was kept awake and hand-held and injected with 100–150 μl volume of the prepared semen in a mouse oral sonde (5202SS, Fuchigami) attached to a 1 ml syringe near the uterine opening approximately 4 cm from the vagina. Embryos were collected using a combination of previously reported nonsurgical intrauterine embryo collection procedures49. Surgery was performed on days 5–7 from the expected ovulation date of the females that underwent AI; 5 ml of Waymouth medium (11220-035, Thermo Fisher Scientific) was heated to 37 °C and used for intrauterine reflux.
Biopsy of embryos
Embryos that had reached the six-cell stage or higher were soaked in embryo biopsy medium for 10 min, and a section of the clear zone was cut open using XYclone (AR Brown). A single blastomere was collected using a glass capillary and transferred to test tubes for genetic analysis. After the embryo biopsy, embryos were cultured again in Sequential Cleav (CooperSurgical) and those that developed normally were prepared for embryo transfer or embryo vitrification.
Vitrification of embryos
Until blastomere analyses were completed, the embryos were vitrified. Before vitrification, the embryos were washed with PB1 (136.9 mM NaCl, 2.68 mM KCl, 1.08 mM CaCl2, 1.47 mM KH2PO4, 491.9 µM MgCl2∙6H2O, 327.1 µM sodium pyruvate, 5.55 mM d(+)-glucose, 201.4 µM penicillin G potassium salt and 0.3% bovine serum albumin) on a plate heater set at 37 °C and were acclimated to the new PB1 medium for 1 min. Next, the embryos were washed in DE10S (5% dimethyl sulfoxide, 5% ethylene glycol, 50% PB1 and 40% 0.75 M sucrose) on a plate heater set at 37 °C and acclimated to the new DE10S for 1 min. Embryos were transferred to a cryovial containing 5 µl DE10S. The vial was cooled on ice for 1 min, and 95 µl DESP1 (5% dimethyl sulfoxide, 25% ethylene glycol, 50% 1 M sucrose and 20% Percoll) was added and kept on ice for another 1 min. The vial was wiped off the surrounding water, and the bottom of the vial was immersed in liquid nitrogen for 15 s to freeze.
Warming the embryos
The cryovial lid was opened, liquid nitrogen in the vial was discarded and the vial was warmed for approximately 30 s by clamping it between the hands. After warming, 900 μl SPB2 (50% 1 M sucrose and 50% PB1) heated to 37 °C was poured into a vial and gently pipetted five times. The thawed embryos were collected, washed four times with PB1 and then acclimatized to the appropriate medium depending on the application.
Statistical analysis
The Shapiro–Wilk test was performed as a preliminary statistical analysis to confirm that the data were normally distributed. Statistical analysis was performed using Student’s two-tailed t-test or Mann–Whitney U test with the GraphPad Prism 9 software (GraphPad Software).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41684-024-01424-0.
Supplementary information
Supplementary Figs. 1–5 and Tables 1–12.
Raw data and statistical source data of Aβ measurements.
Source data
Unprocessed agarose gels.
Unprocessed agarose gel.
Unprocessed western blots.
Raw data of amplicon sequencing using next generation sequencing.
Acknowledgements
We thank T. Tomita, University of Tokyo, for providing antibodies to PSEN1. We thank Y. Nagai of RIKEN CBS for secretarial assistance. We thank Y. Yamada, T. Ishibuchi, M. Togashi, Y. Sawada and members of the Center of Basic Technology in Marmosets, CIEM for their technical assistance in producing and maintaining the AD models. We also thank T. Mineshige, K. Mukasa and T. Yurimoto of the CIEM for their veterinary assistance. This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant number JP20dm0207001 (Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS)) (T.C.S.), JP20dm0207065 (Study on developing neurodegenerative model marmosets and establishment of novel reproductive methodology; E.S.), Grant-in-Aid for Scientific Research (A) ‘Development of an Automated Behavioral Analysis System for Evaluation of Genetically Modified Disease Model Marmosets’ JSPS KAKENHI grant numbers JP21H04756 to E.S., the AMED under grant number 24zf0127007h0003 (Understanding and harnessing the role of the gut microbiome in healthy longevity; E.S.), and a portion of the genome sequencing was supported by a generous donation from S. Sengi to CIEM.
Author contributions
K.S., H.S., W.K., K.N., T.C.S. and E.S. designed this study. K.S. and H.S. performed the experimental group assignment, and the biochemical experiments were blinded to the marmoset genotype. T.S. and T.Y. constructed the Platinum TALEN vector used in this study. K.S., H.S., W.K., T.M., T.I., Y.K. N.M. and E.S. performed experiments. M.T., R.M., K.O., M.S. and Y.O. performed whole-genome analyses, including off-target analysis on the obtained animals. K.S., H.S., W.K., K.N., T.S., T.Y., M.T., R.M., K.O., Y.O., T.C.S. and E.S. jointly analyzed and interpreted the data. K.S., H.S., W.K., K.N., T.S., T.Y., M.T., R.M., K.O., Y.O., T.C.S. and E.S. wrote and edited the manuscript. All authors provided feedback and agreed on the final version of the manuscript.
Peer review
Peer review information
Lab Animal thanks Keith Kazuo Murai, Yuyu Niu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
Raw data were generated at the Central Institute for Experimental Animals and RIKEN. Derived data supporting the findings of this study are available from the corresponding authors on request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Kenya Sato, Hiroki Sasaguri.
Contributor Information
Takaomi C. Saido, Email: takaomi.saido@riken.jp
Erika Sasaki, Email: esasaki@ciea.or.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s41684-024-01424-0.
References
- 1.Sasaki, E. et al. Generation of transgenic non-human primates with germline transmission. Nature459, 523–527 (2009). 10.1038/nature08090 [DOI] [PubMed] [Google Scholar]
- 2.Sato, K. et al. Generation of a nonhuman primate model of severe combined immunodeficiency using highly efficient genome editing. Cell Stem Cell19, 127–138 (2016). 10.1016/j.stem.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 3.Tsukiyama, T. et al. Monkeys mutant for PKD1 recapitulate human autosomal dominant polycystic kidney disease. Nat. Commun.10, 5517 (2019). 10.1038/s41467-019-13398-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou, Y. et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature570, 326–331 (2019). 10.1038/s41586-019-1278-0 [DOI] [PubMed] [Google Scholar]
- 5.Kim, Y. G., Cha, J. & Chandrasegaran, S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc. Natl Acad. Sci. USA93, 1156–1160 (1996). 10.1073/pnas.93.3.1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christian, M. et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics186, 757–761 (2010). 10.1534/genetics.110.120717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle, E. L. et al. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res.40, W117–W122 (2012). 10.1093/nar/gks608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science339, 819–823 (2013). 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science337, 816–821 (2012). 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mali, P. et al. RNA-guided human genome engineering via Cas9. Science339, 823–826 (2013). 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature551, 464–471 (2017). 10.1038/nature24644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature533, 420–424 (2016). 10.1038/nature17946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishida, K. et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science353, aaf8729 (2016). 10.1126/science.aaf8729 [DOI] [PubMed] [Google Scholar]
- 14.Miller, J. C. et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol.29, 143–148 (2011). 10.1038/nbt.1755 [DOI] [PubMed] [Google Scholar]
- 15.Sakuma, T. et al. Repeating pattern of non-RVD variations in DNA-binding modules enhances TALEN activity. Sci. Rep.3, 3379 (2013). 10.1038/srep03379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumita, W. et al. Efficient generation of knock-in/knock-out marmoset embryo via CRISPR/Cas9 gene editing. Sci. Rep.9, 12719 (2019). 10.1038/s41598-019-49110-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson, M. E. et al. High-throughput discovery of novel developmental phenotypes. Nature537, 508–514 (2016). 10.1038/nature19356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winblad, B. et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol.15, 455–532 (2016). 10.1016/S1474-4422(16)00062-4 [DOI] [PubMed] [Google Scholar]
- 19.Crook, R. et al. A variant of Alzheimer’s disease with spastic paraparesis and unusual plaques due to deletion of exon 9 of presenilin 1. Nat. Med.4, 452–455 (1998). 10.1038/nm0498-452 [DOI] [PubMed] [Google Scholar]
- 20.Iwatsubo, T. et al. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ42(43). Neuron13, 45–53 (1994). 10.1016/0896-6273(94)90458-8 [DOI] [PubMed] [Google Scholar]
- 21.Scheuner, D. et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med.2, 864–870 (1996). 10.1038/nm0896-864 [DOI] [PubMed] [Google Scholar]
- 22.Tomita, T. et al. The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid β protein ending at the 42nd (or 43rd) residue. Proc. Natl Acad. Sci. USA94, 2025–2030 (1997). 10.1073/pnas.94.5.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cacace, R., Sleegers, K. & Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement.12, 733–748 (2016). 10.1016/j.jalz.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 24.Prihar, G. et al. Alzheimer disease PS-1 exon 9 deletion defined. Nat. Med.5, 1090 (1999). 10.1038/13383 [DOI] [PubMed] [Google Scholar]
- 25.Thinakaran, G. et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron17, 181–190 (1996). 10.1016/S0896-6273(00)80291-3 [DOI] [PubMed] [Google Scholar]
- 26.Yang, M. et al. Optimized two-step electroporation process to achieve efficient nonviral-mediated gene insertion into primary T cells. FEBS Open Bio12, 38–50 (2022). 10.1002/2211-5463.13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, X. et al. Rapid and highly efficient mammalian cell engineering via Cas9 protein transfection. J. Biotechnol.208, 44–53 (2015). 10.1016/j.jbiotec.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 28.Tu, Z. et al. Promoting Cas9 degradation reduces mosaic mutations in non-human primate embryos. Sci. Rep.7, 42081 (2017). 10.1038/srep42081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su, X. et al. Production of non-mosaic genome edited porcine embryos by injection of CRISPR/Cas9 into germinal vesicle oocytes. J. Genet. Genomics46, 335–342 (2019). 10.1016/j.jgg.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Su, X. et al. Effective generation of maternal genome point mutated porcine embryos by injection of cytosine base editor into germinal vesicle oocytes. Sci. China Life Sci.63, 996–1005 (2020). 10.1007/s11427-019-1611-1 [DOI] [PubMed] [Google Scholar]
- 31.Saito, T. et al. Potent amyloidogenicity and pathogenicity of Aβ43. Nat. Neurosci.14, 1023–1032 (2011). 10.1038/nn.2858 [DOI] [PubMed] [Google Scholar]
- 32.Sasaguri, H. et al. Introduction of pathogenic mutations into the mouse Psen1 gene by base editor and target-AID. Nat. Commun.9, 2892 (2018). 10.1038/s41467-018-05262-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donoviel, D. B. et al. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev.13, 2801–2810 (1999). 10.1101/gad.13.21.2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banas, K. et al. Exon skipping induced by CRISPR-directed gene editing regulates the response to chemotherapy in non-small cell lung carcinoma cells. Gene Ther.29, 357–367 (2022). 10.1038/s41434-022-00324-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chemello, F. et al. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci. Adv.7, eabg4910 (2021). 10.1126/sciadv.abg4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sui, T. et al. CRISPR-induced exon skipping is dependent on premature termination codon mutations. Genome Biol.19, 164 (2018). 10.1186/s13059-018-1532-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uddin, B., Chen, N. P., Panic, M. & Schiebel, E. Genome editing through large insertion leads to the skipping of targeted exon. BMC Genomics16, 1082 (2015). 10.1186/s12864-015-2284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu, X. X. et al. Adenine base-editing-mediated exon skipping induces gene knockout in cultured pig cells. Biotechnol. Lett.44, 59–76 (2022). 10.1007/s10529-021-03214-x [DOI] [PubMed] [Google Scholar]
- 39.Quiroz, Y. T. et al. Brain imaging and blood biomarker abnormalities in children with autosomal dominant Alzheimer disease: a cross-sectional study. JAMA Neurol.72, 912–919 (2015). 10.1001/jamaneurol.2015.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner, H. et al. The biological and pathological function of the presenilin-1 Δexon 9 mutation is independent of its defect to undergo proteolytic processing. J. Biol. Chem.274, 7615–7618 (1999). 10.1074/jbc.274.12.7615 [DOI] [PubMed] [Google Scholar]
- 41.Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med.367, 795–804 (2012). 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Tur, J. et al. A mutation in Alzheimer’s disease destroying a splice acceptor site in the presenilin-1 gene. NeuroReport7, 297–301 (1995). 10.1097/00001756-199512000-00071 [DOI] [PubMed] [Google Scholar]
- 43.Geula, C., Nagykery, N. & Wu, C. K. Amyloid-β deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): incidence and chemical composition. Acta Neuropathol.103, 48–58 (2002). 10.1007/s004010100429 [DOI] [PubMed] [Google Scholar]
- 44.Sakuma, T. et al. Efficient TALEN construction and evaluation methods for human cell and animal applications. Genes Cells18, 315–326 (2013). 10.1111/gtc.12037 [DOI] [PubMed] [Google Scholar]
- 45.Kurotaki, Y. & Sasaki, E. Practical reproductive techniques for the common marmoset. J. Mamm. Ova Res.34, 3–12 (2017). 10.1274/032.034.0103 [DOI] [Google Scholar]
- 46.Takahashi, T. et al. Birth of healthy offspring following ICSI in in vitro-matured common marmoset (Callithrix jacchus) oocytes. PLoS One9, e95560 (2014). 10.1371/journal.pone.0095560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomioka, I., Takahashi, T., Shimada, A., Yoshioka, K. & Sasaki, E. Birth of common marmoset (Callithrix jacchus) offspring derived from in vitro-matured oocytes in chemically defined medium. Theriogenology78, 1487–1493 (2012). 10.1016/j.theriogenology.2012.06.024 [DOI] [PubMed] [Google Scholar]
- 48.Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol.28, 595–599 (2010). 10.1038/nbt.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takabayashi, S., Suzuki, Y. & Katoh, H. Development of a modified artificial insemination technique combining penile vibration stimulation and the swim-up method in the common marmoset. Theriogenology83, 1304–1309.e2 (2015). 10.1016/j.theriogenology.2015.01.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–5 and Tables 1–12.
Raw data and statistical source data of Aβ measurements.
Unprocessed agarose gels.
Unprocessed agarose gel.
Unprocessed western blots.
Raw data of amplicon sequencing using next generation sequencing.
Data Availability Statement
Raw data were generated at the Central Institute for Experimental Animals and RIKEN. Derived data supporting the findings of this study are available from the corresponding authors on request. Source data are provided with this paper.