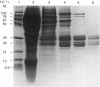
Abstract
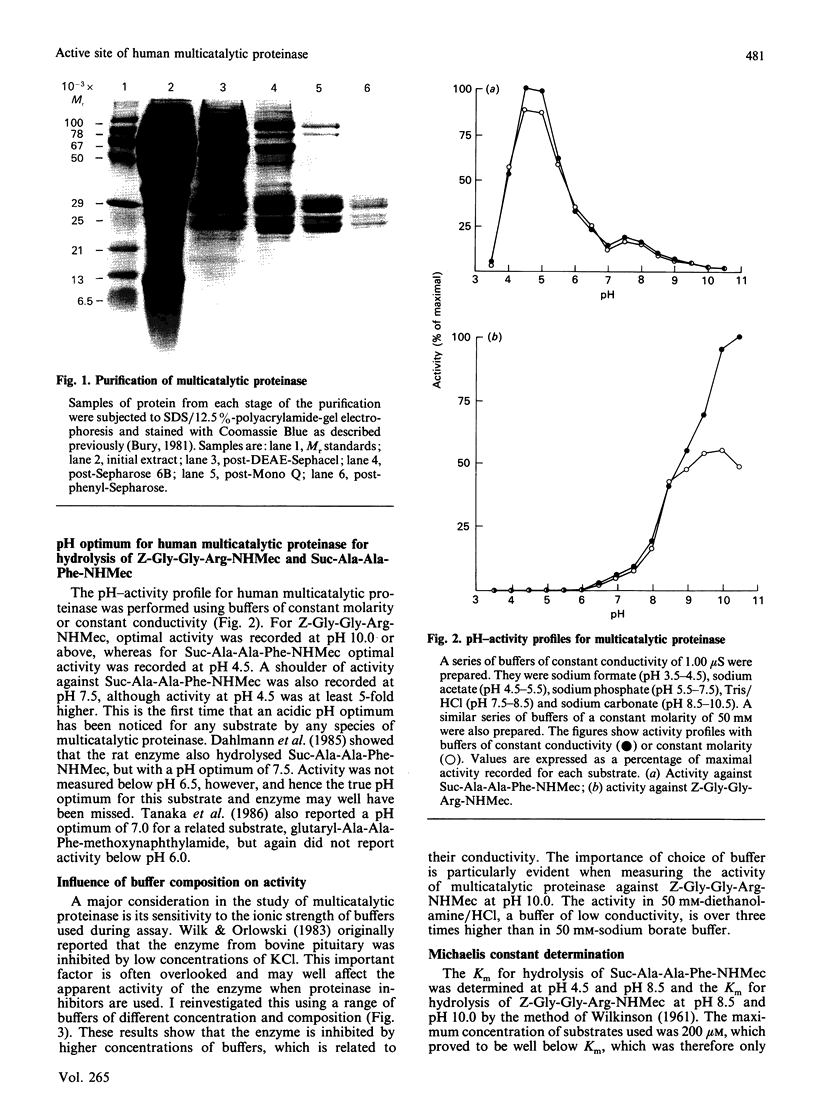
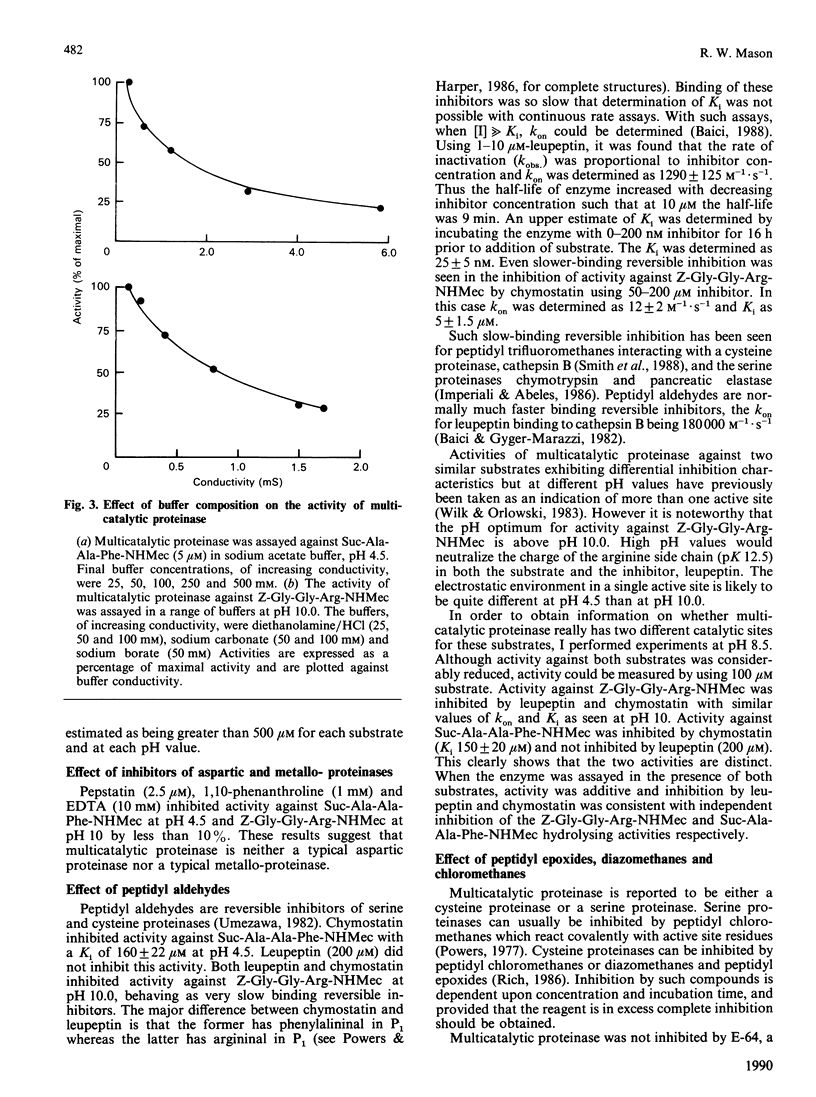
The activity of multicatalytic proteinase against synthetic substrates and the kinetics of its inhibition by a range of class-specific inhibitors have been investigated. The enzyme was found to have a broader pH activity profile than previously noted, being active against succinyl-Ala-Ala-Phe-7-amino-4-methylcoumarin optimally at pH 4.5 and against benzyloxycarbonyl-Gly-Gly-Arg-7-amino-4-methylcoumarin optimally at pH 10.5. Neither activity was inhibited by the class-specific inhibitors 1,10-phenanthroline, EDTA, pepstatin, di-isopropyl fluorophosphate, peptidyl chloromethanes, peptidyl diazomethanes or L-3-carboxy-2,3-trans-epoxypropionyl-leucylamido-(4-guanidin o)butane (E-64), indicating that the enzyme is not a typical metallo-, aspartic, serine or cysteine proteinase. Inhibition by HgCl2, iodoacetamide and N-ethylmaleimide suggests that free thiols are necessary for the enzyme to maintain activity, but that these thiols are not particularly reactive as is the case for cysteine proteinases of the papain superfamily. The peptidyl aldehydes chymostatin and leupeptin were found to be reversible inhibitors of multicatalytic proteinase. Chymostatin inhibited activity against succinyl-Ala-Ala-Phe-7-amino-4-methylcoumarin at pH 4.5 (Ki 160 +/- 22 microM) whereas leupeptin (200 microM) was not inhibitory. Inhibition of activity against benzyloxycarbonyl-Gly-Gly-Arg-7-amino-4-methylcoumarin by these compounds was more complex, in that they behaved as slow tight-binding inhibitors. kon values were determined to be 12 +/- 2 M-1.s-1 and 1290 +/- 125 M-1.s-1 for chymostatin and leupeptin, respectively. The upper limit for Ki values for these two inhibitors was estimated as 5 +/- 1.5 microM and 25 +/- 5 nM, respectively. The different inhibition characteristics for each substrate were also apparent at an intermediate pH of 8.5, showing that the two activities are distinct. Dichloroisocoumarin, a mechanism-based inhibitor of serine proteinases, did inhibit activity against succinyl-Ala-Ala-Phe-7-amino-4-methylcoumarin with a rate constant of 250 M-1.s-1, suggesting that multicatalytic proteinase is an atypical serine proteinase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baici A., Gyger-Marazzi M. The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect. Eur J Biochem. 1982 Dec;129(1):33–41. doi: 10.1111/j.1432-1033.1982.tb07017.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Crawford C., Mason R. W., Wikstrom P., Shaw E. The design of peptidyldiazomethane inhibitors to distinguish between the cysteine proteinases calpain II, cathepsin L and cathepsin B. Biochem J. 1988 Aug 1;253(3):751–758. doi: 10.1042/bj2530751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlmann B., Kuehn L., Rutschmann M., Reinauer H. Purification and characterization of a multicatalytic high-molecular-mass proteinase from rat skeletal muscle. Biochem J. 1985 May 15;228(1):161–170. doi: 10.1042/bj2280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász P., Polgár L. Negatively charged reactants as probes in the study of the essential mercaptide-imidazolium ion-pair of thiolenzymes. Eur J Biochem. 1977 Oct 3;79(2):491–494. doi: 10.1111/j.1432-1033.1977.tb11832.x. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Hemmi K., Powers J. C. Reaction of serine proteases with substituted isocoumarins: discovery of 3,4-dichloroisocoumarin, a new general mechanism based serine protease inhibitor. Biochemistry. 1985 Apr 9;24(8):1831–1841. doi: 10.1021/bi00329a005. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiali B., Abeles R. H. Inhibition of serine proteases by peptidyl fluoromethyl ketones. Biochemistry. 1986 Jul 1;25(13):3760–3767. doi: 10.1021/bi00361a005. [DOI] [PubMed] [Google Scholar]
- Luaces A. L., Barrett A. J. Affinity purification and biochemical characterization of histolysin, the major cysteine proteinase of Entamoeba histolytica. Biochem J. 1988 Mar 15;250(3):903–909. doi: 10.1042/bj2500903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár L., Csoma C. Dissociation of ionizing groups in the binding cleft inversely controls the endo- and exopeptidase activities of cathepsin B. J Biol Chem. 1987 Oct 25;262(30):14448–14453. [PubMed] [Google Scholar]
- Smith R. A., Copp L. J., Donnelly S. L., Spencer R. W., Krantz A. Inhibition of cathepsin B by peptidyl aldehydes and ketones: slow-binding behavior of a trifluoromethyl ketone. Biochemistry. 1988 Aug 23;27(17):6568–6573. doi: 10.1021/bi00417a056. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ii K., Ichihara A., Waxman L., Goldberg A. L. A high molecular weight protease in the cytosol of rat liver. I. Purification, enzymological properties, and tissue distribution. J Biol Chem. 1986 Nov 15;261(32):15197–15203. [PubMed] [Google Scholar]
- The multicatalytic proteinase: a high-Mr endopeptidase. Biochem J. 1988 Oct 15;255(2):750–751. [PMC free article] [PubMed] [Google Scholar]
- Tian W. X., Tsou C. L. Determination of the rate constant of enzyme modification by measuring the substrate reaction in the presence of the modifier. Biochemistry. 1982 Mar 2;21(5):1028–1032. doi: 10.1021/bi00534a031. [DOI] [PubMed] [Google Scholar]
- Umezawa H. Low-molecular-weight enzyme inhibitors of microbial origin. Annu Rev Microbiol. 1982;36:75–99. doi: 10.1146/annurev.mi.36.100182.000451. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J Neurochem. 1983 Mar;40(3):842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]
- Zolfaghari R., Baker C. R., Jr, Canizaro P. C., Amirgholami A., Behal F. J. A high-molecular-mass neutral endopeptidase-24.5 from human lung. Biochem J. 1987 Jan 1;241(1):129–135. doi: 10.1042/bj2410129. [DOI] [PMC free article] [PubMed] [Google Scholar]