Abstract
Objective
Meniscal tears treated with a partial meniscectomy could induce knee osteoarthritis, thereby altering or damaging knee kinetics and biomechanics. We have developed a meniscal scaffold made of polyglycolic acid (PGA) coated with polylactic acid/caprolactone (PGA scaffold), which could induce new tissue growth of meniscus-like tissue. This study aimed to evaluate the safety and efficacy of a novel meniscal scaffold for the treatment of irreparable meniscal injuries.
Design
This study describes the findings of a cyclic torque test and first clinical trial of a PGA scaffold for inducing meniscus-like tissue in humans. As the first step, biomechanical testing of the PGA scaffold was performed using a cyclic torque test. Six patients underwent arthroscopic implantation of the PGA scaffold. Furthermore, the patients underwent preoperative clinical, serological, radiographic, and magnetic resonance imaging examinations at 3, 6, and 12 months postoperatively. The patients also underwent a second-look arthroscopy 12 months after implantation.
Results
Torque increased with increasing cyclic loading. However, no structural damage to the sample was noted after 70,000 loading cycles. All patients showed improvement in pain, Lysholm scores, Tegner activity scores, International Knee Documentation Committee, and knee injury and osteoarthritis outcome. The second-look arthroscopy revealed that meniscal tissue had regenerated in 5 patients (83%). Radiography and magnetic resonance imaging confirmed no progression of degenerative joint disease.
Conclusions
The PGA scaffold could tolerate shear forces, did not produce safety concerns, and may have therapeutic potentials for irreparable meniscal tears in humans.
Keywords: meniscus, tissue scaffold, clinical trial, polyglycolic acid
Introduction
The meniscus plays an essential role in knee function by facilitating force transmission, shock absorption, joint stability, lubrication, and proprioception.1,2 However, loss of meniscal function could occur due to traumatic or degenerative reasons. Several surgical treatments of injured menisci have been reported, including suturing, partial or total meniscectomy, implantation of artificial and allogenic menisci,3,4 and meniscal scaffolds.5-7 Most treatments for meniscal tears involve suturing or excision of the degenerative meniscus. In cases wherein the meniscus could be sutured, the re-rupture rate has been reported to be 15% to 30%.8,9 Notwithstanding, partial meniscectomy could accelerate osteoarthritis (OA) 10 due to the decreased contact area and increased contact pressure.11-14 Preserving or reconstructing the meniscus structure might be an essential factor for knee homeostasis.
In recent years, meniscal scaffolds have been studied for meniscal regeneration using tissue engineering techniques.15-17 A meniscal scaffold could be used for replacing degenerated menisci and preserve the biomechanical properties of the meniscus. Although Zaffagnini et al. 7 reported that treatment with a scaffold had been superior to partial meniscectomy, these scaffolds did not yield long-term regeneration. 18 Several materials have been evaluated as scaffolds for meniscal implants.16,17,19 Bioabsorbable materials are attractive options, particularly in younger patients undergoing meniscectomy. Murakami et al. 20 previously demonstrated that polyglycolic acid (PGA) was a useful material for tissue regeneration of the meniscus and reported the effect of a novel meniscal scaffold made of PGA laminate coated with an L-lactide-ε-caprolactone copolymer P(LA/CL) sponge in a mini pig model. 21 Recently, a clinical trial has been started and introduced this surgical procedure. 22 This study describes the findings of a cyclic torque test and first clinical trial of a PGA scaffold for inducing meniscus-like tissue in humans.
Methods
Study Design
Six consecutive patients who underwent partial meniscectomy followed by meniscal scaffold implantation between May 2021 and January 2022 were included in this case series. The meniscal scaffold was created as previously described.20,21 This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Osaka Medical and Pharmaceutical University (no. 21-3-08-474; date of approval: April 21, 2021). Informed consent was obtained from the patients. The inclusion criteria for the clinical trial were: patients aged between 18 and 60 years with degenerative and irreparable meniscal tears; after partial meniscectomy of degenerated irreparable lesions, the peripheral rim must have been preserved; and the defect size should have been less than one-third of the circumference length because a large defect area might induce instability of the implanted scaffold. Furthermore, the hip-knee-ankle angle should have been within ±3° from the neutral angle since the coronal knee alignment should be normal. The preoperative exclusion criteria were as follows: allergic symptoms such as bronchial asthma and urticaria, obesity, body mass index (BMI) ≥ 30 kg/m2, knee instability with dysfunctional cruciate ligaments, and underwent ipsilateral knee surgery such as ligament repair or osteotomy within 6 months. The intraoperative exclusion criteria were as follows: cartilage damage > 1 cm2 with International Cartilage Research Society (ICRS) classification grade III or higher.
Fabrication of PGA Scaffold
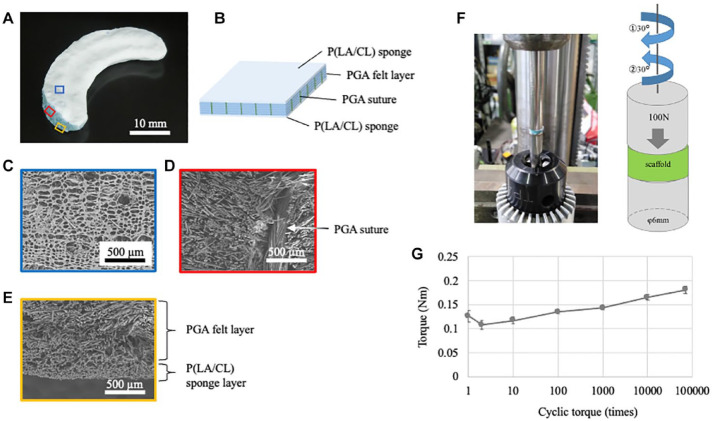
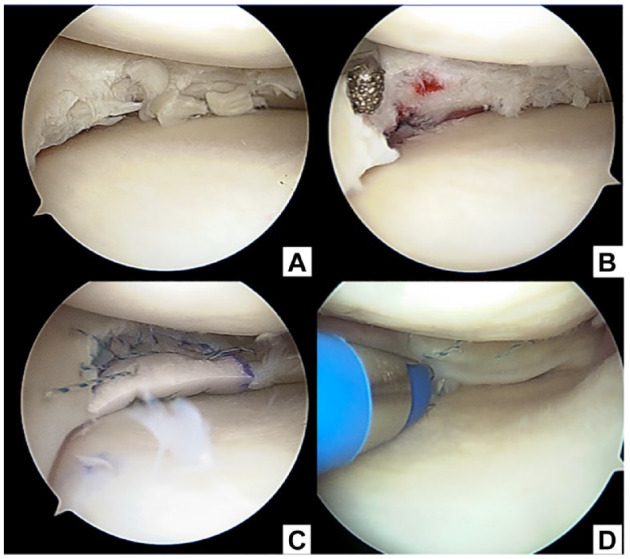
The PGA scaffold was set using the PGA laminate coated with a P (LA/CL) sponge ( Fig. 1A and B ). The PGA laminate was fabricated by stacking PGA non-woven fabrics (NEOVEIL®; Gunze Medical Ltd., Osaka, Japan) and securing them with 4-0 PGA sutures (Gunze Medical Ltd.). The PGA laminate was then immersed in a solution of the copolymer (polyester with a 50:50 molar composition of lactate and ε-caprolactone) in 1,4-dioxane and freeze-dried to develop a sponge texture. The scaffold was trimmed to form a final C-shaped scaffold, with the average size of the medial meniscus as that of the human specimen: diameter, 45 mm; central body width, 14 mm; and maximum thickness, 7 mm.23,24 Electron microscopy was used for showing the details of the PGA scaffold ( Fig. 1C-E ).
Figure 1.
PGA scaffold and torque test. PGA = polyglycolic acid.
Shear Properties of the PGA Scaffold
To detect shear stress, we performed a cyclic torque test using a modified torque machine (NITTOSEIKO Co., Ltd., Kyoto, Japan). The test pieces were prepared by punching the PGA scaffold using a φ6 mm biopsy punch (Kai Industries Co., Ltd., Gifu, Japan). The sample was set in a φ6 mm cylindrical jig. Under a load of 100 N, it was pre-rotated 15° counterclockwise. Thereafter, 30° clockwise and 30° counterclockwise movements were repeated 70,000 times. The torque value was defined as the difference between the maximum and minimum values in 1 cycle ( Fig. 1F ).
Surgical Technique
The surgical technique for scaffold implantation has been previously described. 22 Briefly, an arthroscopic view from the anterolateral portal was obtained for diagnostic evaluation to ensure an irreparable degenerative meniscus. The degenerated meniscus was subjected to partial meniscectomy and the defect area was measured. The meniscal scaffold was trimmed by 1 to 2 mm larger than the defect area to ensure sufficient contact with the native meniscus and brought through the anteromedial portal of the arthroscopy. The scaffold was fixed next to the native meniscus using the inside-out technique with a double-armed meniscus needle® (CONMED, NY). The cartilage, synovium, and meniscus were evaluated using ICRS score, Klint classification, 25 and International Society of Arthroscopy, Knee Surgery and Orthopedic Sports Medicine (ISAKOS) classification, 26 respectively. A second-look arthroscopy was performed 12 months after the initial surgery to evaluate synovitis, cartilage, and meniscus-like tissue. 27 Furthermore, quantitative analysis was performed as described by Sekiya et al. 28
Postoperative Rehabilitation
The patients underwent postoperative rehabilitation referred to as the rehabilitation program of the previously described scaffold.6,18,29 The range of motion (ROM) was then increased to 60° during the second week. At week 5, an ROM of 0° to 90° was permitted, and passive motion was allowed starting at week 6. The patients were instructed to avoid weightbearing for 3 weeks. After this period, progressive weightbearing was encouraged. At week 8, full and unrestricted weightbearing was permitted. Quadriceps isometric exercises were performed starting on the second postoperative day. Walking was allowed after 8 weeks within 2,500 steps/day. Return to sports and cutting activity were permitted 6 months after surgery.
Safety and Outcome Measurements
To evaluate the safety of the meniscal scaffold, C-reactive protein (CRP) and white blood cell (WBC) count analysis were performed to evaluate the safety and inflammation based on preoperative blood examination to postoperative blood examination at 12 months. Clinical outcomes, including the International Knee Documentation Committee (IKDC), knee injury and osteoarthritis outcome (KOOS), Lysholm, visual analog scale (VAS), and knee ROM were identified preoperatively and postoperatively at weeks 1 and 2 and at 1, 2, 3, 6 and 12 months. In addition, the Tegner activity level was evaluated preoperatively and after the implantation surgery until 12 months.
Radiographic Analysis
Knee x-ray in the standing position was performed for assessing OA progression after implantation. Meniscus-like tissue and accumulation of joint fluid after scaffold implantation were evaluated with magnetic resonance imaging (MRI) using a high-field MRI machine with a knee coil (3 Tesla, Philips) from the preoperative to the postoperative period of 12 months. T1- and T2-weighted images and proton density-weighted images optimized for human knee imaging in the sagittal, axial, and coronary-angled directions were obtained. The slice thickness was 0.72 mm without any gaps. The MRI images of the knee joints were evaluated by 2 orthopedic surgeons and a radiologist based on the description by Efe et al. 30 With respect to the preoperative tear pattern, meniscal evaluation was performed based on the methods of the ISAKOS classification. 26 The researchers assessed the images together when their individual evaluations were not in agreement. The volume of joint fluid was evaluated as the sum of 3 consecutive slices, which were selected based on the highest intensity on sagittal short T inversion recovery.
Statistical Analysis
Data were expressed as mean ± standard deviation. The statistical significance of the results was determined using a 2-tailed Student’s t test between the preoperative and postoperative groups or the Mann-Whitney test and repeated-measures tests with Bonferroni correction. All the data analyses were performed using JMP Pro version 15.1.0 (SAS 196 Institute Japan, Tokyo, Japan). The differences between means were considered statistically significant at P < 0.05.
Results
Regarding the biomechanical analysis of the scaffold, a cyclic torque test was performed. The torque value gradually increased with cyclic loading. However, there was no structural damage to the sample after 70,000 cycles ( Fig. 1G ).
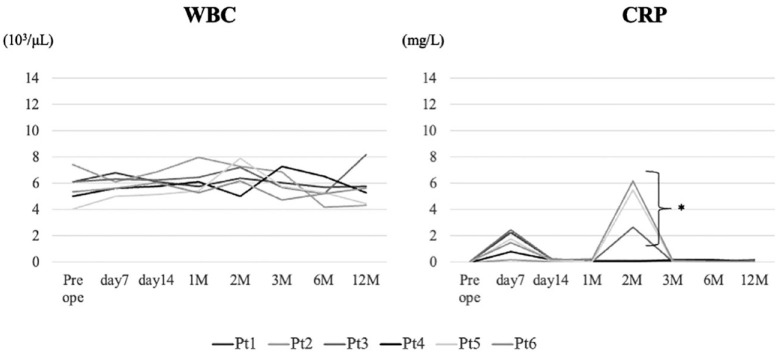
In the current clinical trial, 4 male and 2 female participants with a mean age of 41.0 ± 10.2 years (range: 30-59 years) were included. The median BMI was 23.0 ± 3.3 kg/m2 (range: 19.4-27.5 kg/m2). Examinations of WBC count and CRP level were performed to evaluate inflammation after implantation ( Fig. 2 ). WBC was within the normal range, and CRP had increased in 50% of the patients at 2 months postoperatively; after that, it was within the normal range ( Fig. 2 ).
Figure 2.
Inflammatory changes. WBC count and CRP levels are evaluated from preoperative to 12 months postoperatively: in this area. Three cases show increased CRP level 2 months after implantation; however, all cases demonstrate CRP levels within the normal range until 12 months. WBC = white blood cell; CRP = C-reactive protein.
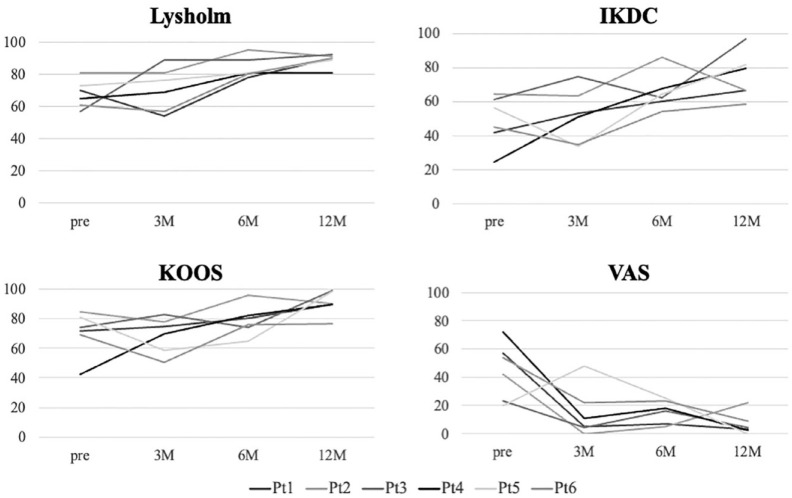
All patients showed improved clinical outcomes with Lysholm score, IKDC, KOOS, and VAS ( Fig. 3 ). Notably, VAS was significantly decreased at 12 months after implantation.
Figure 3.
Changes in clinical outcomes. Lysholm score and patient-reported outcome measures (IKDC, KOOS, and VAS) are evaluated and improvement is observed. IKDC = International Knee Documentation Committee; KOOS = Knee Osteoarthritis and Outcome Score; VAS = visual analogue scale.
Except KOOS and VAS, the clinical outcome measures improved within 6 months, and all outcomes improved at 12 months postimplantation (P < 0.05, Table 1 ).
Table 1.
Changes in Clinical Outcome After Scaffold Implantation.
| Preoperative | Postoperative 6 Months |
P Value (Preope vs. 6 Months) |
Postoperative 12 Months |
P Value (Preope vs. 12 Months) |
|
|---|---|---|---|---|---|
| Lysholm | 67.8 ± 8.7 | 84.0 ± 6.6 | 0.007 | 88.8 ± 4.0 | 0.003 |
| IKDC | 48.7 ± 15.0 | 65.7 ± 11.1 | 0.039 | 74.9 ± 13.7 | 0.017 |
| KOOS | 70.4 ± 15.0 | 79.0 ± 10.2 | 0.304 | 90.7 ± 8.0 | 0.021 |
| VAS | 41.3 ± 26.0 | 15.7 ± 8.2 | 0.089 | 6.7 ± 8.1 | 0.022 |
IKDC = International Knee Documentation Committee; KOOS = Knee Osteoarthritis and Outcome Score; VAS = visual analogue scale.
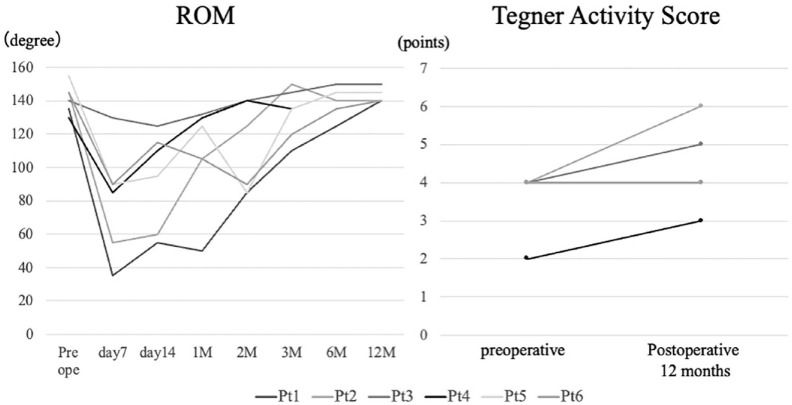
ROM recovered to the preoperative level at 12 months after implantation. In addition, the Tegner activity score improved in 3 cases and remained unchanged in the other 3 cases ( Fig. 4 ).
Figure 4.
ROM and Tegner activity score after implantation. ROM = range of motion.
No OA progression was found during the follow-up period based on x-ray examination. The implant interface, morphology, and bone bruise did not alter in the postoperative MRI findings ( Table 2 ). However, the scaffold signal changed from iso- to low-intensity.
Table 2.
MRI Analysis at 3, 6, and 12 Months After Implantation.
| Implant Interface |
Implant Morphology |
Bone Bruise |
Scaffold Signal Intensity |
|||||
|---|---|---|---|---|---|---|---|---|
| C | S | C | S | MF | MT | C | S | |
| 3 months | ||||||||
| Case 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 2 |
| Case 2 | 0 | 0 | 3 | 1 | 1 | 0 | X | 1 |
| Case 3 | 0 | 0 | 2a | 1 | 0 | 0 | 1 | 1 |
| Case 4 | 0 | 0 | 2a | 1 | 0 | 0 | 1 | 1 |
| Case 5 | 0 | 0 | 2a | 1 | 0 | 0 | 1 | 1 |
| Case 6 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 |
| 6 months | ||||||||
| Case 1 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 2 |
| Case 2 | 0 | 0 | 3 | 1 | 1 | 0 | X | 1 |
| Case 3 | 0 | 0 | 2a | 1 | 0 | 0 | 1 | 2 |
| Case 4 | 0 | 0 | 2a | 2a | 0 | 0 | 1 | 2 |
| Case 5 | 0 | 0 | 2a | 1 | 0 | 0 | 1 | 1 |
| Case 6 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 |
| 12 months | ||||||||
| Case 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 |
| Case 2 | 0 | 0 | 3 | 1 | 1 | 0 | X | 1 |
| Case 3 | 0 | 0 | 2a | 1 | 0 | 0 | 1 | 2 |
| Case 4 | 0 | 0 | 2a | 2a | 0 | 0 | 2 | 2 |
| Case 5 | 0 | 0 | 2a | 1 | 0 | 0 | 1 | 0 |
| Case 6 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
C = coronal; S = sagittal; MF = medial femoral condyle; MT = medial tibial plateau.
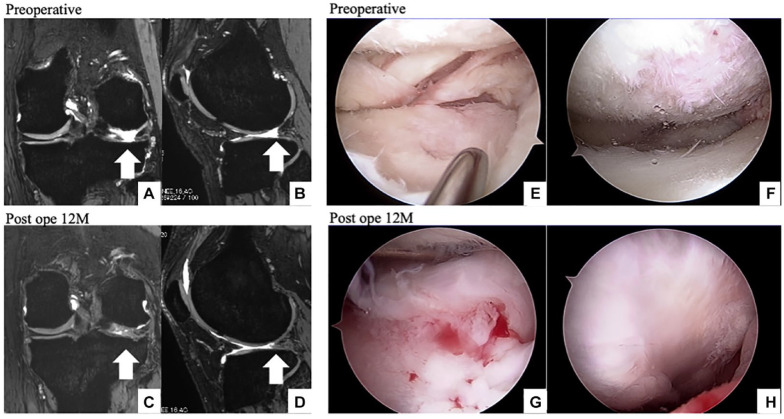
The second-look arthroscopy revealed that meniscal tissue had regenerated in 5 patients (83%). A representative image is shown in Figure 5 . This is case 1 and degenerated meniscus was detected on preoperative arthroscopy ( Fig. 5A ), partial meniscectomy was performed, and implants were placed ( Fig. 5B and C ). The meniscus-like tissue regenerated on second-look arthroscopy ( Fig. 5D ).
Figure 5.
Arthroscopic findings of medial meniscus degeneration in a 44-year-old patient (case 1). Initial arthroscopy (A-C) and second-look arthroscopy (D) are shown. The irreparable meniscal tear is detected, and a partial meniscectomy (A) and rasping are performed (B). A polyglycolic acid scaffold is implanted (C). The implanted meniscal scaffold is replaced with meniscus tissue at 12 months postoperatively (D).
Another example of cartilage-like tissue is shown in Figure 6 (case 6). Partial meniscectomy at the lateral meniscus was performed 14 years prior, in which an MRI of the preoperative knee is shown in Figure 6A and B. The defect area occupied the high-intensity soft tissue 12 months after implantation ( Fig. 6C and D ). Preoperative arthroscopy revealed a loss of the meniscus at the posterolateral corner with cartilage degeneration ( Fig. 6 and F ). Second-look arthroscopy revealed new soft meniscus- and cartilage-like tissues ( Fig. 6G and H ).
Figure 6.
Scaffold implantation after lateral meniscectomy (case 6). A 30-year-old male patient undergoes partial meniscectomy at the lateral posterior horn at the age of 17 years. The meniscus at the lateral posterior corner, which is lost, is seen on the MRI (A, B), and cartilage degeneration was detected during the initial arthroscopy (E, F). After scaffold implantation, the lateral meniscus is covered with new meniscus-like tissue, as seen on the MRI (C, D). Growth of new soft tissue at the lateral posterior corner and improvement in cartilage fibrillation is confirmed during the second-look arthroscopy (G, H). MRI = magnetic resonance imaging.
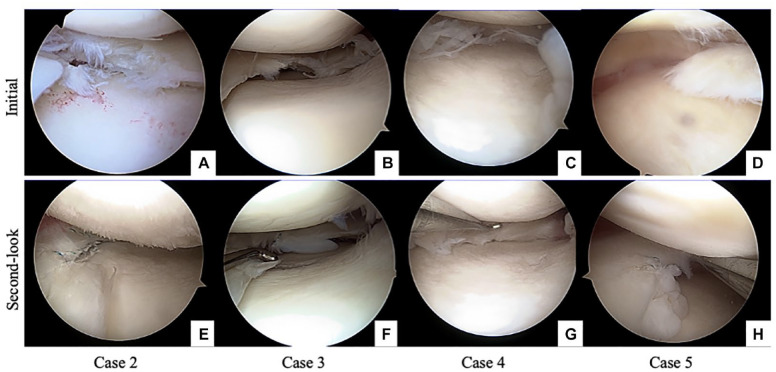
Initial arthroscopy and second-look arthroscopy of the other 4 cases are shown in Figure 7A-H . The results of the quantitative analysis are presented in Table 3 . Second-look arthroscopy showed better scores relative to those of initial arthroscopy ( Table 3 , P < 0.01). The scores for synovitis, cartilage degeneration, and meniscal repair are shown. Five participants demonstrated good or excellent meniscal repair. However, 1 patient was reduced in size, and another demonstrated cheese wiring at suture fixation (Supplemental Table S1).
Figure 7.
Initial arthroscopy and second-look arthroscopy of the other 4 cases are shown. Case 2 shows degenerative medial meniscus in panel A and implanted scaffold is absorbed on the second-look arthroscopy, indicating poor meniscal repair (E). Case 3 shows degenerative medial meniscus in panel B and panel F shows new soft tissue at the implanted area. However, cheese-cut wiring is found. In case 4, the degenerative medial meniscus is shown in panel C and preserved meniscus-like tissue in panel G. Case 5 shows a degenerative flap tear in panel D; new meniscus-like tissue is observed in panel H.
Table 3.
Evaluation of Initial Arthroscopy and Second-Look Arthroscopy.
| Initial |
Second-Look |
|||||||
|---|---|---|---|---|---|---|---|---|
| Presence | Stability | Smoothness | Total | Presence | Stability | Smoothness | Total | |
| Case 1 | 1 | 0 | 0 | 1 | 2 | 2 | 2 | 6 |
| Case 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Case 3 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 4 |
| Case 4 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 5 |
| Case 5 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 5 |
| Case 6 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 5 |
| Mean ± SD | 0.67 ± 0.52 | 4.2 ± 2.1 | ||||||
| P value | 0.0030 | |||||||
Scoring was referred by Sekiya et al. Paired t test was used for statistical analysis.
Discussion
The main finding of this study was that the PGA meniscal scaffold, which tolerated over 70,000 times of share force testing, successfully achieved the primary endpoint of safety, based on blood examination and MRI findings in the clinical trial. Furthermore, the ROM was preserved or improved in all patients whose clinical outcomes were generally favorable. Second-look arthroscopy was performed in all patients at 12 months after the initial implantation; it was observed that the scaffold had facilitated the growth of autologous meniscus-like tissue in 5 of the 6 cases.
The current PGA scaffold tolerated over 70,000 torques and might allow at least 8 weeks of 2,500 steps/day based on the in vivo analysis results. This might be useful information for considering the level of walking during rehabilitation program after allowing full weightbearing permission However, in only 1 case, the tissue was lost at implanted area, which might be an indication for paying more careful attention before allowing increases in activity level in the future trial.
The main functions of the meniscus are shock absorption and stability, and the purpose of meniscal repair was to restore hoop strength to its native size. 11 Meniscal allograft transplantation is a reasonable treatment option for symptomatic meniscal deficiency. Fresh-frozen or deep-frozen grafts, despite their low cell viability, have commonly been used with good success in both total 31 and segmental 32 meniscus allografts. However, the use of allografts for meniscus repair is not recommended due to insufficient recellularization and stiffness of the graft. 33 Meniscal scaffolds are an attractive treatment option due to low immunogenicity, low infection rates, and the ability to reconstruct the size of the native meniscus. Developing a reliable meniscal scaffold for treating irreparable and degenerative meniscal injuries has been a major challenge for the medical community. However, considerable progress has been made over the last 20 years in establishing novel therapeutic approaches.15,34 Considering clinical use, meniscal scaffolds come in 2 varieties: collagen-based, such as collagen meniscal implants (CMI; Ivy Sports Medicine, Grafelfing, Germany),5,7,18,19,29 and polyurethane-based (Actifit; Orteq Ltd., London, UK).6,30,35 Although both have demonstrated good clinical outcomes 36 and mean 10 years clinical follow-up 37 , they showed variable failure rates ranging between 0 and 38% at 4 years. 38 No data are available on the shear force of the 2 available scaffolds. In addition, the current study indicated that the PGA coated with P(LA/CL) scaffold could bear shear force over 70,000 torques. The current scaffold had shown proved biomechanical strength regarding its compressive forces.13,20 Furthermore, this current scaffold had also been shown to be completely bioabsorbable based on proof-of-concept studies in rabbit and porcine models.20,21 Recently, the surgical technique for implantation by arthroscopy and the appropriate implant size have been established to achieve better clinical outcomes.13,22 This study was human clinical trial of meniscal scaffolds and demonstrated the safety of the implant. Furthermore, it may provide another option for the surgical treatment of irreparable meniscal injury.
However, 1 participant had a reduced meniscal size after implantation, and another experienced suture rupture. These cases might have been affected by the rehabilitation protocol and size of the implanted scaffold. One patient started some exercises 2 months after implantation, and the other might have had increased suture tightness. Sezaki et al. 13 reported better outcomes of a novel meniscal scaffold regarding compression force distribution compared with partial meniscectomy. ROM exercises may induce suture rupture in cases of same-size implantation as well as the defect size. Strict rehabilitation program and appropriate size of scaffold implantation might be needed to prevent failure after implantation. Despite this, this novel PGA scaffold may provide a new therapeutic option for irreparable meniscal tears and degeneration.
This study has several limitations. This case series included a small sample size with short follow-up. The long-term results were less predictable. Meniscal scaffolds have been reported to be non-functional, owing to fragmentation, shrinkage, and extrusion, which result in a failure in increasing the articular cartilage coverage, reducing the peak pressure, and achieving a balanced load distribution. 32 Preservation of the peripheral meniscus is an essential indication for this surgery as the hoop tension does not reach sufficient strength for scaffold implantation. Additional treatment may be required in cases of meniscal extrusion with peripheral reconstruction. 39 Although more issues may appear in meniscal scaffold implantation, appropriate surgical techniques with strict indications may induce better long-term outcomes after scaffold implantation.
To conclude, a novel meniscal scaffold, made of PGA coated with polylactate and caprolactone, achieved safe postoperative ROM and clinical outcomes at 12-months follow-up. Furthermore, the scaffold was replaced with autologous tissue based on the second-look arthroscopy. Although additional studies are required, this novel scaffold may provide an additional surgical option for irreparable meniscal injuries.
Supplemental Material
Supplemental material, sj-docx-1-car-10.1177_19476035231193087 for Safety and Efficacy of a Novel Polyglycolic Acid Meniscal Scaffold for Irreparable Meniscal Tear by Shuhei Otsuki, Shunsuke Sezaki, Yoshinori Okamoto, Takashi Ishitani, Hitoshi Wakama and Masashi Neo in CARTILAGE
Footnotes
Acknowledgments and Funding: The authors thank Yoshie Seki and Noriko Kinoshita for their expert technical assistance. We thank Editage for English language editing. The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Shuhei Otsuki  https://orcid.org/0000-0002-2752-8484
https://orcid.org/0000-0002-2752-8484
Masashi Neo  https://orcid.org/0000-0001-9208-6419
https://orcid.org/0000-0001-9208-6419
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
References
- 1. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975;109:184-92. [DOI] [PubMed] [Google Scholar]
- 3. Verdonk PC, Verstraete KL, Almqvist KF, De Cuyper K, Veys EM, Verbruggen G, et al. Meniscal allograft transplantation: long-term clinical results with radiological and magnetic resonance imaging correlations. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):694-706. [DOI] [PubMed] [Google Scholar]
- 4. Zaslav KR, Farr J, Alfred R, Alley RM, Dyle M, Gomoll AH, et al. Treatment of post-meniscectomy knee symptoms with medial meniscus replacement results in greater pain reduction and functional improvement than non-surgical care. Knee Surg Sports Traumatol Arthrosc. 2022;30(4):1325-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold. J Bone Joint Surg Am. 1997;79(12):1770-7. [DOI] [PubMed] [Google Scholar]
- 6. Verdonk R, Verdonk P, Huysse W, Forsyth R, Heinrichs EL. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am J Sports Med. 2011;39(4):774-82. [DOI] [PubMed] [Google Scholar]
- 7. Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, Bruni D, Giordano G, Ravazzolo G, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977-85. [DOI] [PubMed] [Google Scholar]
- 8. Paxton ES, Stock MV, Brophy RH. Meniscal repair versus partial meniscectomy: a systematic review comparing reoperation rates and clinical outcomes. Arthroscopy. 2011;27(9):1275-88. [DOI] [PubMed] [Google Scholar]
- 9. Schweizer C, Hanreich C, Tscholl PM, Ristl R, Apprich S, Windhager R, et al. Nineteen percent of meniscus repairs are being revised and failures frequently occur after the second postoperative year: a systematic review and meta-analysis with a minimum follow-up of 5 years. Knee Surg Sports Traumatol Arthrosc. 2022;30(7):2267-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masaracchio MF, Kirker K, Loghmani P, Gramling J, Mattia M, States R. The prevalence of tibiofemoral knee osteoarthritis following arthroscopic partial meniscectomy is variably reported in general, and over time: a systematic review with a minimum of 5-year follow-up. Arthrosc Sports Med Rehabil. 2022;25:e1203-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beamer BS, Walley KC, Okajima S, Manoukian OS, Perez-Viloria M, DeAngelis JP, et al. Changes in contact area in meniscus horizontal cleavage tears subjected to repair and resection. Arthroscopy. 2017;33(3):617-24. [DOI] [PubMed] [Google Scholar]
- 12. Koh JL, Yi SJ, Ren Y, Zimmerman TA, Zhang LQ. Tibiofemoral contact mechanics with horizontal cleavage tear and resection of the medial meniscus in the human knee. J Bone Joint Surg Am. 2016;98:1829-36. [DOI] [PubMed] [Google Scholar]
- 13. Sezaki S, Otsuki S, Ikeda K, Ishitani T, Okamoto Y, Wakama H, et al. Biomechanical assessment of a novel meniscal scaffold compared to partial meniscectomy: a study on porcine meniscal injury. J Biomed Mater Res B Appl Biomater. 2023;111(4):895-902. [DOI] [PubMed] [Google Scholar]
- 14. Sezaki S, Otsuki S, Ikeda K, Okuno N, Okamoto Y, Wakama H, et al. Development of a pressure-sensitive conductive rubber sensor for analyzing meniscal injury in porcine models. Appl Bionics Biomech. 2021;2021:4931092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peng Y, Lu M, Zhou Z, Wang C, Liu E, Zhang Y, et al. Natural biopolymer scaffold for meniscus tissue engineering. Front Bioeng Biotechnol. 2022;10:1003484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rongen JJ, van Tienen TG, van Bochove B, Grijpma DW, Buma P. Biomaterials in search of a meniscus substitute. Biomaterials. 2014;35(11):3527-40. [DOI] [PubMed] [Google Scholar]
- 17. Shimomura K, Rothrauff BB, Hart DA, Hamamoto S, Kobayashi M, Yoshikawa H, et al. Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell-derived tissue engineered construct. Biomaterials. 2019;192:346-54. [DOI] [PubMed] [Google Scholar]
- 18. Lucidi GA, Grassi A, Agostinone P, Di Paolo S, Dal Fabbro G, D’Alberton C, et al. Risk factors affecting the survival rate of collagen meniscal implant for partial meniscal deficiency: an analysis of 156 consecutive cases at a mean 10 years of follow-up. Am J Sports Med. 2022;50(11):2900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stone KR, Rodkey WG, Webber R, McKinney L, Steadman JR. Meniscal regeneration with copolymeric collagen scaffolds: in vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am J Sports Med. 1992;20(2):104-11. [DOI] [PubMed] [Google Scholar]
- 20. Murakami T, Otsuki S, Nakagawa K, Okamoto Y, Inoue T, Sakamoto Y, et al. Establishment of novel meniscal scaffold structures using polyglycolic and poly-l-lactic acids. J Biomater Appl. 2017;32(2):150-61. [DOI] [PubMed] [Google Scholar]
- 21. Otsuki S, Nakagawa K, Murakami T, Sezaki S, Sato H, Suzuki M, et al. Evaluation of meniscal regeneration in a mini pig model treated with a novel polyglycolic acid meniscal scaffold. Am J Sports Med. 2019;47(8):1804-15. [DOI] [PubMed] [Google Scholar]
- 22. Otsuki S, Ikeda K, Tanaka K, Okamoto Y, Sezaki S, Neo M. Implantation of novel meniscus scaffold for irreparable meniscal tear. Arthrosc Tech. 2022;11(5):e775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDermott ID, Sharifi F, Bull AM, Gupte CM, Thomas RW, Amis AA. An anatomical study of meniscal allograft sizing. Knee Surg Sports Traumatol Arthrosc. 2004;12(2):130-5. [DOI] [PubMed] [Google Scholar]
- 24. Takroni T, Laouar L, Adesida A, Elliott JA, Jomha NM. Anatomical study: comparing the human, sheep and pig knee meniscus. J Exp Orthop. 2016;3(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. af Klint E, Catrina AI, Matt P, Neregråd P, Lampa J, Ulfgren AK, et al. Evaluation of arthroscopy and macroscopic scoring. Arthritis Res Ther. 2009;11(3):R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson AF, Irrgang JJ, Dunn W, Beaufils P, Cohen M, Cole BJ, et al. Interobserver reliability of the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) classification of meniscal tears. Am J Sports Med. 2011;39(5):926-32. [DOI] [PubMed] [Google Scholar]
- 27. Horibe S, Shino K, Maeda A, Nakamura N, Matsumoto N, Ochi T. Results of isolated meniscal repair evaluated by second-look arthroscopy. Arthroscopy. 1996;12(2):150-5. [DOI] [PubMed] [Google Scholar]
- 28. Sekiya I, Koga H, Katano H, Mizuno M, Kohno Y, Otabe K, et al. Second-look arthroscopy after meniscus repair and synovial mesenchymal stem cell transplantation to treat degenerative flaps and radial tears of the medial meniscus: a case report. J Orthop Sci. 2022;27(4):821-34. [DOI] [PubMed] [Google Scholar]
- 29. Rodkey WG, DeHaven KE, Montgomery WH, III, Baker CL, Jr, Beck CL, Jr, Hormel SE, et al. Comparison of the collagen meniscus implant with partial meniscectomy: a prospective randomized trial. J Bone Joint Surg Am. 2008;90(7):1413-26. [DOI] [PubMed] [Google Scholar]
- 30. Efe T, Getgood A, Schofer MD, Fuchs-Winkelmann S, Mann D, Paletta JR, et al. The safety and short-term efficacy of a novel polyurethane meniscal scaffold for the treatment of segmental medial meniscus deficiency. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1822-30. [DOI] [PubMed] [Google Scholar]
- 31. Rodeo SA, Seneviratne A, Suzuki K, Felker K, Wickiewicz TL, Warren RF. Histological analysis of human meniscal allografts. A preliminary report. J Bone Joint Surg Am. 2000;82(8):1071-82. [DOI] [PubMed] [Google Scholar]
- 32. Seiter MN, Haber DB, Ruzbarsky JJ, Arner JW, Peebles AM, Provencher MT. Segmental meniscus allograft transplantation. Arthrosc Tech. 2021;10(3):e697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gelse K, Körber L, Schöne M, Raum K, Koch P, Pachowsky M, et al. Transplantation of chemically processed decellularized meniscal allografts: a pilot sheep study. Cartilage. 2017;8:180-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Veronesi F, Di Matteo B, Vitale ND, Filardo G, Visani A, Kon E, et al. Biosynthetic scaffolds for partial meniscal loss: a systematic review from animal models to clinical practice. Bioact Mater. 2021;6(11):3782-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Toanen C, Dhollander A, Bulgheroni P, Filardo G, Zaffagnini S, Spalding T, et al. Polyurethane meniscal scaffold for the treatment of partial meniscal deficiency: 5-year follow-up outcomes: a European multicentric study. Am J Sports Med. 2020;48(6):1347-55. [DOI] [PubMed] [Google Scholar]
- 36. Reale D, Previtali D, Andriolo L, Grassi A, Candrian C, Zaffagnini S, et al. No differences in clinical outcome between CMI and Actifit meniscal scaffolds: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2022;30(1):328-48. [DOI] [PubMed] [Google Scholar]
- 37. Reale D, Lucidi GA, Grassi A, Poggi A, Filardo G, Zaffagnini S. A comparison between polyurethane and collagen meniscal scaffold for partial meniscal defects: similar positive clinical results at a mean of 10 years of follow-up. Arthroscopy. 2022;38(4):1279-87. [DOI] [PubMed] [Google Scholar]
- 38. Houck DA, Kraeutler MJ, Belk JW, McCarty EC, Bravman JT. Similar clinical outcomes following collagen or polyurethane meniscal scaffold implantation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2018;26(8):2259-69. [DOI] [PubMed] [Google Scholar]
- 39. Koga H, Muneta T, Watanabe T, Mochizuki T, Horie M, Nakamura T, et al. Two-year outcomes after arthroscopic lateral meniscus centralization. Arthroscopy. 2016;32(10):2000-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-car-10.1177_19476035231193087 for Safety and Efficacy of a Novel Polyglycolic Acid Meniscal Scaffold for Irreparable Meniscal Tear by Shuhei Otsuki, Shunsuke Sezaki, Yoshinori Okamoto, Takashi Ishitani, Hitoshi Wakama and Masashi Neo in CARTILAGE