Abstract
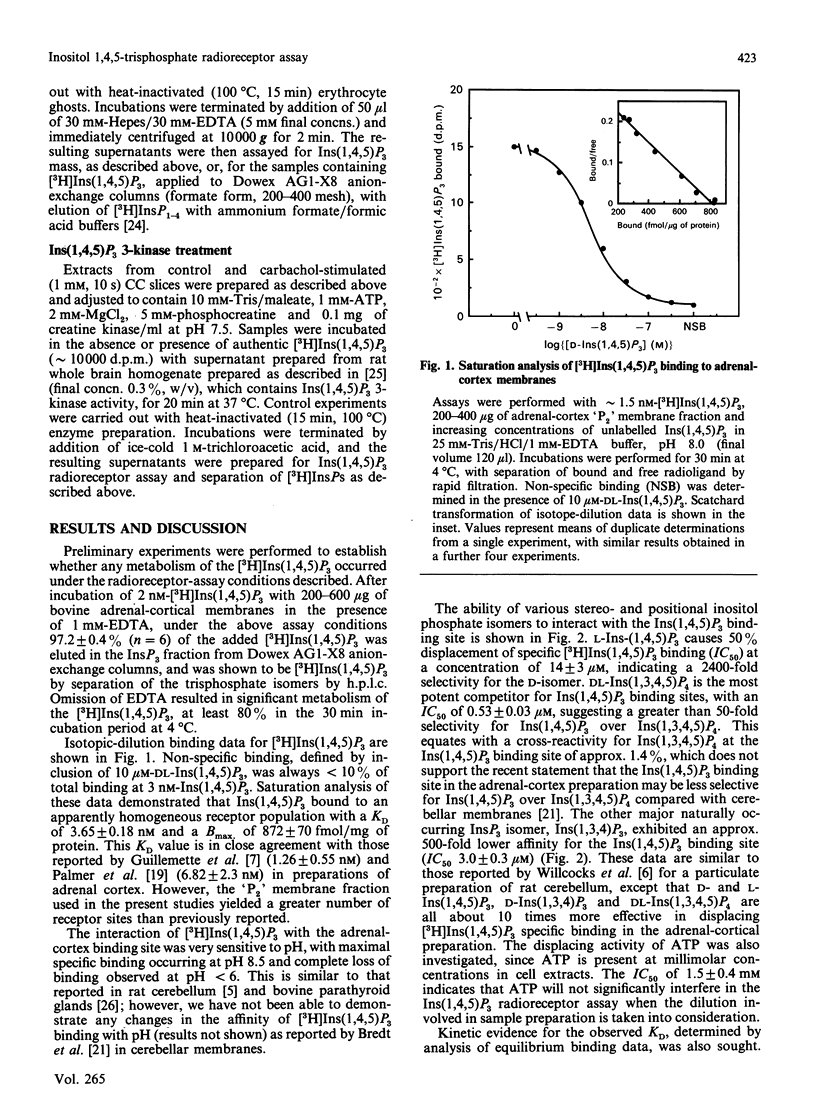
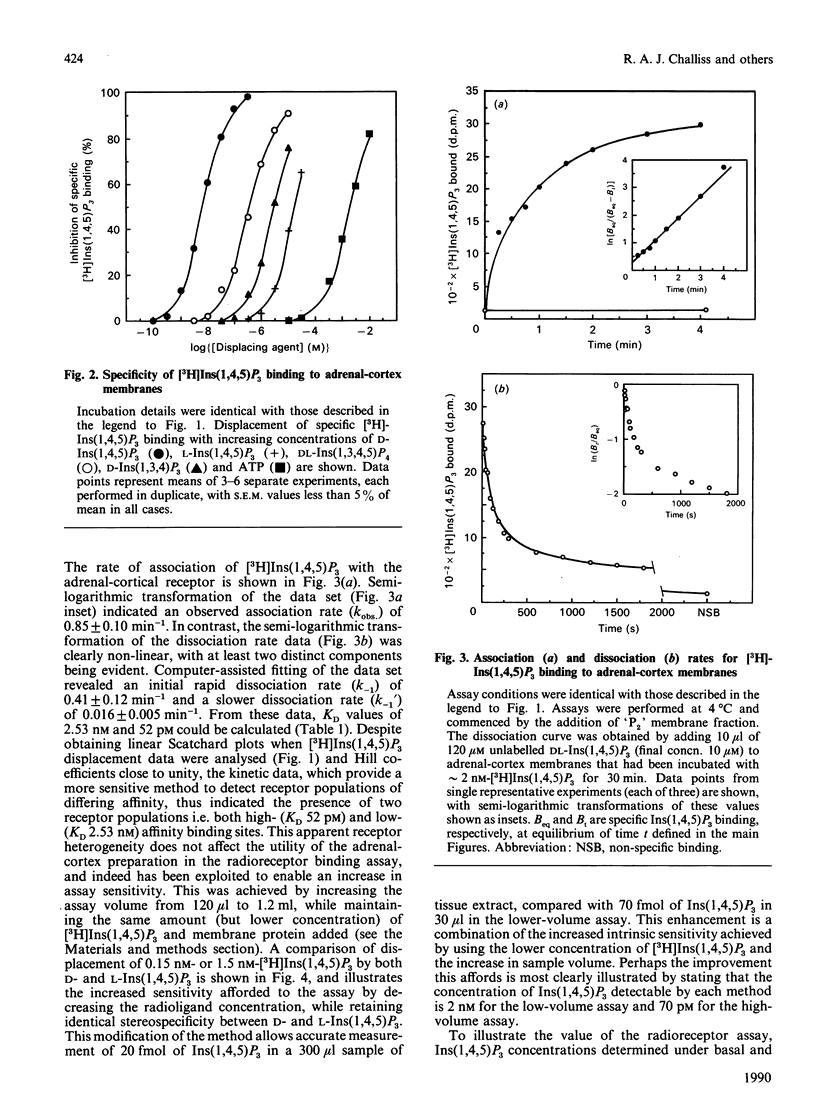
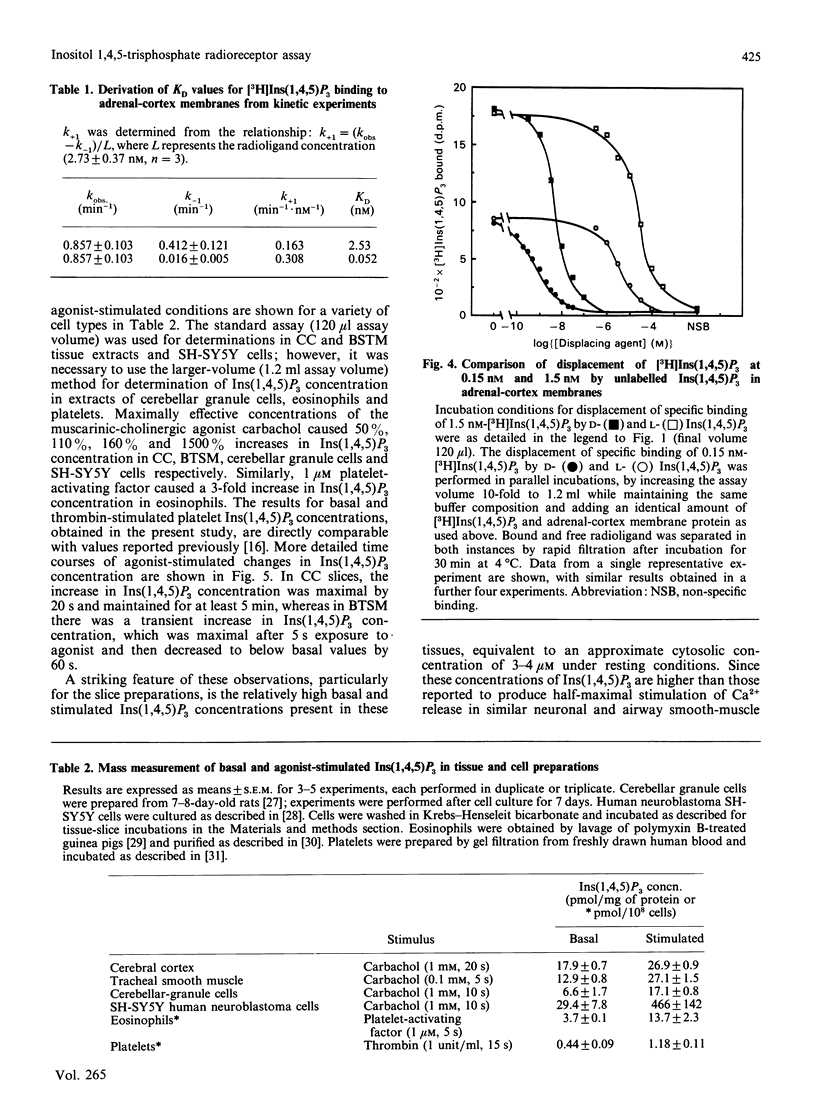
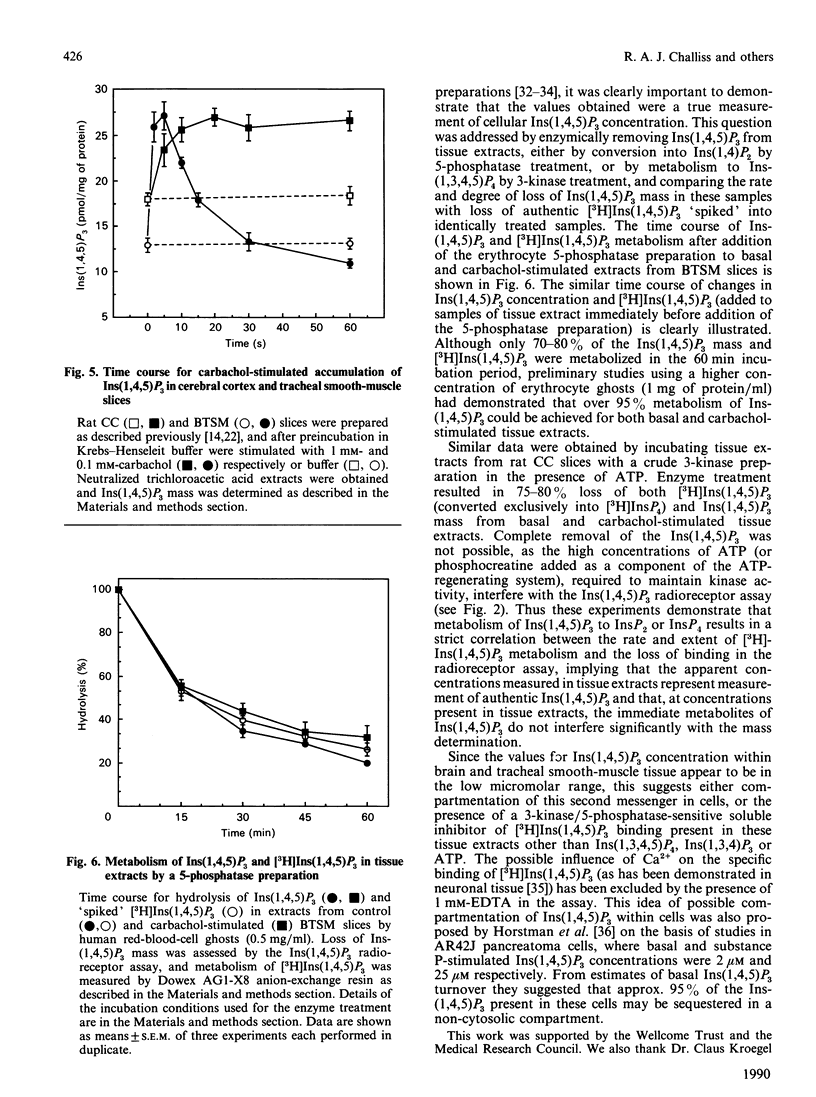
1. The characterization of a radioreceptor assay for determining Ins(1,4,5)P3 concentration in tissue extracts is described which utilizes the binding of [3H]Ins(1,4,5)P3 to an adrenal-cortex membrane fraction. 2. Analysis of [3H]Ins(1,4,5)P3 binding by isotope dilution demonstrated an apparent single population of binding sites (KD 3.65 +/- 0.18 nM, Bmax. 872 +/- 70 fmol/mg of protein). Specific binding of [3H]Ins(1,4,5)P3 was enhanced at alkaline pH values (maximum at pH 8.5), with complete loss of specific binding at pH less than 6. These binding sites displayed strict stereo- and positional specificity for Ins(1,4,5)P3, with L-Ins(1,4,5)P3, Ins(1,3,4)P3 and DL-Ins(1,3,4,5)P4 causing 50% displacement of specific [3H]Ins(1,4,5)P3 binding (IC50 values) at concentrations of 14 +/- 3 microM, 3.0 +/- 0.3 microM and 0.53 +/- 0.03 microM respectively. 3. Kinetic analysis of binding data, however, revealed a high-affinity [3H]Ins(1,4,5)P3 binding site (KD 0.052 nM) in addition to the lower-affinity site (KD 2.53 nM) already demonstrated in displacement studies. 4. It is shown that the presence of the high-affinity site can be exploited to increase the sensitivity of the [3H]Ins(1,4,5)P3 radioreceptor assay, allowing accurate detection of 20 fmol of Ins(1,4,5)P3 in 300 microliters of tissue extract. 5. Further validation of the specificity of the above assay for Ins(1,4,5)P3 was provided by incubating tissue extracts with either a 5-phosphatase or 3-kinase preparation. It was shown that identical loss occurred of both Ins(1,4,5)P3 mass and [3H]Ins(1,4,5)P3, added to parallel incubations. 6. The ability of the assay to measure basal and agonist-stimulated increases in Ins(1,4,5)P3 concentration has been demonstrated with rat cerebral cortex and bovine tracheal smooth-muscle slices and a range of cultured and isolated cell preparations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukal A. J., Guillemette G., Rubin R., Spät A., Catt K. J. Binding sites for inositol trisphosphate in the bovine adrenal cortex. Biochem Biophys Res Commun. 1985 Dec 17;133(2):532–538. doi: 10.1016/0006-291x(85)90939-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Quantitative changes in inositol 1,4,5-trisphosphate in chemoattractant-stimulated neutrophils. J Biol Chem. 1986 Nov 25;261(33):15644–15647. [PubMed] [Google Scholar]
- Bredt D. S., Mourey R. J., Snyder S. H. A simple, sensitive, and specific radioreceptor assay for inositol 1,4,5-trisphosphate in biological tissues. Biochem Biophys Res Commun. 1989 Mar 31;159(3):976–982. doi: 10.1016/0006-291x(89)92204-3. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Batty I. H., Nahorski S. R. Mass measurements of inositol(1,4,5)trisphosphate in rat cerebral cortex slices using a radioreceptor assay: effects of neurotransmitters and depolarization. Biochem Biophys Res Commun. 1988 Dec 15;157(2):684–691. doi: 10.1016/s0006-291x(88)80304-8. [DOI] [PubMed] [Google Scholar]
- Chilvers E. R., Challiss R. A., Barnes P. J., Nahorski S. R. Mass changes of inositol(1,4,5)trisphosphate in trachealis muscle following agonist stimulation. Eur J Pharmacol. 1989 May 30;164(3):587–590. doi: 10.1016/0014-2999(89)90269-0. [DOI] [PubMed] [Google Scholar]
- Danoff S. K., Supattapone S., Snyder S. H. Characterization of a membrane protein from brain mediating the inhibition of inositol 1,4,5-trisphosphate receptor binding by calcium. Biochem J. 1988 Sep 15;254(3):701–705. doi: 10.1042/bj2540701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Brown E., Williams G. Distinct binding sites for Ins(1,4,5)P3 and Ins(1,3,4,5)P4 in bovine parathyroid glands. Biochem Biophys Res Commun. 1989 Feb 28;159(1):200–208. doi: 10.1016/0006-291x(89)92423-6. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Spät A., Catt K. J. Intracellular receptors for inositol 1,4,5-trisphosphate in angiotensin II target tissues. J Biol Chem. 1987 Jan 25;262(3):1010–1015. [PubMed] [Google Scholar]
- Gärtner I. Separation of human eosinophils in density gradients of polyvinylpyrrolidone-coated silica gel (Percoll). Immunology. 1980 May;40(1):133–136. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Ito Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br J Pharmacol. 1985 Sep;86(1):191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstman D. A., Takemura H., Putney J. W., Jr Formation and metabolism of [3H]inositol phosphates in AR42J pancreatoma cells. Substance P-induced Ca2+ mobilization in the apparent absence of inositol 1,4,5-trisphosphate 3-kinase activity. J Biol Chem. 1988 Oct 25;263(30):15297–15303. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Rice H. L. The relationship between inositol trisphosphate receptor density and calcium release in brain microsomes. Mol Pharmacol. 1989 Mar;35(3):355–359. [PubMed] [Google Scholar]
- Joseph S. K., Rice H. L., Williamson J. R. The effect of external calcium and pH on inositol trisphosphate-mediated calcium release from cerebellum microsomal fractions. Biochem J. 1989 Feb 15;258(1):261–265. doi: 10.1042/bj2580261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. G., Ghataorre A. S., Nahorski S. R. Muscarinic receptor binding characteristics of a human neuroblastoma SK-N-SH and its clones SH-SY5Y and SH-EP1. Eur J Pharmacol. 1989 Jun 8;165(1):71–77. doi: 10.1016/0014-2999(89)90771-1. [DOI] [PubMed] [Google Scholar]
- Mayr G. W. A novel metal-dye detection system permits picomolar-range h.p.l.c. analysis of inositol polyphosphates from non-radioactively labelled cell or tissue specimens. Biochem J. 1988 Sep 1;254(2):585–591. doi: 10.1042/bj2540585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek J. L. Inositol bis-, tris-, and tetrakis(phosphate)s: analysis in tissues by HPLC. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4162–4166. doi: 10.1073/pnas.83.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski S. R., Potter B. V. Molecular recognition of inositol polyphosphates by intracellular receptors and metabolic enzymes. Trends Pharmacol Sci. 1989 Apr;10(4):139–144. doi: 10.1016/0165-6147(89)90165-x. [DOI] [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Pincus S. H. Production of eosinophil-rich guinea pig peritoneal exudates. Blood. 1978 Jul;52(1):127–134. [PubMed] [Google Scholar]
- Rittenhouse S. E. Activation of human platelet phospholipase C by ionophore A23187 is totally dependent upon cyclo-oxygenase products and ADP. Biochem J. 1984 Aug 15;222(1):103–110. doi: 10.1042/bj2220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Spät A., Bradford P. G., McKinney J. S., Rubin R. P., Putney J. W., Jr A saturable receptor for 32P-inositol-1,4,5-triphosphate in hepatocytes and neutrophils. Nature. 1986 Feb 6;319(6053):514–516. doi: 10.1038/319514a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarver A. P., King W. G., Rittenhouse S. E. Inositol 1,4,5-trisphosphate and inositol 1,2-cyclic 4,5-trisphosphate are minor components of total mass of inositol trisphosphate in thrombin-stimulated platelets. Rapid formation of inositol 1,3,4-trisphosphate. J Biol Chem. 1987 Dec 25;262(36):17268–17271. [PubMed] [Google Scholar]
- Verhoeven A. J., Tysnes O. B., Horvli O., Cook C. A., Holmsen H. Stimulation of phosphate uptake in human platelets by thrombin and collagen. Changes in specific 32P labeling of metabolic ATP and polyphosphoinositides. J Biol Chem. 1987 May 25;262(15):7047–7052. [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks A. L., Cooke A. M., Potter B. V., Nahorski S. R. Stereospecific recognition sites for [3H]inositol(1,4,5)-triphosphate in particulate preparations of rat cerebellum. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1071–1078. doi: 10.1016/0006-291x(87)90756-x. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Snyder S. H. Inositol 1,4,5-trisphosphate receptor binding: autoradiographic localization in rat brain. J Neurosci. 1989 Jan;9(1):339–346. doi: 10.1523/JNEUROSCI.09-01-00339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]


