Abstract
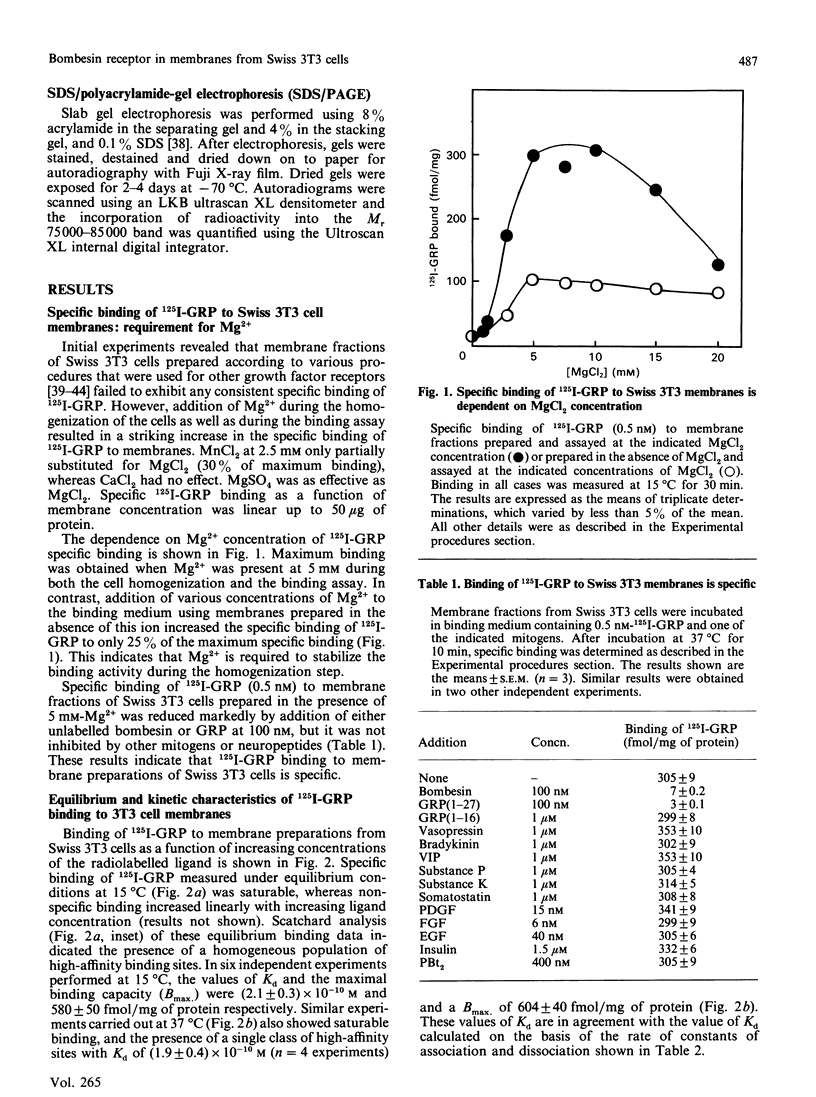
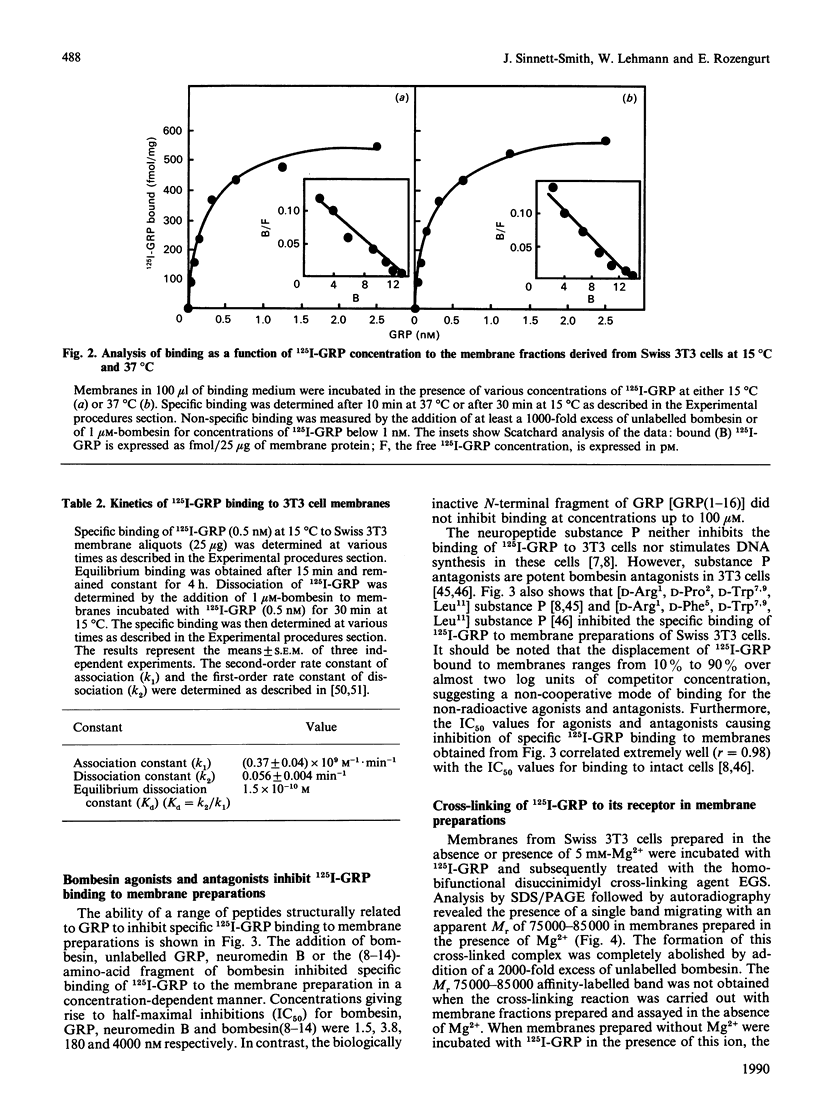
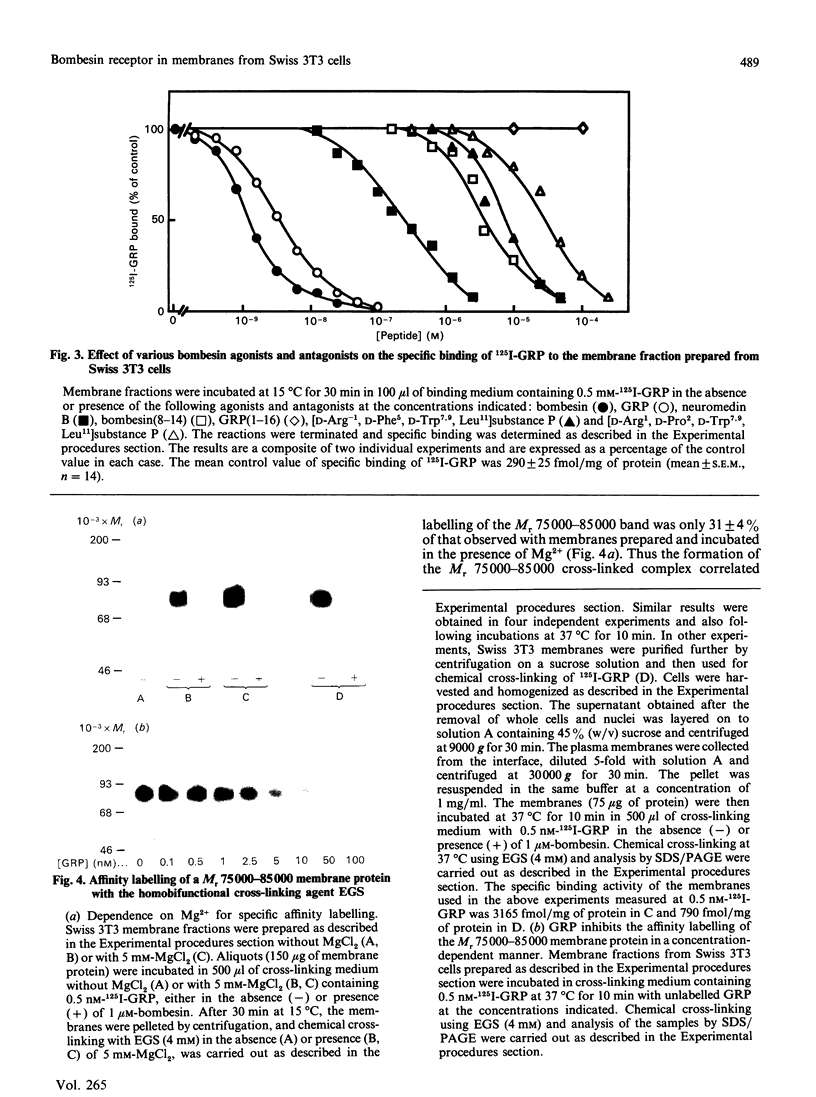
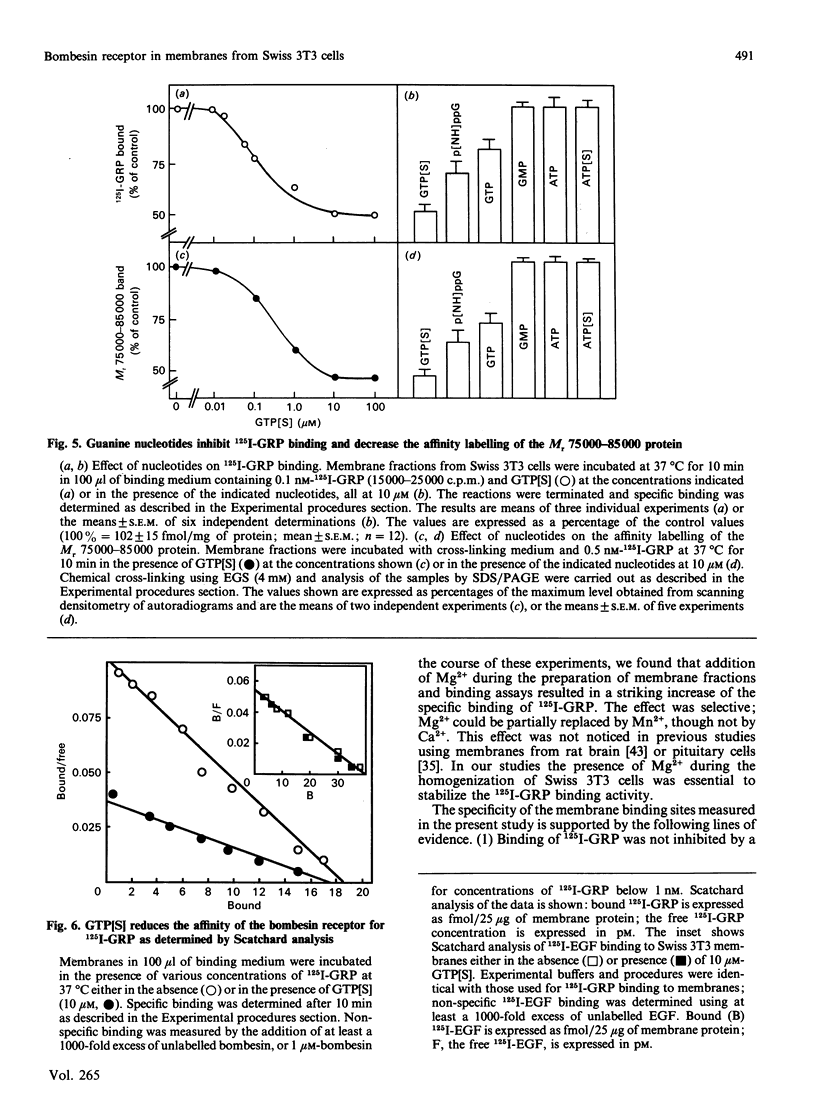
Bombesin-like neuropeptides, including mammalian gastrin-releasing peptide (GRP), are potent mitogens for Swiss 3T3 cells. In this study, we have characterized the bombesin receptor in membrane preparations from these cells. Addition of Mg2+ during cell homogenization was essential to preserve 125I-GRP binding activity in the resulting membrane preparation. The effect of Mg2+ was concentration dependent, with a maximum at 5 mM. Specific binding of 125I-GRP was saturable; Scatchard analysis indicated a single class of high-affinity sites of Kd = (2.1 +/- 0.3) x 10(-10) M at 15 degrees C and Kd = (1.9 +/- 0.4) x 10(-10) M at 37 degrees C, and a maximum binding capacity of 580 +/- 50 fmol/mg of protein (15 degrees C) or 604 +/- 40 fmol/mg of protein (37 degrees C). The kinetically derived dissociation constant was 1.5 x 10(-10) M. 125I-GRP binding was inhibited in a concentration-dependent manner by various peptides containing the highly conserved C-terminal heptapeptide of the bombesin family, including bombesin, GRP, neuromedin B and the 8-14 fragment of bombesin. In contrast, a variety of structurally unrelated mitogens and neuropeptides had no effect. The cross-linking agent ethyleneglycolbis(succinimidylsuccinate) covalently linked 125I-GRP to a single Mr 75 000-85 000 protein in membrane preparations of 3T3 cells. Affinity labelling of this molecule was specific and dependent on the presence of Mg2+ during membrane preparation. Finally, the non-hydrolysable GTP analogue guanosine-5'-[gamma-thio]triphosphate (GTP[S]) caused a concentration-dependent inhibition of 125I-GRP binding and cross-linking to 3T3 cell membranes [concentration giving half-maximal inhibition (IC50) approximately 0.2 microM]. The inhibitory effect was specific (GMP, ATP or ATP[S] had no effect at 10 microM) and was due to an increase in Kd from (1.7 +/- 0.2) x 10(-10) M to (4.3 +/- 0.6) x 10(-10) M in the presence of 10 microM-GTP[S]. This modulation of ligand affinity and cross-linking implies that the bombesin receptors that mediate mitogenesis in Swiss 3T3 cells are coupled to a guanine-nucleotide-binding-protein signal-transduction pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Erspamer V., Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971 Feb 15;27(2):166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Blay J., Irvine R. F., Heslop J. P., Berridge M. J. Reduction of epidermal growth factor receptor affinity by heterologous ligands: evidence for a mechanism involving the breakdown of phosphoinositides and the activation of protein kinase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):377–384. doi: 10.1016/0006-291x(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Laurie M. S., Littlewood C. J., Blakeley D. M., Corps A. N. Characterization of the high-affinity receptors on Swiss 3T3 cells which mediate the binding, internalization and degradation of the mitogenic peptide bombesin. Biochem J. 1988 May 15;252(1):227–235. doi: 10.1042/bj2520227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Cuttitta F., Moody T. W., Minna J. D. Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin-releasing peptide. Cancer Res. 1987 Feb 1;47(3):821–825. [PubMed] [Google Scholar]
- Cirillo D. M., Gaudino G., Naldini L., Comoglio P. M. Receptor for bombesin with associated tyrosine kinase activity. Mol Cell Biol. 1986 Dec;6(12):4641–4649. doi: 10.1128/mcb.6.12.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kochenburger R. J., Castagna M., Blumberg P. M. Kinetics and subcellular localization of specific [3H]phorbol 12, 13-dibutyrate binding by mouse brain. Cancer Res. 1981 Jul;41(7):2640–2647. [PubMed] [Google Scholar]
- Erisman M. D., Linnoila R. I., Hernandez O., DiAugustine R. P., Lazarus L. H. Human lung small-cell carcinoma contains bombesin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2379–2383. doi: 10.1073/pnas.79.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky J. D., Friedberg I., Rozengurt E. Bombesin, diacylglycerols, and phorbol esters rapidly stimulate the phosphorylation of an Mr = 80,000 protein kinase C substrate in permeabilized 3T3 cells. Effect of guanine nucleotides. J Biol Chem. 1988 Dec 15;263(35):19188–19194. [PubMed] [Google Scholar]
- Fanger B. O., Sporn M. B. A binding assay for the solubilized receptors of type beta transforming growth factor: adsorption and removal of free ligand by dextran-coated charcoal. Anal Biochem. 1986 Aug 1;156(2):444–453. doi: 10.1016/0003-2697(86)90278-2. [DOI] [PubMed] [Google Scholar]
- Fischer J. B., Schonbrunn A. The bombesin receptor is coupled to a guanine nucleotide-binding protein which is insensitive to pertussis and cholera toxins. J Biol Chem. 1988 Feb 25;263(6):2808–2816. [PubMed] [Google Scholar]
- Harshman S., Conlin J. G. A simplified procedure for isolating plasma membranes from cultured mouse fibroblast cells: 3T3 and SV-3T3. Anal Biochem. 1978 Oct 1;90(1):98–106. doi: 10.1016/0003-2697(78)90012-x. [DOI] [PubMed] [Google Scholar]
- Heslop J. P., Blakeley D. M., Brown K. D., Irvine R. F., Berridge M. J. Effects of bombesin and insulin on inositol (1,4,5)trisphosphate and inositol (1,3,4)trisphosphate formation in Swiss 3T3 cells. Cell. 1986 Dec 5;47(5):703–709. doi: 10.1016/0092-8674(86)90513-1. [DOI] [PubMed] [Google Scholar]
- Isacke C. M., Meisenhelder J., Brown K. D., Gould K. L., Gould S. J., Hunter T. Early phosphorylation events following the treatment of Swiss 3T3 cells with bombesin and the mammalian bombesin-related peptide, gastrin-releasing peptide. EMBO J. 1986 Nov;5(11):2889–2898. doi: 10.1002/j.1460-2075.1986.tb04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris R. M., Hazan R., Villines J., Moody T. W., Schlessinger J. Identification of the bombesin receptor on murine and human cells by cross-linking experiments. J Biol Chem. 1987 Aug 15;262(23):11215–11220. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letterio J. J., Coughlin S. R., Williams L. T. Pertussis toxin-sensitive pathway in the stimulation of c-myc expression and DNA synthesis by bombesin. Science. 1986 Nov 28;234(4780):1117–1119. doi: 10.1126/science.3465038. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Mendoza S. A., Nånberg E., Sinnett-Smith J., Rozengurt E. Ca2+-mobilizing actions of platelet-derived growth factor differ from those of bombesin and vasopressin in Swiss 3T3 mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5768–5772. doi: 10.1073/pnas.84.16.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelicke A., Fulpius B. W., Klett R. P., Reich E. Acetylcholine receptor. Responses to drug binding. J Biol Chem. 1977 Jul 25;252(14):4811–4830. [PubMed] [Google Scholar]
- McDonald T. J., Jörnvall H., Nilsson G., Vagne M., Ghatei M., Bloom S. R., Mutt V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979 Sep 12;90(1):227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Schneider J. A., Lopez-Rivas A., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. II. Changes in Na+ and Ca2+ fluxes, Na+/K+ pump activity, and intracellular pH. J Cell Biol. 1986 Jun;102(6):2223–2233. doi: 10.1083/jcb.102.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar J. B., Rozengurt E. Bombesin enhancement of cAMP accumulation in Swiss 3T3 cells: evidence of a dual mechanism of action. J Cell Physiol. 1988 Nov;137(2):214–222. doi: 10.1002/jcp.1041370203. [DOI] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. Neuromedin B: a novel bombesin-like peptide identified in porcine spinal cord. Biochem Biophys Res Commun. 1983 Jul 29;114(2):541–548. doi: 10.1016/0006-291x(83)90814-8. [DOI] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. Neuromedin C: a bombesin-like peptide identified in porcine spinal cord. Biochem Biophys Res Commun. 1984 Feb 29;119(1):14–20. doi: 10.1016/0006-291x(84)91611-5. [DOI] [PubMed] [Google Scholar]
- Minamino N., Sudoh T., Kangawa K., Matsuo H. Neuromedin B-32 and B-30: two "big" neuromedin B identified in porcine brain and spinal cord. Biochem Biophys Res Commun. 1985 Jul 31;130(2):685–691. doi: 10.1016/0006-291x(85)90471-1. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Gazdar A. F., Carney D. N., Minna J. D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981 Dec 11;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Rivier J., Brown M. R. Bomebesin: specific binding to rat brain membranes. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5372–5376. doi: 10.1073/pnas.75.11.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand J. N., Kent C. A one-step technique for the subcellular fractionation of total cell homogenates. Anal Biochem. 1986 Nov 15;159(1):157–162. doi: 10.1016/0003-2697(86)90321-0. [DOI] [PubMed] [Google Scholar]
- Nånberg E., Rozengurt E. Temporal relationship between inositol polyphosphate formation and increases in cytosolic Ca2+ in quiescent 3T3 cells stimulated by platelet-derived growth factor, bombesin and vasopressin. EMBO J. 1988 Sep;7(9):2741–2747. doi: 10.1002/j.1460-2075.1988.tb03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Zachary I., Rozengurt E. Rapid dephosphorylation of a Mr 80,000 protein, a specific substrate of protein kinase C upon removal of phorbol esters, bombesin and vasopressin. Biochem Biophys Res Commun. 1986 Oct 15;140(1):379–385. doi: 10.1016/0006-291x(86)91101-0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. W. Bombesin induction of c-fos and c-myc proto-oncogenes in Swiss 3T3 cells: significance for the mitogenic response. J Cell Physiol. 1987 May;131(2):218–225. doi: 10.1002/jcp.1041310211. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemama J. L., Zahidi A., Fourmy D., Fagot-Revurat P., Vaysse N., Pradayrol L., Ribet A. Interaction of [125I]-Tyr4-bombesin with specific receptors on normal human pancreatic membranes. Regul Pept. 1986 Jan;13(2):125–132. doi: 10.1016/0167-0115(86)90220-x. [DOI] [PubMed] [Google Scholar]
- Sinnett-Smith J., Zachary I., Rozengurt E. Characterization of a bombesin receptor on Swiss mouse 3T3 cells by affinity cross-linking. J Cell Biochem. 1988 Dec;38(4):237–249. doi: 10.1002/jcb.240380403. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M. Guanine nucleotide binding proteins and signal transduction. Vitam Horm. 1988;44:47–101. doi: 10.1016/s0083-6729(08)60693-7. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Bollag W. E., Rasmussen H. The effects of bombesin on polyphosphoinositide and calcium metabolism in Swiss 3T3 cells. J Biol Chem. 1987 Jan 5;262(1):182–188. [PubMed] [Google Scholar]
- Thom D., Powell A. J., Lloyd C. W., Rees D. A. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977 Nov 15;168(2):187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll P. J., Rozengurt E. Bombesin and bombesin antagonists: studies in Swiss 3T3 cells and human small cell lung cancer. Br J Cancer. 1988 Jun;57(6):579–586. doi: 10.1038/bjc.1988.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll P. J., Rozengurt E. [D-Arg1,D-Phe5,D-Trp7,9,Leu11]substance P, a potent bombesin antagonist in murine Swiss 3T3 cells, inhibits the growth of human small cell lung cancer cells in vitro. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1859–1863. doi: 10.1073/pnas.85.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. M., Wood J. R., Ghatei M. A., Lee Y. C., O'Shaughnessy D., Bloom S. R. Bombesin, somatostatin and neurotensin-like immunoreactivity in bronchial carcinoma. J Clin Endocrinol Metab. 1981 Dec;53(6):1310–1312. doi: 10.1210/jcem-53-6-1310. [DOI] [PubMed] [Google Scholar]
- Zachary I., Millar J., Nånberg E., Higgins T., Rozengurt E. Inhibition of bombesin-induced mitogenesis by pertussis toxin: dissociation from phospholipase C pathway. Biochem Biophys Res Commun. 1987 Jul 31;146(2):456–463. doi: 10.1016/0006-291x(87)90551-1. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. A substance P antagonist also inhibits specific binding and mitogenic effects of vasopressin and bombesin-related peptides in Swiss 3T3 cells. Biochem Biophys Res Commun. 1986 May 29;137(1):135–141. doi: 10.1016/0006-291x(86)91186-1. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. High-affinity receptors for peptides of the bombesin family in Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7616–7620. doi: 10.1073/pnas.82.22.7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Identification of a receptor for peptides of the bombesin family in Swiss 3T3 cells by affinity cross-linking. J Biol Chem. 1987 Mar 25;262(9):3947–3950. [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Internalization and degradation of peptides of the bombesin family in Swiss 3T3 cells occurs without ligand-induced receptor down-regulation. EMBO J. 1987 Aug;6(8):2233–2239. doi: 10.1002/j.1460-2075.1987.tb02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Modulation of the epidermal growth factor receptor by mitogenic ligands: effects of bombesin and role of protein kinase C. Cancer Surv. 1985;4(4):729–765. [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Woll P. J., Rozengurt E. A role for neuropeptides in the control of cell proliferation. Dev Biol. 1987 Dec;124(2):295–308. doi: 10.1016/0012-1606(87)90483-0. [DOI] [PubMed] [Google Scholar]