Abstract
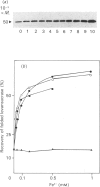
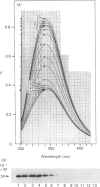
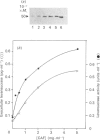
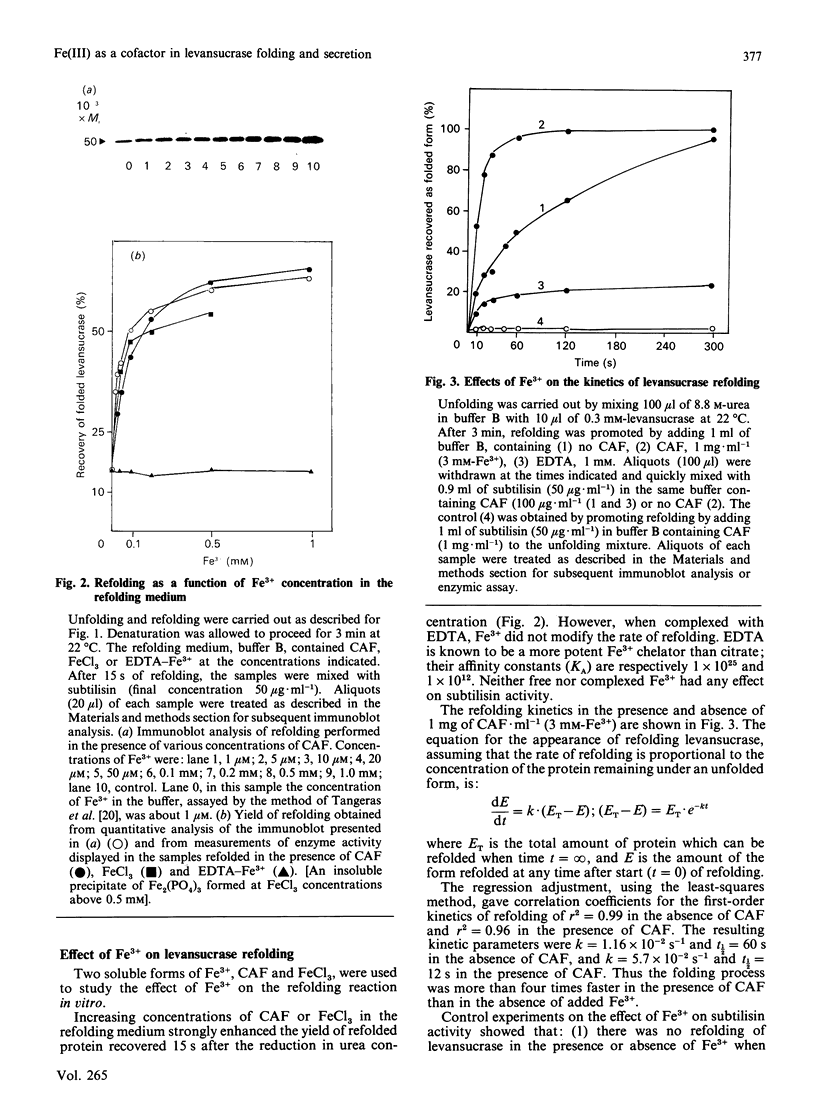
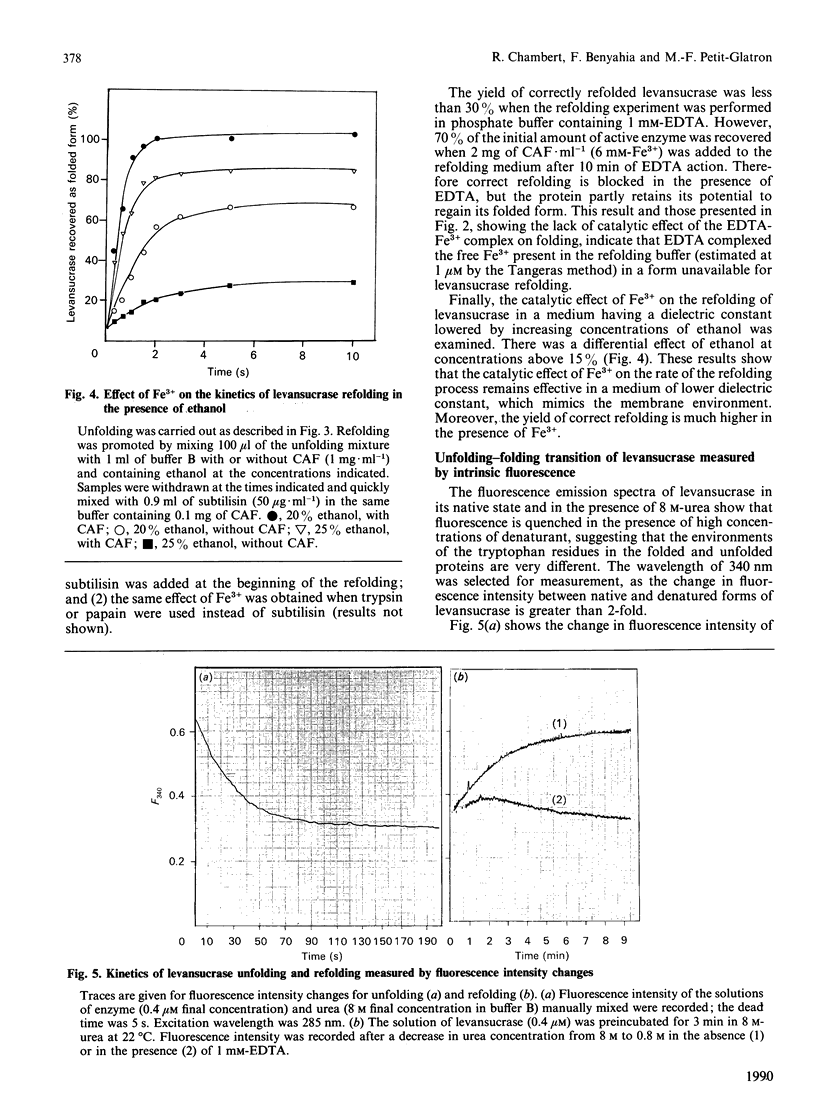
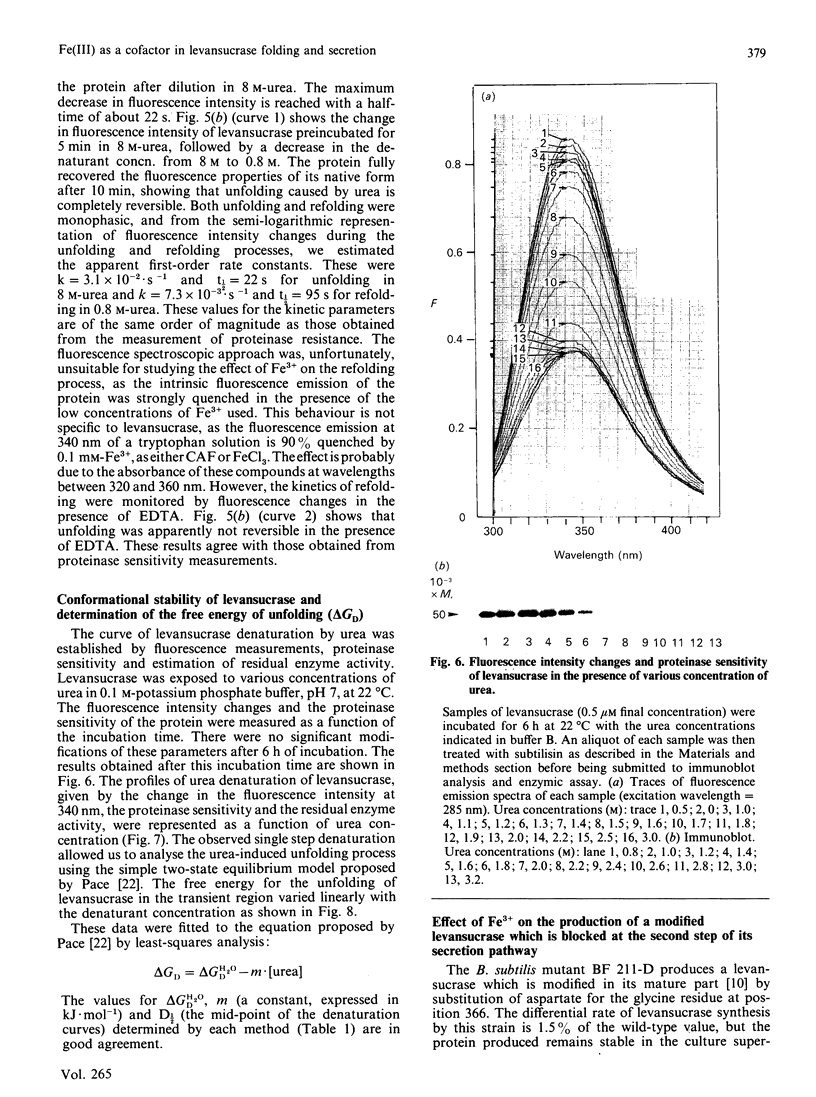
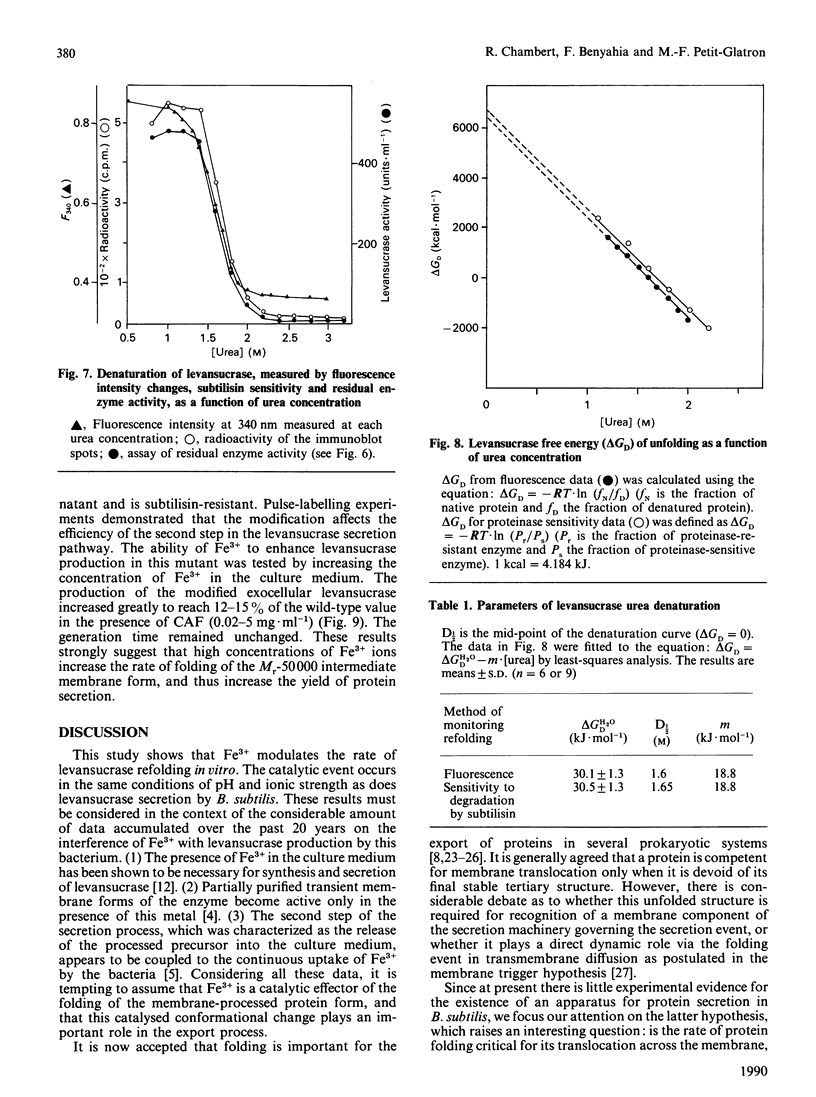
The refolding of levansucrase denatured by urea was studied as a possible model for the second step of the secretion pathway of this protein. The folding-unfolding transition was monitored by measuring intrinsic fluorescence and resistance to proteolysis. Both methods provided the same estimation for the unfolding free energy of levansucrase, delta GD, which was 30.1 +/- 1.7 kJ.mol-1 (7.2 +/- 0.4 kcal.mol-1) at pH 7 in 0.1 M-potassium phosphate buffer. The rate of refolding was greatly enhanced by Fe3+, whereas the Fe3+ chelator EDTA prevented correct refolding. Fe3+ allowed the protein to reach its folded form in medium in which the dielectric constant had been lowered by ethanol. The efficiency in vivo of the export of levansucrase bearing an amino acid modification which blocks the second step of the translocation pathway was greatly increased by high concentrations of Fe3+ in the culture medium. Assuming that the protein folding governs the second step of the secretion process of levansucrase, we discuss from an irreversible thermodynamic point of view the possible role of Fe3+ in the efficient coupling of the two events.
Full text
PDF
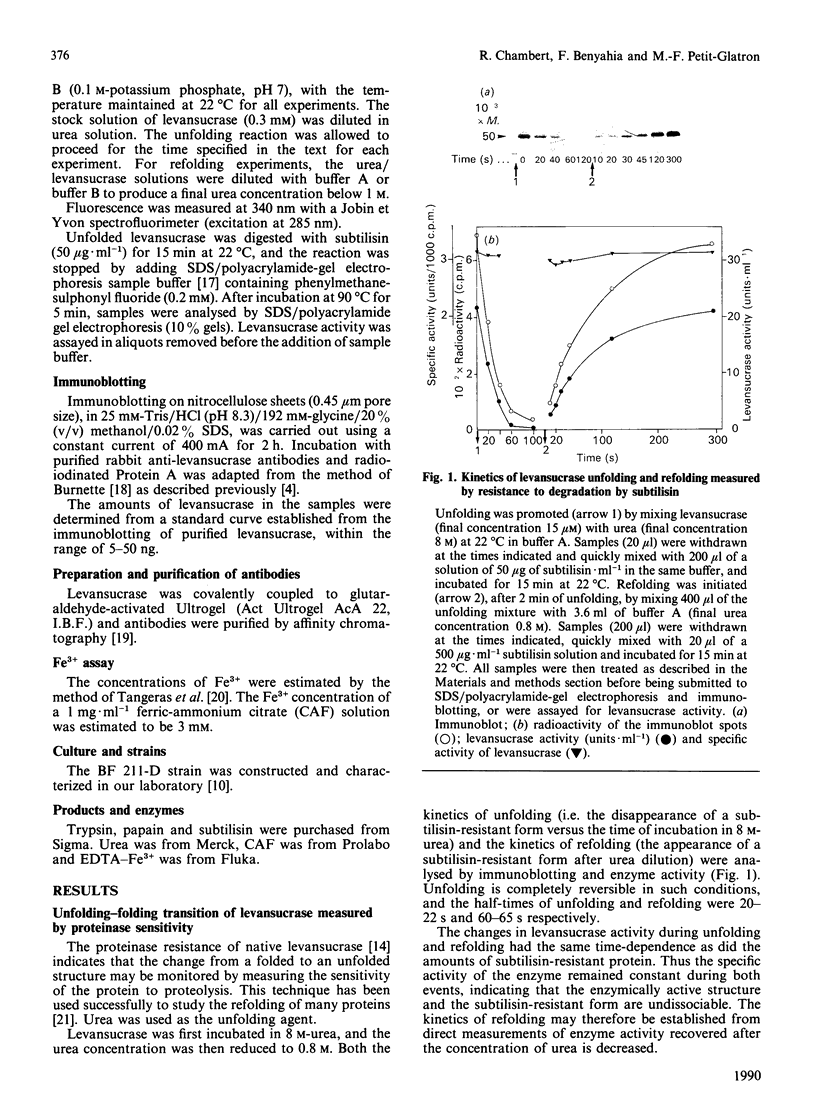

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Baker K., Mackman N., Holland I. B. Genetics and biochemistry of the assembly of proteins into the outer membrane of E. coli. Prog Biophys Mol Biol. 1987;49(2-3):89–115. doi: 10.1016/0079-6107(87)90010-1. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Bychkova V. E., Pain R. H., Ptitsyn O. B. The 'molten globule' state is involved in the translocation of proteins across membranes? FEBS Lett. 1988 Oct 10;238(2):231–234. doi: 10.1016/0014-5793(88)80485-x. [DOI] [PubMed] [Google Scholar]
- Chambert R., Gonzy-Tréboul G. Levansucrase of Bacillus subtilis: kinetic and thermodynamic aspects of transfructosylation processes. Eur J Biochem. 1976 Feb 2;62(1):55–64. doi: 10.1111/j.1432-1033.1976.tb10097.x. [DOI] [PubMed] [Google Scholar]
- Chambert R., Petit-Glatron M. F. Hyperproduction of exocellular levansucrase by Bacillus subtilis: examination of the phenotype of a sacUh strain. J Gen Microbiol. 1984 Dec;130(12):3143–3152. doi: 10.1099/00221287-130-12-3143. [DOI] [PubMed] [Google Scholar]
- Chambert R., Petit-Glatron M. F. Secretion mechanism of Bacillus subtilis levansucrase: characterization of the second step. J Gen Microbiol. 1988 May;134(5):1205–1214. doi: 10.1099/00221287-134-5-1205. [DOI] [PubMed] [Google Scholar]
- Delobbe A. Rôle du fer dans la production, la purification de la lévane-sucrase. Effets sur l'enzyme purifié. Bull Soc Chim Biol (Paris) 1968;50(3):641–674. [PubMed] [Google Scholar]
- Eilers M., Schatz G. Protein unfolding and the energetics of protein translocation across biological membranes. Cell. 1988 Feb 26;52(4):481–483. doi: 10.1016/0092-8674(88)90458-8. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Silhavy T. J. Sequence information required for protein translocation from the cytoplasm. J Bacteriol. 1987 Dec;169(12):5339–5342. doi: 10.1128/jb.169.12.5339-5342.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzy-Treboul G., Chambert R., Dedonder R. Levansucrase of Bacillus subtilis : reexamination of some physical and chemical properties. Biochimie. 1975;57(1):17–28. doi: 10.1016/s0300-9084(75)80105-2. [DOI] [PubMed] [Google Scholar]
- Kontinen V. P., Sarvas M. Mutants of Bacillus subtilis defective in protein export. J Gen Microbiol. 1988 Aug;134(8):2333–2344. doi: 10.1099/00221287-134-8-2333. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeBrun E., van Rapenbusch R. The structure of BAcillus subtilis levansucrase at 3.8 A resolution. J Biol Chem. 1980 Dec 25;255(24):12034–12036. [PubMed] [Google Scholar]
- Liu G. P., Topping T. B., Cover W. H., Randall L. L. Retardation of folding as a possible means of suppression of a mutation in the leader sequence of an exported protein. J Biol Chem. 1988 Oct 15;263(29):14790–14793. [PubMed] [Google Scholar]
- Meyer D. I. Preprotein conformation: the year's major theme in translocation studies. Trends Biochem Sci. 1988 Dec;13(12):471–474. doi: 10.1016/0968-0004(88)90233-2. [DOI] [PubMed] [Google Scholar]
- Minsky A., Summers R. G., Knowles J. R. Secretion of beta-lactamase into the periplasm of Escherichia coli: evidence for a distinct release step associated with a conformational change. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4180–4184. doi: 10.1073/pnas.83.12.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Extracellular and membrane-bound proteases from Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):493–501. doi: 10.1128/jb.141.2.493-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C. N. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 1986;131:266–280. doi: 10.1016/0076-6879(86)31045-0. [DOI] [PubMed] [Google Scholar]
- Petit-Glatron M. F., Benyahia F., Chambert R. Secretion of Bacillus subtilis levansucrase: a possible two-step mechanism. Eur J Biochem. 1987 Mar 2;163(2):379–387. doi: 10.1111/j.1432-1033.1987.tb10810.x. [DOI] [PubMed] [Google Scholar]
- Petit-Glatron M. F., Chambert R., Steinmetz M. Levansucrase of Bacillus subtilis. Characterization of a form isolated from phenol-treated cells and activated by Triton X-100. Eur J Biochem. 1980 Jan;103(1):189–195. doi: 10.1111/j.1432-1033.1980.tb04303.x. [DOI] [PubMed] [Google Scholar]
- Price N. C. The proteolysis of proteins during folding. Biochem Soc Trans. 1987 Oct;15(5):818–820. doi: 10.1042/bst0150818. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Tangerås A., Flatmark T., Bäckström D., Ehrenberg A. Mitochondrial iron not bound in heme and iron-sulfur centers. Estimation, compartmentation and redox state. Biochim Biophys Acta. 1980 Feb 8;589(2):162–175. doi: 10.1016/0005-2728(80)90035-3. [DOI] [PubMed] [Google Scholar]
- Wickner W. Secretion and membrane assembly. Trends Biochem Sci. 1989 Jul;14(7):280–283. doi: 10.1016/0968-0004(89)90064-9. [DOI] [PubMed] [Google Scholar]