Abstract
Enzymically isolated rabbit aortic smooth-muscle cells (SMC) in the first few days of primary culture express a 'contractile phenotype', but with time these cells modulate to a 'synthetic phenotype'. Synthetic-state SMC are able to proliferate, and, provided that they undergo fewer than 5 cumulative population doublings, return to the contractile phenotype after reaching confluency [Campbell, Kocher, Skalli, Gabbiani & Campbell (1989) Arteriosclerosis 9, 633-643]. The present study has determined the synthesis of collagen, at the protein and mRNA levels, by cultured SMC as they undergo a change in phenotypic state. The results show that, upon modulating to the synthetic phenotype, SMC synthesized 25-30 times more collagen than did contractile cells. At the same time, non-collagen-protein synthesis increased only 5-6-fold, indicating a specific stimulation of collagen synthesis. Steady-state mRNA levels are also elevated, with alpha 2(I) and alpha 1(III) mRNA levels 30 times and 20 times higher respectively, probably reflecting increased transcriptional activity. Phenotypic modulation was also associated with an alteration in the relative proportions of type I and III collagens synthesized, contractile SMC synthesizing 78.1 +/- 3.6% (mean +/- S.D.) type I collagen and 17.5 +/- 4.7% type III collagen, and synthetic cells synthesizing 90.3 +/- 2.0% type I collagen and 5.8% +/- 1.8% type III collagen. Enrichment of type I collagen was similarly noted at the mRNA level. On return to the contractile state, at confluency, collagen production and the percentage of type I collagen decreased. This further illustrates the close association between the phenotypic state of SMC and their collagen-biosynthetic phenotype.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allebach E. S., Boettiger D., Pacifici M., Adams S. L. Control of types I and II collagen and fibronectin gene expression in chondrocytes delineated by viral transformation. Mol Cell Biol. 1985 May;5(5):1002–1008. doi: 10.1128/mcb.5.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. J. Collagens in atherosclerosis. Coll Relat Res. 1985 Jan;5(1):65–97. doi: 10.1016/s0174-173x(85)80048-0. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Morton L. F., Levene C. I. Synthesis of collagens types I and III by pig medial smooth muscle cells in culture. Biochem Biophys Res Commun. 1976 May 17;70(2):339–347. doi: 10.1016/0006-291x(76)91051-2. [DOI] [PubMed] [Google Scholar]
- Bartos F., Ledvina M. Collagen, elastin and desmosines in three layers of bovine aortas of different ages. Exp Gerontol. 1979;14(1):21–26. doi: 10.1016/0531-5565(79)90004-4. [DOI] [PubMed] [Google Scholar]
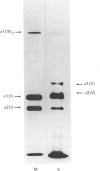
- Bateman J. F., Harley V., Chan D., Cole W. G. Comprehensive analysis of collagen metabolism in vitro using [4(3H)]/[14C]proline dual-labeling and polyacrylamide gel electrophoresis. Anal Biochem. 1988 Jan;168(1):171–175. doi: 10.1016/0003-2697(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Peterkofsky B. Mechanisms of Kirsten murine sarcoma virus transformation-induced changes in the collagen phenotype and synthetic rate of BALB 3T3 cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6028–6032. doi: 10.1073/pnas.78.10.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldekas J. C., Gerstenfeld L., Sonenshein G. E., Franzblau C. Cell density and estradiol modulation of procollagen type III in cultured calf smooth muscle cells. J Biol Chem. 1982 Oct 25;257(20):12252–12256. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E. New collagens, new concepts. Annu Rev Cell Biol. 1988;4:551–577. doi: 10.1146/annurev.cb.04.110188.003003. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Balian G., Ross R., Bornstein P. Synthesis of types I and III procollagen and collagen by monkey aortic smooth muscle cells in vitro. Biochemistry. 1977 Jul 12;16(14):3243–3249. doi: 10.1021/bi00633a031. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Ross R. Synthesis of connective tissue macromolecules by smooth muscle. Int Rev Connect Tissue Res. 1979;8:119–157. doi: 10.1016/b978-0-12-363708-6.50010-2. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Campbell J. H., Manderson J. A., Horrigan S., Rennick R. E. Arterial smooth muscle. A multifunctional mesenchymal cell. Arch Pathol Lab Med. 1988 Oct;112(10):977–986. [PubMed] [Google Scholar]
- Campbell G. R., Campbell J. H. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis. Exp Mol Pathol. 1985 Apr;42(2):139–162. doi: 10.1016/0014-4800(85)90023-1. [DOI] [PubMed] [Google Scholar]
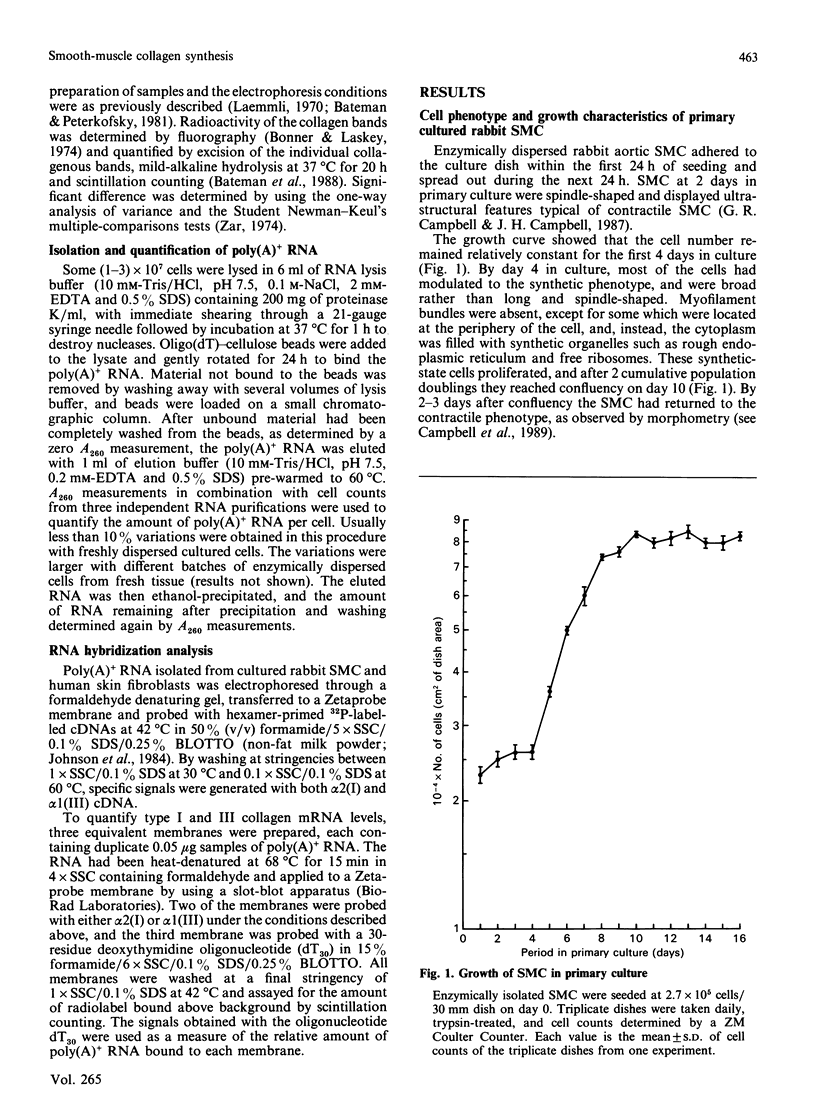
- Campbell J. H., Kocher O., Skalli O., Gabbiani G., Campbell G. R. Cytodifferentiation and expression of alpha-smooth muscle actin mRNA and protein during primary culture of aortic smooth muscle cells. Correlation with cell density and proliferative state. Arteriosclerosis. 1989 Sep-Oct;9(5):633–643. doi: 10.1161/01.atv.9.5.633. [DOI] [PubMed] [Google Scholar]
- Campbell J. H., Reardon M. F., Campbell G. R., Nestel P. J. Metabolism of atherogenic lipoproteins by smooth muscle cells of different phenotype in culture. Arteriosclerosis. 1985 Jul-Aug;5(4):318–328. doi: 10.1161/01.atv.5.4.318. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R., Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981 May;89(2):379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R., McConnell J. D., Gröschel-Stewart U. Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell Tissue Res. 1977 Feb 14;177(4):503–522. doi: 10.1007/BF00220611. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Ehrhart L. A., Holderbaum D. Aortic collagen, elastin and non-fibrous protein synthesis in rabbits red cholesterol and peanut oil. Atherosclerosis. 1980 Nov;37(3):423–432. doi: 10.1016/0021-9150(80)90147-1. [DOI] [PubMed] [Google Scholar]
- Ehrhart L. A., Holderbaum D. Stimulation of aortic protein synthesis in experimental rabbit atherosclerosis. Atherosclerosis. 1977 Aug;27(4):477–485. doi: 10.1016/0021-9150(77)90165-4. [DOI] [PubMed] [Google Scholar]
- Fischer G. M., Swain M. L., Cherian K. Increased vascular collagen and elastin synthesis in experimental atherosclerosis in the rabbit. Variation in synthesis among major vessels. Atherosclerosis. 1980 Jan;35(1):11–20. doi: 10.1016/0021-9150(80)90023-4. [DOI] [PubMed] [Google Scholar]
- Geesin J. C., Darr D., Kaufman R., Murad S., Pinnell S. R. Ascorbic acid specifically increases type I and type III procollagen messenger RNA levels in human skin fibroblast. J Invest Dermatol. 1988 Apr;90(4):420–424. doi: 10.1111/1523-1747.ep12460849. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Adams E. P., Cliff W. J. The aortic tunica media of the developing rat. II. Incorporation by medial cells 3-H-proline into collagen and elastin: autoradiographic and chemical studies. Lab Invest. 1975 May;32(5):601–609. [PubMed] [Google Scholar]
- Grant R. A. Content and distribution of aortic collagen, elastin and carbohydrate in different species. J Atheroscler Res. 1967 Jul-Aug;7(4):463–472. doi: 10.1016/s0368-1319(67)80024-3. [DOI] [PubMed] [Google Scholar]
- Halme T., Peltonen J., Sims T. J., Vihersaari T., Penttinen R. Collagen in human aorta. Changes in the type III/I ratio and concentration of the reducible crosslink, dehydrohydroxylysinonorleucine in ascending aorta from healthy subjects of different age and patients with annulo-aortic ectasia. Biochim Biophys Acta. 1986 Apr 11;881(2):222–228. doi: 10.1016/0304-4165(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Hanson A. N., Bentley J. P. Quantitation of type I to type III collagen ratios in small samples of human tendon, blood vessels, and atherosclerotic plaque. Anal Biochem. 1983 Apr 1;130(1):32–40. doi: 10.1016/0003-2697(83)90646-2. [DOI] [PubMed] [Google Scholar]
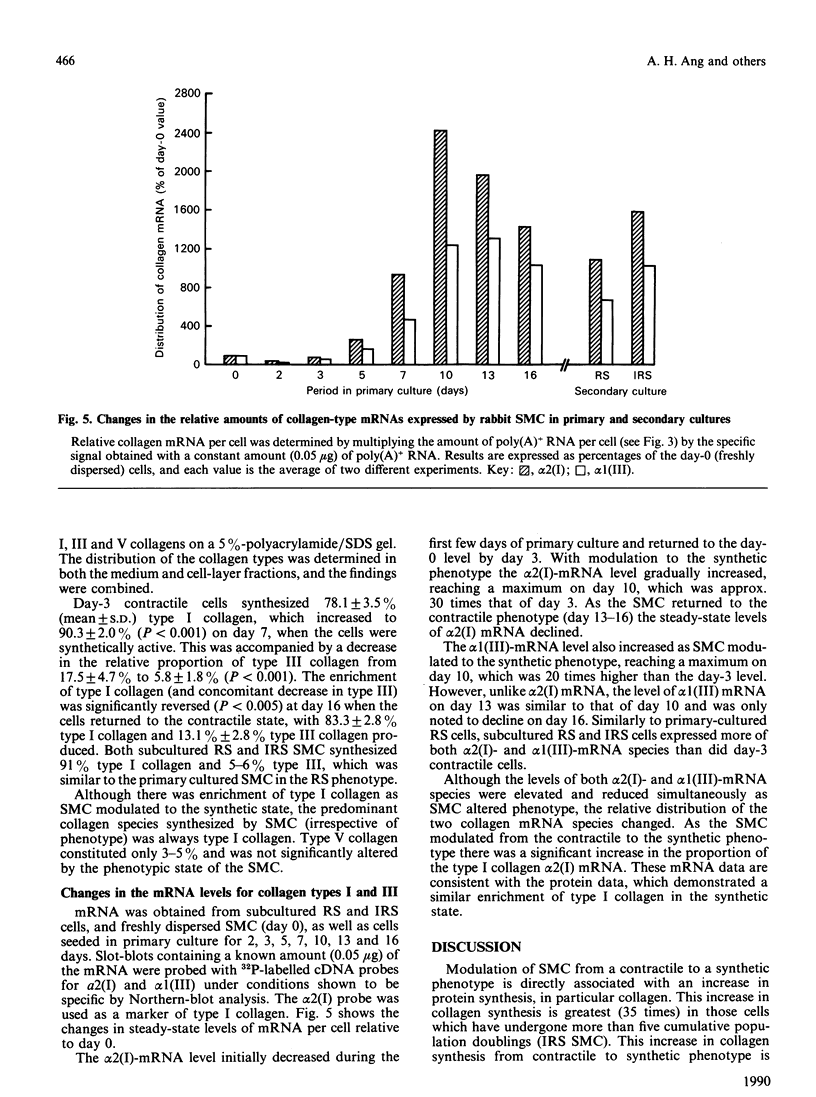
- Holderbaum D., Ehrhart L. A. Modulation of types I and III procollagen synthesis at various stages of arterial smooth muscle cell growth in vitro. Exp Cell Res. 1984 Jul;153(1):16–24. doi: 10.1016/0014-4827(84)90443-9. [DOI] [PubMed] [Google Scholar]
- Hosoda Y., Kawano K., Yamasawa F., Ishii T., Shibata T., Inayama S. Age-dependent changes of collagen and elastin content in human aorta and pulmonary artery. Angiology. 1984 Oct;35(10):615–621. doi: 10.1177/000331978403501001. [DOI] [PubMed] [Google Scholar]
- Issler R. W. The arterial medial cell, smooth muscle or multifunctional mesenchyme? J Atheroscler Res. 1968 Mar-Apr;8(2):201–213. doi: 10.1016/s0368-1319(68)80056-0. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Jaakkola O., Nikkari S. T., Nikkari T. Growth properties and composition of cytoskeletal and cytocontractile proteins in aortic cells isolated and cultured from normal and atherosclerotic rabbits. Atherosclerosis. 1984 Jul;52(1):13–26. doi: 10.1016/0021-9150(84)90153-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layman D. L., Epstein E. H., Jr, Dodson R. F., Titus J. L. Biosynthesis of type I and III collagens by cultured smooth muscle cells from human aorta. Proc Natl Acad Sci U S A. 1977 Feb;74(2):671–675. doi: 10.1073/pnas.74.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D. L., Titus J. L. Synthesis of type I collagen by human smooth muscle cells in vitro. Lab Invest. 1975 Aug;33(2):103–107. [PubMed] [Google Scholar]
- Leushner J. R., Haust M. D. Characterization of basement membrane collagens of bovine aortae. Atherosclerosis. 1984 Jan;50(1):11–27. doi: 10.1016/0021-9150(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Leushner J. R., Haust M. D. Interstitial collagens in fibrous atherosclerotic lesions of human aorta. Pathol Biol (Paris) 1986 Jan;34(1):14–18. [PubMed] [Google Scholar]
- Liau G., Chan L. M. Regulation of extracellular matrix RNA levels in cultured smooth muscle cells. Relationship to cellular quiescence. J Biol Chem. 1989 Jun 15;264(17):10315–10320. [PubMed] [Google Scholar]
- Manderson J. A., Mosse P. R., Safstrom J. A., Young S. B., Campbell G. R. Balloon catheter injury to rabbit carotid artery. I. Changes in smooth muscle phenotype. Arteriosclerosis. 1989 May-Jun;9(3):289–298. doi: 10.1161/01.atv.9.3.289. [DOI] [PubMed] [Google Scholar]
- Mankoo B. S., Dalgleish R. Human pro alpha 1(III) collagen: cDNA sequence for the 3' end. Nucleic Acids Res. 1988 Mar 25;16(5):2337–2337. doi: 10.1093/nar/16.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R. Collagenous proteins of blood vessels. Arteriosclerosis. 1986 Nov-Dec;6(6):585–593. doi: 10.1161/01.atv.6.6.585. [DOI] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J., Blose S. H., Chacko S. Collagen polymorphism in cell cultures derived from guinea pig aortic smooth muscle: comparison with three populations of fibroblasts. Arch Biochem Biophys. 1977 Jun;181(2):462–469. doi: 10.1016/0003-9861(77)90252-1. [DOI] [PubMed] [Google Scholar]
- Mayne R., Zettergren J. G., Mayne P. M., Bedwell N. W. Isolation and partial characterization of basement membrane-like collagens from bovine thoracic aorta. Artery. 1980;7(4):262–280. [PubMed] [Google Scholar]
- McCloskey D. I., Cleary E. G. Chemical composition of the rabbit aorta during development. Circ Res. 1974 Jun;34(6):828–835. doi: 10.1161/01.res.34.6.828. [DOI] [PubMed] [Google Scholar]
- McCullagh K. A., Balian G. Collagen characterisation and cell transformation in human atherosclerosis. Nature. 1975 Nov 6;258(5530):73–75. doi: 10.1038/258073a0. [DOI] [PubMed] [Google Scholar]
- McCullagh K. G., Duance V. C., Bishop K. A. The distribution of collagen types I, III and V (AB) in normal and atherosclerotic human aorta. J Pathol. 1980 Jan;130(1):45–55. doi: 10.1002/path.1711300107. [DOI] [PubMed] [Google Scholar]
- McCullagh K. G., Ehrhart L. A. Enhanced synthesis and accumulation of collagen in cholesterol-aggravated pigeon atherosclerosis. Atherosclerosis. 1977 Mar;26(3):341–352. doi: 10.1016/0021-9150(77)90087-9. [DOI] [PubMed] [Google Scholar]
- McCullagh K. G., Ehrhart L. A. Increased arterial collagen synthesis in experimental canine atherosclerosis. Atherosclerosis. 1974 Jan-Feb;19(1):13–28. doi: 10.1016/0021-9150(74)90040-9. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Gay S. The collagens: an overview and update. Methods Enzymol. 1987;144:3–41. doi: 10.1016/0076-6879(87)44170-0. [DOI] [PubMed] [Google Scholar]
- Miskulin M., Dalgleish R., Kluve-Beckerman B., Rennard S. I., Tolstoshev P., Brantly M., Crystal R. G. Human type III collagen gene expression is coordinately modulated with the type I collagen genes during fibroblast growth. Biochemistry. 1986 Mar 25;25(6):1408–1413. doi: 10.1021/bi00354a033. [DOI] [PubMed] [Google Scholar]
- Morton L. F., Barnes M. J. Collagen polymorphism in the normal and diseased blood vessel wall. Investigation of collagens types I, III and V. Atherosclerosis. 1982 Mar;42(1):41–51. doi: 10.1016/0021-9150(82)90124-1. [DOI] [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. II. Atherosclerosis-free diffuse intimal thickenings compared with the media. Arteriosclerosis. 1986 Nov-Dec;6(6):664–669. doi: 10.1161/01.atv.6.6.664. [DOI] [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Wang Z. L., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest. 1985 Nov;53(5):556–562. [PubMed] [Google Scholar]
- Murata K., Kotake C., Motoyama T. Collagen species in human aorta: with special reference to basement membrane-associated collagens in the intima and media and their alteration with atherosclerosis. Artery. 1987;14(4):229–247. [PubMed] [Google Scholar]
- Murata K., Motayama T., Kotake C. Collagen types in various layers of the human aorta and their changes with the atherosclerotic process. Atherosclerosis. 1986 Jun;60(3):251–262. doi: 10.1016/0021-9150(86)90172-3. [DOI] [PubMed] [Google Scholar]
- Opsahl W. P., DeLuca D. J., Ehrhart L. A. Accelerated rates of collagen synthesis in atherosclerotic arteries quantified in vivo. Arteriosclerosis. 1987 Sep-Oct;7(5):470–476. doi: 10.1161/01.atv.7.5.470. [DOI] [PubMed] [Google Scholar]
- PEASE D. C., PAULE W. J. Electron microscopy of elastic arteries; the thoracic aorta of the rat. J Ultrastruct Res. 1960 Jun;3:469–483. doi: 10.1016/s0022-5320(60)90023-x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pietilä K., Nikkari T. Enhanced synthesis of collagen and total protein by smooth muscle cells from atherosclerotic rabbit aortas in culture. Atherosclerosis. 1980 Sep;37(1):11–19. doi: 10.1016/0021-9150(80)90089-1. [DOI] [PubMed] [Google Scholar]
- Ross R., Klebanoff S. J. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol. 1971 Jul;50(1):159–171. doi: 10.1083/jcb.50.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schofield J. D., Harwood R. Procollagen biosynthesis in embryonic chick arteries. Biochem Soc Trans. 1975;3(1):143–145. doi: 10.1042/bst0030143. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Scott D. M., Harwood R., Grant M. E., Jackson D. S. Characterisation of the major collagen species present in porcine aortae and the synthesis of their precursors by smooth muscle cells in culture. Connect Tissue Res. 1977;5(1):7–13. doi: 10.3109/03008207709152606. [DOI] [PubMed] [Google Scholar]
- Sjölund M., Hedin U., Sejersen T., Heldin C. H., Thyberg J. Arterial smooth muscle cells express platelet-derived growth factor (PDGF) A chain mRNA, secrete a PDGF-like mitogen, and bind exogenous PDGF in a phenotype- and growth state-dependent manner. J Cell Biol. 1988 Feb;106(2):403–413. doi: 10.1083/jcb.106.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund M., Madsen K., von der Mark K., Thyberg J. Phenotype modulation in primary cultures of smooth-muscle cells from rat aorta. Synthesis of collagen and elastin. Differentiation. 1986;32(2):173–180. doi: 10.1111/j.1432-0436.1986.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Kindy M. S., Franzblau C., Sonenshein G. E. Complex regulation of collagen gene expression in cultured bovine aortic smooth muscle cells. J Biol Chem. 1986 May 15;261(14):6542–6547. [PubMed] [Google Scholar]
- Thyberg J., Nilsson J., Palmberg L., Sjölund M. Adult human arterial smooth muscle cells in primary culture. Modulation from contractile to synthetic phenotype. Cell Tissue Res. 1985;239(1):69–74. doi: 10.1007/BF00214904. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Palmberg L., Nilsson J., Ksiazek T., Sjölund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation. 1983;25(2):156–167. doi: 10.1111/j.1432-0436.1984.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L. Human aorta collagens: evidence for three distinct species. Biochem Biophys Res Commun. 1974 Apr 8;57(3):717–725. doi: 10.1016/0006-291x(74)90605-6. [DOI] [PubMed] [Google Scholar]
- Uitto J., Hoffmann H-P, Prockop D. J. Synthesis of elastin and procallagen by cells from embryonic aorta. Differences in the role of hydroxyproline and the effects of proline analogs on the secretion of the two proteins. Arch Biochem Biophys. 1976 Mar;173(1):187–200. doi: 10.1016/0003-9861(76)90249-6. [DOI] [PubMed] [Google Scholar]
- Velican C., Velican D. The precursors of coronary atherosclerotic plaques in subjects up to 40 years old. Atherosclerosis. 1980 Sep;37(1):33–46. doi: 10.1016/0021-9150(80)90091-x. [DOI] [PubMed] [Google Scholar]