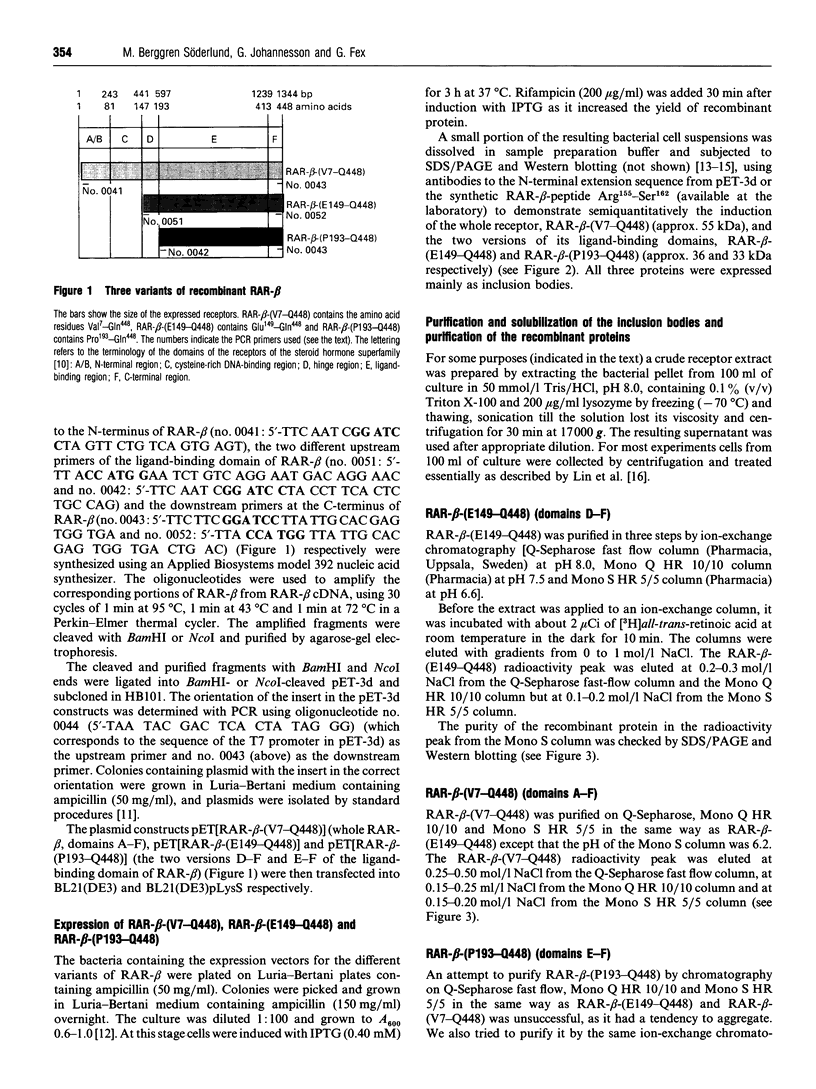
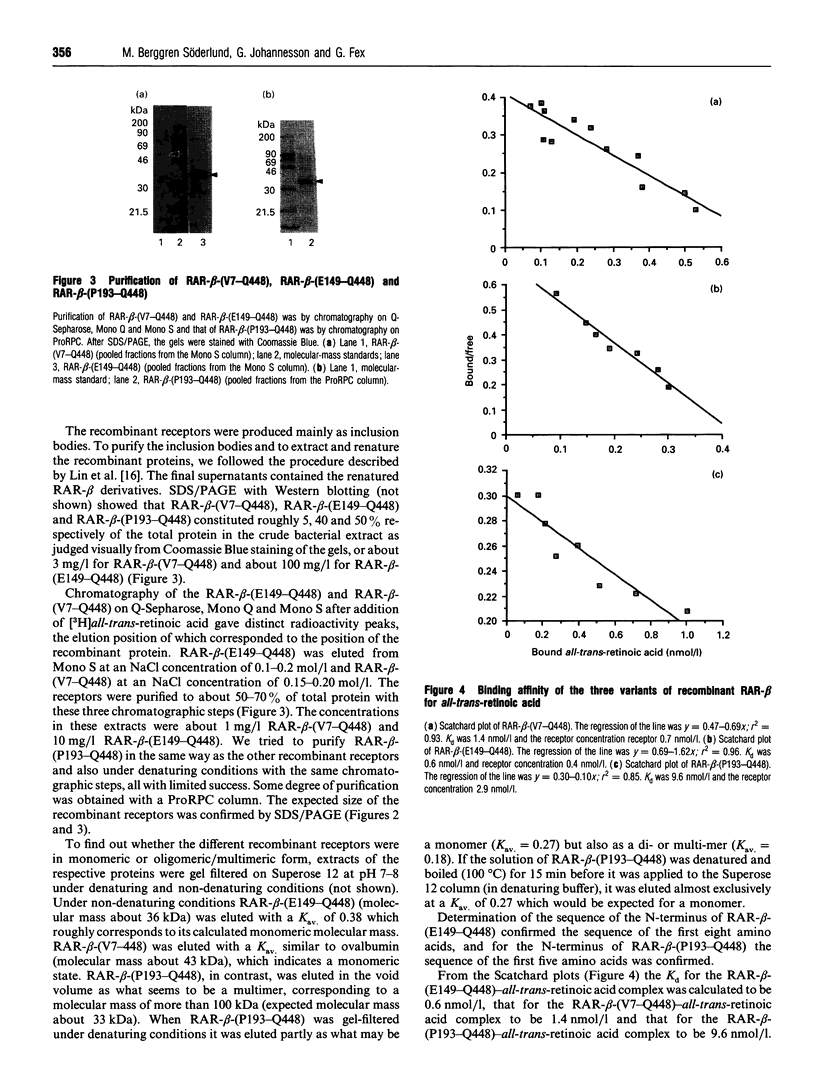
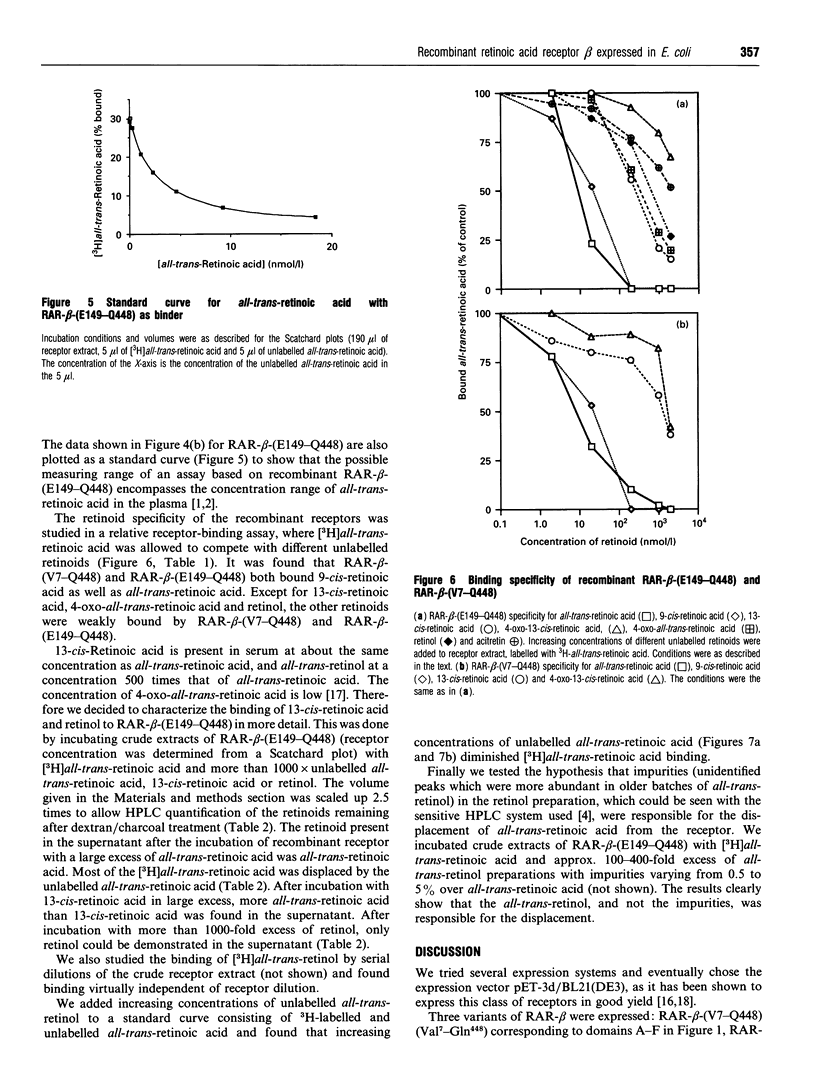
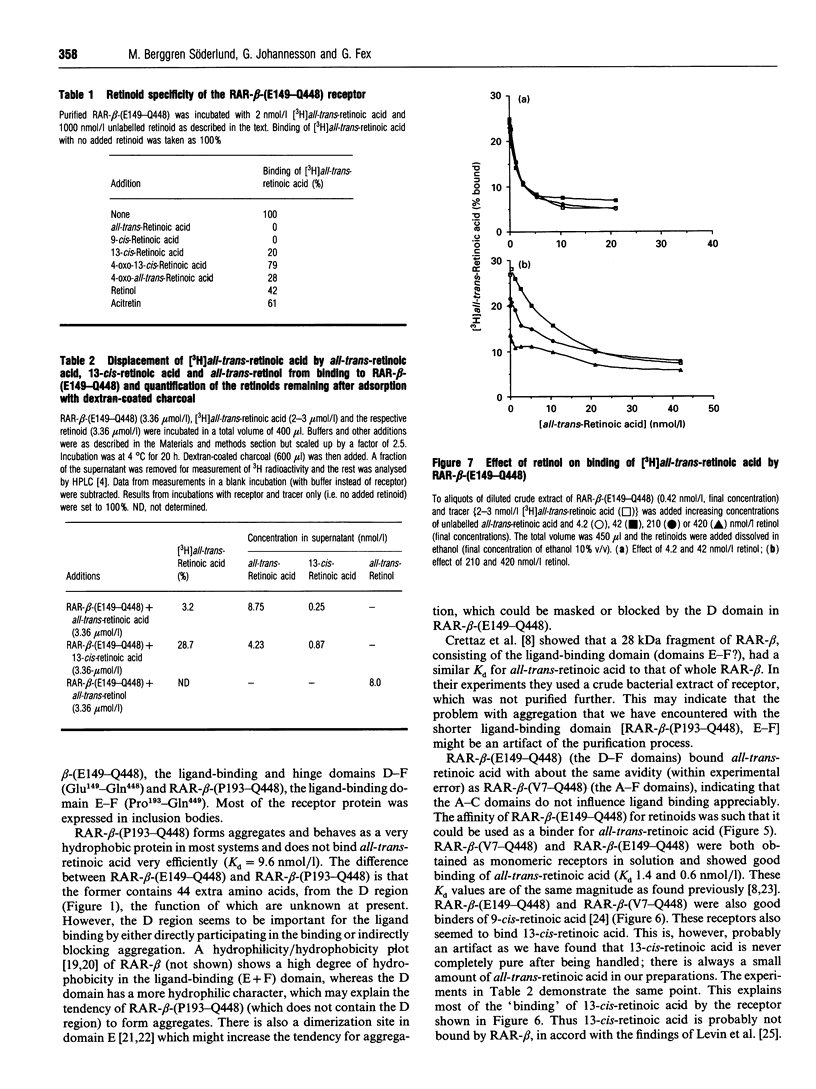
Abstract
all-trans-Retinoic acid, one of the hormonally active derivatives of vitamin A, occurs physiologically in plasma at a concentration below 10 nmol/l. The methods currently used for its quantification are based on HPLC, need about 1 ml of serum, are relatively laborious and thus not well suited for mass analysis. The affinity and specificity of retinoic acid receptors for all-trans-retinoic acid encouraged us to express both the entire human retinoic acid receptor beta (RAR-beta) and two versions of its retinoic acid-binding domain in Escherichia coli in the hope that these recombinant proteins might be used as binders in a ligand-binding assay for all-trans-retinoic acid. The recombinant receptors, the whole receptor [RAR-beta-(V7-Q448)], corresponding to domains A-F, and the ligand-binding domain [RAR-beta-(E149-Q448)], corresponding to domains D-F, were expressed in the vector pET 3d/BL21 (DE3) as inclusion bodies, solubilized with guanidinium chloride, renatured and purified by ion-exchange chromatography. RAR-beta-(P193-Q448), corresponding to domains E-F, was expressed in the vector pET 3d/BL21(DE3)pLysS, and purified by reversed-phase chromatography. Under non-denaturing conditions, the expressed whole receptor [RAR-beta-(V7-Q448)] and the D-F construct (RAR-beta-(E149-Q448)] behaved chromatographically as monomeric proteins whereas the E-F construct [RAR-beta-(P193-Q448)] had a strong tendency to aggregate. RAR-beta-(V7-Q448) and RAR-beta-(E149-Q448) had similar Kd values for all-trans-retinoic acid (1.4 and 0.6 nmol/l respectively) whereas RAR-beta-(P193-Q448) bound all-trans-retinoic acid less avidly (Kd 9.6 nmol/l). 9-cis-Retinoic acid bound to RAR-beta-(E149-Q448) and RAR-beta-(V7-Q448) as avidly as all-trans-retinoic acid. Competition experiments showed weak or no binding of 4-oxo-all-trans-retinoic acid, 4-oxo-13-cis-retinoic acid, 13-cis-retinoic acid, acitretin and retinol by RAR-beta-(E149-Q448).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au-Fliegner M., Helmer E., Casanova J., Raaka B. M., Samuels H. H. The conserved ninth C-terminal heptad in thyroid hormone and retinoic acid receptors mediates diverse responses by affecting heterodimer but not homodimer formation. Mol Cell Biol. 1993 Sep;13(9):5725–5737. doi: 10.1128/mcb.13.9.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Baron A., Siegenthaler G., Hunziker W. Ligand specificities of recombinant retinoic acid receptors RAR alpha and RAR beta. Biochem J. 1990 Dec 1;272(2):391–397. doi: 10.1042/bj2720391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leenheer A. P., Lambert W. E., Claeys I. All-trans-retinoic acid: measurement of reference values in human serum by high performance liquid chromatography. J Lipid Res. 1982 Dec;23(9):1362–1367. [PubMed] [Google Scholar]
- Eckhoff C., Collins M. D., Nau H. Human plasma all-trans-, 13-cis- and 13-cis-4-oxoretinoic acid profiles during subchronic vitamin A supplementation: comparison to retinol and retinyl ester plasma levels. J Nutr. 1991 Jul;121(7):1016–1025. doi: 10.1093/jn/121.7.1016. [DOI] [PubMed] [Google Scholar]
- Eckhoff C., Nau H. Identification and quantitation of all-trans- and 13-cis-retinoic acid and 13-cis-4-oxoretinoic acid in human plasma. J Lipid Res. 1990 Aug;31(8):1445–1454. [PubMed] [Google Scholar]
- Fiorella P. D., Napoli J. L. Expression of cellular retinoic acid binding protein (CRABP) in Escherichia coli. Characterization and evidence that holo-CRABP is a substrate in retinoic acid metabolism. J Biol Chem. 1991 Sep 5;266(25):16572–16579. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidel S., Rupp E., Szardenings M. Recombinant human retinoic acid receptor alpha. Binding of DNA and synthetic retinoids to the protein expressed in Escherichia coli. Eur J Biochem. 1992 Mar 15;204(3):1141–1148. doi: 10.1111/j.1432-1033.1992.tb16739.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin K. H., Fukuda T., Cheng S. Y. Hormone and DNA binding activity of a purified human thyroid hormone nuclear receptor expressed in Escherichia coli. J Biol Chem. 1990 Mar 25;265(9):5161–5165. [PubMed] [Google Scholar]
- Lotan R., Clifford J. L. Nuclear receptors for retinoids: mediators of retinoid effects on normal and malignant cells. Biomed Pharmacother. 1991;45(4-5):145–156. doi: 10.1016/0753-3322(91)90102-y. [DOI] [PubMed] [Google Scholar]
- Perlmann T., Rangarajan P. N., Umesono K., Evans R. M. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993 Jul;7(7B):1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- Pijnappel W. W., Hendriks H. F., Folkers G. E., van den Brink C. E., Dekker E. J., Edelenbosch C., van der Saag P. T., Durston A. J. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature. 1993 Nov 25;366(6453):340–344. doi: 10.1038/366340a0. [DOI] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L., Orf J. W., Hollis B. W. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984 Jan;58(1):91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- Shidoji Y., Hosoya N. Competitive protein-binding radioassay for retinoic acid. Anal Biochem. 1980 May 15;104(2):457–463. doi: 10.1016/0003-2697(80)90099-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tang G. W., Russell R. M. 13-cis-retinoic acid is an endogenous compound in human serum. J Lipid Res. 1990 Feb;31(2):175–182. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss R., Bucheli F. Quantitative analysis of retinoids in biological fluids by high-performance liquid chromatography using column switching. I. Determination of isotretinoin and tretinoin and their 4-oxo metabolites in plasma. J Chromatogr. 1988 Feb 26;424(2):303–314. doi: 10.1016/s0378-4347(00)81107-x. [DOI] [PubMed] [Google Scholar]
- Yang N., Schüle R., Mangelsdorf D. J., Evans R. M. Characterization of DNA binding and retinoic acid binding properties of retinoic acid receptor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3559–3563. doi: 10.1073/pnas.88.9.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]





