Abstract
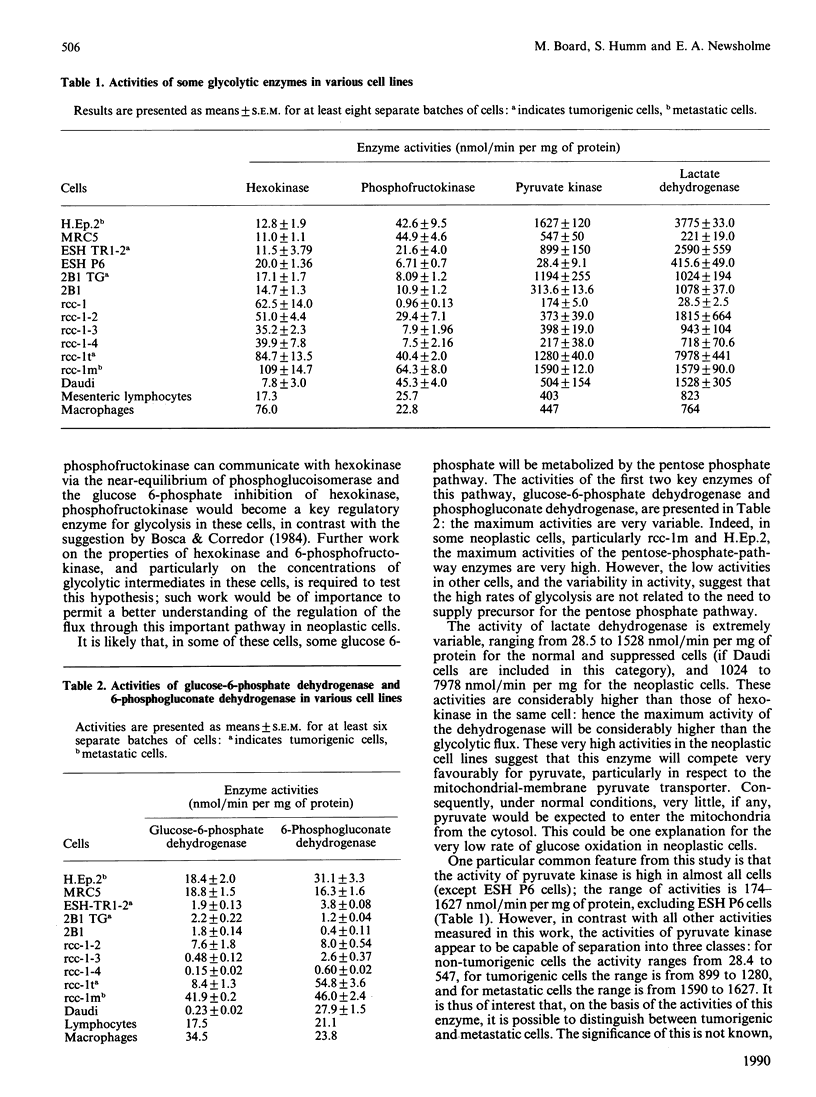
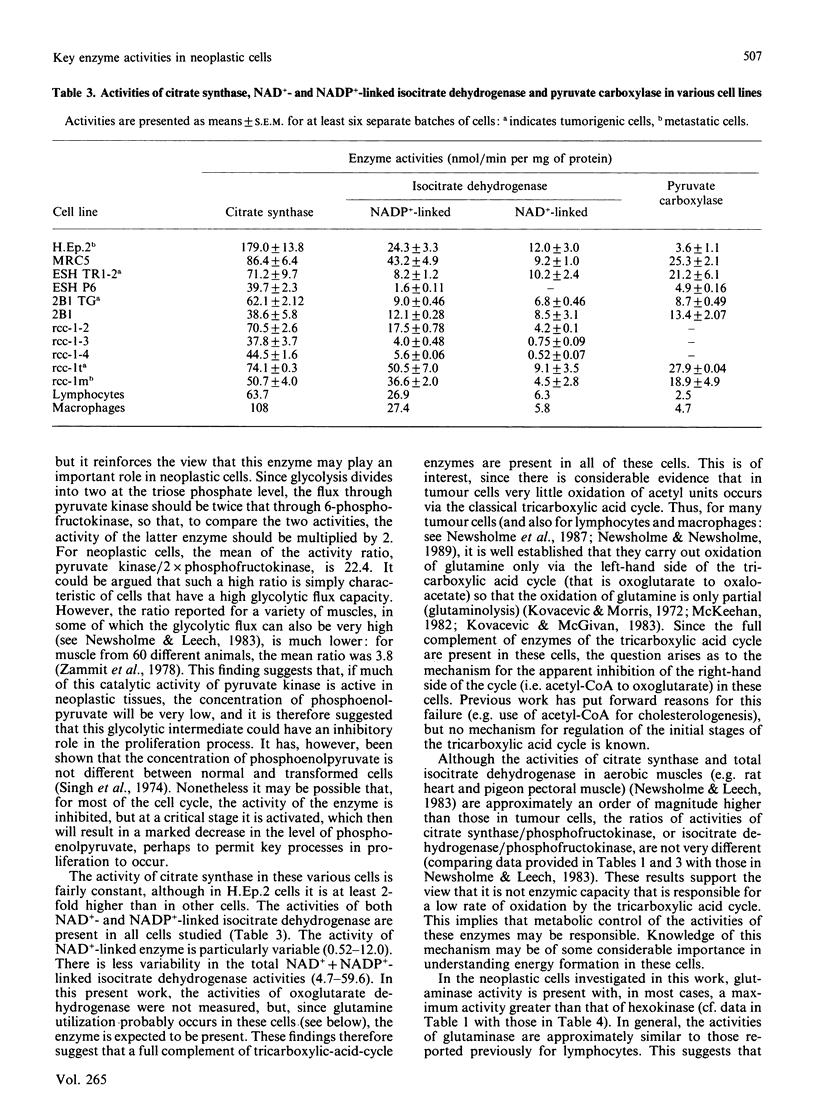
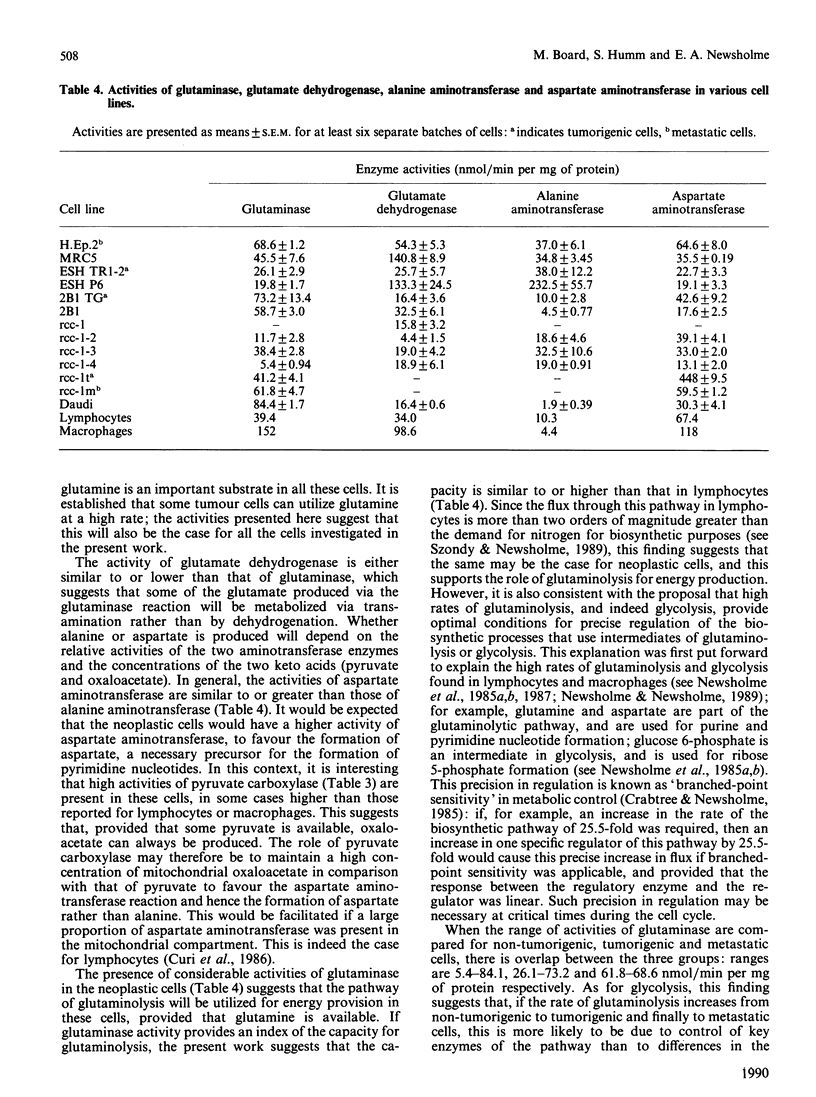
1. Maximal activities of some key enzymes of glycolysis, the pentose phosphate pathway, the tricarboxylic acid cycle and glutaminolysis were measured in homogenates from a variety of normal, neoplastic and suppressed cells. 2. The relative activities of hexokinase and 6-phosphofructokinase suggest that, particularly in neoplastic cells, in which the capacity for glucose transport is high, hexokinase could approach saturation in respect to intracellular glucose; consequently, hexokinase and phosphofructokinase could play an important role in the regulation of glycolytic flux in these cells. 3. The activity of pyruvate kinase is considerably higher in tumorigenic cells than in non-tumorigenic cells and higher in metastatic cells than in tumorigenic cells: for non-tumorigenic cells the activities range from 28.4 to 574, for tumorigenic cells from 899 to 1280, and for metastatic cells from 1590 to 1627 nmol/min per mg of protein. 4. The ratio of pyruvate kinase activity to 2 x phosphofructokinase activity is very high in neoplastic cells. The mean is 22.4 for neoplastic cells, whereas for muscle from 60 different animals it is only 3.8. 5. Both citrate synthase and isocitrate dehydrogenase activities are present in non-neoplastic and neoplastic cells, suggesting that the full complement of tricarboxylic-acid-cycle enzymes are present in these latter cells. 6. In neoplastic cells, the activity of glutaminase is similar to or greater than that of hexokinase, which suggests that glutamine may be as important as glucose for energy generation in these cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardawi M. S., Newsholme E. A. Glutamine metabolism in lymphocytes of the rat. Biochem J. 1983 Jun 15;212(3):835–842. doi: 10.1042/bj2120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardawi M. S., Newsholme E. A. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochem J. 1982 Dec 15;208(3):743–748. doi: 10.1042/bj2080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardawi M. S., Newsholme E. A. Metabolism in lymphocytes and its importance in the immune response. Essays Biochem. 1985;21:1–44. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coleman P. S., Lavietes B. B. Membrane cholesterol, tumorigenesis, and the biochemical phenotype of neoplasia. CRC Crit Rev Biochem. 1981;11(4):341–393. doi: 10.1080/10409238109104421. [DOI] [PubMed] [Google Scholar]
- Cooney G. J., Taegtmeyer H., Newsholme E. A. Tricarboxylic acid cycle flux and enzyme activities in the isolated working rat heart. Biochem J. 1981 Dec 15;200(3):701–703. doi: 10.1042/bj2000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Reiss N. A., Schwartz R. J., Hunter T. Three glycolytic enzymes are phosphorylated at tyrosine in cells transformed by Rous sarcoma virus. Nature. 1983 Mar 17;302(5905):218–223. doi: 10.1038/302218a0. [DOI] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. A quantitative approach to metabolic control. Curr Top Cell Regul. 1985;25:21–76. doi: 10.1016/b978-0-12-152825-6.50006-0. [DOI] [PubMed] [Google Scholar]
- Culvenor J. G., Weidemann M. J. Phytohaemagglutinin stimulation of rat thymus lymphocytes glycolysis. Biochim Biophys Acta. 1976 Jul 21;437(2):354–363. doi: 10.1016/0304-4165(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Curi R., Newsholme P., Newsholme E. A. Intracellular distribution of some enzymes of the glutamine utilisation pathway in rat lymphocytes. Biochem Biophys Res Commun. 1986 Jul 16;138(1):318–322. doi: 10.1016/0006-291x(86)90282-2. [DOI] [PubMed] [Google Scholar]
- Harris H., Miller O. J., Klein G., Worst P., Tachibana T. Suppression of malignancy by cell fusion. Nature. 1969 Jul 26;223(5204):363–368. doi: 10.1038/223363a0. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Radik J. L., Ferber E., Weidemann M. J. Aerobic glycolysis and lymphocyte transformation. Biochem J. 1978 Sep 15;174(3):703–709. doi: 10.1042/bj1740703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibsen K. H., Orlando R. A., Garratt K. N., Hernandez A. M., Giorlando S., Nungaray G. Expression of multimolecular forms of pyruvate kinase in normal, benign, and malignant human breast tissue. Cancer Res. 1982 Mar;42(3):888–892. [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- Kelleher J. K., Bryan B. M., 3rd, Mallet R. T., Holleran A. L., Murphy A. N., Fiskum G. Analysis of tricarboxylic acid-cycle metabolism of hepatoma cells by comparison of 14CO2 ratios. Biochem J. 1987 Sep 15;246(3):633–639. doi: 10.1042/bj2460633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surgace IgM specificity on cells derived from a Burkitt's lymphoma. Lancet. 1967 Nov 18;2(7525):1068–1070. doi: 10.1016/s0140-6736(67)90340-6. [DOI] [PubMed] [Google Scholar]
- Klinger H. P. Suppression of tumorigenicity in somatic cell hybrids. I. Suppression and reexpression of tumorigenicity in diploid human X D98AH2 hybrids and independent segregation of tumorigenicity from other cell phenotypes. Cytogenet Cell Genet. 1980;27(4):254–266. doi: 10.1159/000131494. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z., McGivan J. D. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983 Apr;63(2):547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- Kovacević Z., Morris H. P. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972 Feb;32(2):326–333. [PubMed] [Google Scholar]
- Krebs H. A. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34. [PubMed] [Google Scholar]
- Lazo P. A. Amino acids and glucose utilization by different metabolic pathways in ascites-tumour cells. Eur J Biochem. 1981 Jun;117(1):19–25. doi: 10.1111/j.1432-1033.1981.tb06297.x. [DOI] [PubMed] [Google Scholar]
- MOORE A. E., SABACHEWSKY L., TOOLAN H. W. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955 Oct;15(9):598–602. [PubMed] [Google Scholar]
- Mares-Perlman J. A., Shrago E. Energy substrate utilization in freshly isolated Morris Hepatoma 7777 cells. Cancer Res. 1988 Feb 1;48(3):602–608. [PubMed] [Google Scholar]
- McKeehan W. L. Glycolysis, glutaminolysis and cell proliferation. Cell Biol Int Rep. 1982 Jul;6(7):635–650. doi: 10.1016/0309-1651(82)90125-4. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Ardawi M. S. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985 Oct;70(4):473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Ardawi M. S. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985 May;5(5):393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Zammit V. A. Use of enzyme activities as indices of maximum rates of fuel utilization. Ciba Found Symp. 1979;(73):245–258. doi: 10.1002/9780470720561.ch14. [DOI] [PubMed] [Google Scholar]
- Newsholme P., Curi R., Gordon S., Newsholme E. A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem J. 1986 Oct 1;239(1):121–125. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Gordon S., Newsholme E. A. Rates of utilization and fates of glucose, glutamine, pyruvate, fatty acids and ketone bodies by mouse macrophages. Biochem J. 1987 Mar 15;242(3):631–636. doi: 10.1042/bj2420631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Newsholme E. A. Rates of utilization of glucose, glutamine and oleate and formation of end-products by mouse peritoneal macrophages in culture. Biochem J. 1989 Jul 1;261(1):211–218. doi: 10.1042/bj2610211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peehl D. M., Stanbridge E. J. Characterization of human keratinocyte X HeLa somatic cell hybrids. Int J Cancer. 1981 May 15;27(5):625–635. doi: 10.1002/ijc.2910270509. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979 Apr 25;254(8):2669–2676. [PubMed] [Google Scholar]
- Singh V. N., Singh M., August J. T., Horecker B. L. Alterations in glucose metabolism in chick-embryo cells transformed by Rous sarcoma virus: intracellular levels of glycolytic intermediates. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4129–4132. doi: 10.1073/pnas.71.10.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden P. H., Newsholme E. A. Activities of citrate synthase, NAD+-linked and NADP+-linked isocitrate dehydrogenases, glutamate dehydrogenase, aspartate aminotransferase and alanine aminotransferase in nervous tissues from vertebrates and invertebrates. Biochem J. 1975 Jul;150(1):105–111. doi: 10.1042/bj1500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szondy Z., Newsholme E. A. The effect of glutamine concentration on the activity of carbamoyl-phosphate synthase II and on the incorporation of [3H]thymidine into DNA in rat mesenteric lymphocytes stimulated by phytohaemagglutinin. Biochem J. 1989 Aug 1;261(3):979–983. doi: 10.1042/bj2610979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Nakamura K. D., Salter D. W. Molecular events leading to enhanced glucose transport in Rous sarcoma virus-transformed cells. Fed Proc. 1984 May 15;43(8):2246–2250. [PubMed] [Google Scholar]
- Weinhouse S. Glycolysis, respiration, and anomalous gene expression in experimental hepatomas: G.H.A. Clowes memorial lecture. Cancer Res. 1972 Oct;32(10):2007–2016. [PubMed] [Google Scholar]
- White M. K., Bramwell M. E., Harris H. Hexose transport in hybrids between malignant and normal cells. Nature. 1981 Nov 19;294(5838):232–235. doi: 10.1038/294232a0. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Beis I., Newsholme E. A. Maximum activities and effects of fructose bisphosphate on pyruvate kinase from muscles of vertebrates and invertebrates in relation to the control of glycolysis. Biochem J. 1978 Sep 15;174(3):989–998. doi: 10.1042/bj1740989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Zielke C. L., Ozand P. T. Glutamine: a major energy source for cultured mammalian cells. Fed Proc. 1984 Jan;43(1):121–125. [PubMed] [Google Scholar]


