Abstract
Xanthine oxidase (XO) is an enzyme that converts hypoxanthine into xanthine and xanthine into uric acid, which is then eliminated by the kidneys. Serum XO has been linked to diabetes, hypertension, liver dysfunction, and cardiovascular diseases. However, limited information exists on the relationship between serum XO activity and MetS. This study aimed to analyze the relationship between XO activity and metabolic syndrome (MetS) and its components in an adult population group in Bangladesh A total of 601 participants aged ≥18 years were included in the study. MetS was defined based on the criteria set by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III). Serum XO activity was measured using the enzyme-linked immunosorbent assay (ELISA), while other biochemical parameters were measured using colorimetric methods. The relationship between serum XO and MetS levels was determined through multivariate logistic regression analysis. Serum XO activity was found to be significantly higher in females (6.17 ± 3.77 U/L) as compared to males (4.00 ± 2.77 U/L) (p < 0.001). Furthermore, participants with MetS had significantly higher mean levels of serum XO (5.34 ± 3.39 U/L) than those without MetS (3.86 ± 2.90 U/L) (p < 0.001). The prevalence of MetS and its components, such as blood pressure and blood glucose increased across the XO quartiles (p < 0.001). Regression analysis indicated that XO activity was significantly and independently associated with the prevalence of MetS (at least p < 0.05 for all cases) and its components, including elevated blood pressure, high blood glucose, and low HDL-C (at least p < 0.05 for all cases). In conclusion, individuals with MetS had significantly higher XO levels than those without MetS. Serum XO activity showed an independent association with MetS and some of its components. Therefore, XO might serve as a useful marker of MetS. Prospective studies are needed to determine the underlying mechanisms linking XO and MetS.
Keywords: Xanthine oxidase, Association, Metabolic syndrome, Bangladeshi adults
Subject terms: Biomarkers, Diagnostic markers, Predictive markers
Introduction
Xanthine oxidase (XO) is a type of enzyme that belongs to the xanthine oxidoreductase (XOR) family. It generates reactive oxygen species (ROS) and catalyzes the oxidation of hypoxanthine to xanthine and xanthine to uric acid1. Another form of XOR is xanthine dehydrogenase (XDH). Both XDH and XO are single-gene products that exist in separate but inter-convertible forms2. XDH is mostly present inside the cell, whereas XO is found in biological fluids like milk and blood plasma3,4. Apart from the significant role of XO in producing uric acid in the human body, it is also responsible for producing a considerable amount of reactive oxygen species (ROS) such as H2O2 and O2⋅−5,6 in mammalian cells. ROS are linked to increased blood pressure through endothelial dysfunction, vascular inflammation, and structural remodeling7. In recent years, there has been increasing attention given to XO. Animal studies have demonstrated that oxidants produced by XO have a greater impact on arterial blood pressure in spontaneously hypertensive rats8.
It has been reported that XO activity is significantly elevated in the vasculature of patients with coronary artery disease9. Additionally, XO activity has been linked to cardiovascular events and an increased risk of future events in patients with chronic heart failure10.
Metabolic syndrome (MetS) is a complex medical condition that results from the presence of multiple metabolic disorders11. These disorders include obesity, particularly visceral obesity, dyslipidemia with high triglycerides and/or low levels of high-density lipoprotein cholesterol (HDL-C), hypertension, and fasting hyperglycemia11. A person is diagnosed with MetS if they have three or more of these conditions11,12. The risk factors associated with MetS are predictive of both cardiovascular diseases and type 2 diabetes.
It has long been recognized that high levels of uric acid in the blood also called hyperuricemia can increase the risk of various cardiometabolic diseases13,14. However, some previous studies have suggested that hyperuricemia may not be an independent risk factor for such diseases, but rather a marker for other underlying conditions15,16. As a result, there is still ongoing debate about whether hyperuricemia should be considered an independent risk factor for cardiometabolic diseases. In contrast, XO has been suggested to be linked with diabetes and cardiovascular events in humans17,18. A study conducted in Japan showed that plasma XOR activity was positively linked with BMI, liver enzymes, TG and fasting plasma insulin19. Serum XO activity has been suggested to be related to obesity, hypertension, and diabetes in the general population18,20–22. However, there is limited information on the relationship between XO and metabolic parameters and MetS, which needs further clarification. Therefore, we hypothesize that serum XO activity could be a useful marker for MetS. In this study, we measured serum XO activity and determined its associations with MetS in an adult cohort in Bangladesh.
Materials and methods
Study population
The study was conducted at the Department of Biochemistry and Molecular Biology of Shahjalal University of Science and Technology, Sylhet, Bangladesh. It was a cross-sectional design carried out between December 2019 and November 2020. A simple random sampling technique was used to select participants from different backgrounds such as local city people, students, and academic and non-academic staff from the Sylhet and Dhaka regions of Bangladesh. A total of 601 participants, aged 18 years and above, were included in the study. The inclusion criteria for the study were being above 18 years of age, willing to participate and free from severe chronic illness. The study excluded pregnant women, lactating mothers, and those with a history of hepatotoxic drug intake, kidney disease, alcohol intake, and acute or chronic hepatitis. Also, participants with missing anthropometric data or blood samples were excluded. The study was approved by the Internal Ethics Committee at the Department of Biochemistry and Molecular Biology of Shahjalal University of Science and Technology, Bangladesh (Reference no 02/BMB/2019). Prior to being included in the study, all participants provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Data collection
The participants' demographic and lifestyle information were collected using a standard questionnaire. The questionnaire included recording age, gender, weight, and height, following a standard procedure described elsewhere23–29. The participant’s systolic and diastolic blood pressure (SBP and DBP) were measured twice in the left arm using an automated sphygmomanometer (Omron M10, Omron Corporation, Tokyo, Japan) while seated after resting for at least 10 min. Body weight (BW) was measured using a calibrated digital weighing machine (Beurer 700, Germany) to the nearest 0.1 kg, and body height (BH) was recorded to the nearest 0.1 cm using a height measuring tape. The body mass index (BMI) was calculated as weight in kg divided by height in square meters (kg/m2). Waist circumference (WC) was measured using a general tape midway between the lowest border of the ribs and the iliac crest. Hip circumference (HC) was measured at the largest circumference of the buttocks to the nearest 0.5 cm. Measurements were taken without shoes and with minimal clothing.
Sample collection and laboratory analysis
Venous blood samples were collected from each subject after an overnight fast. The samples were then centrifuged and the isolated serum was stored at − 20 °C until laboratory analysis. XO activity in the serum was measured using an enzyme-linked immunosorbent assay (ELISA) with a plate reader (ELISA reader, Apollo 11 LB 913, Berthold, Germany). The Human XO ELISA Kit was purchased from MyBioSource company, USA (Cat No: MBS774009). In addition, other biochemical markers such as serum uric acid (SUA), fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using a colorimetric method and a semi-automatic biochemistry analyzer (Humalyzer 3000, Medicon Services, Germany), following the manufacturer's protocol (HUMAN Gesellschaft für Biochemica und Diagnostica mbH, Germany). The measurements were carried out according to the standard manufacturer's protocols provided within the kit, and the precision of the measurements was maintained regularly by method standard calibration.
Serum XO measurement
The level of Serum XO was measured as per the manufacturing protocol. In brief, standards and serum samples were added to the micro-wells that had previously been coated with antibodies. Subsequently, HRP-conjugate solution was added to these wells to form an immune complex. After an incubation period of 1 h at 37 °C, the unbound enzyme was removed by washing the wells. Substrates were then added, causing the solution to turn blue and eventually yellow. The intensity of the yellow colour was directly proportional to the levels of XO present in the serum sample. After 15 min, the absorbance (OD) of each well was measured at 450 nm using an ELISA reader (Apollo 11 LB 913, Berthold, Germany). Finally, XO activity in the samples was calculated based on the standard graph prepared in an excel sheet.
Diagnostic criteria
MetS was defined using the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) criteria12. It is characterized by having at least three of the following components: WC of ≥ 90 cm in men or ≥ 80 cm in women; TG levels of ≥ 150 mg/dl; HDL-C levels of < 40 mg/dl in men and < 50 mg/dl in women; blood pressure levels of ≥ 130/85 mmHg or taking antihypertensive medication; FBG levels of ≥ 110 mg/dl or taking antidiabetic medicines. All participants were divided into four quartiles based on their serum XO concentrations, with Q1: ≤ 2.23 U/L, Q2: 2.24–3.72 U/L, Q3: 3.73–6.15 U/L, and Q4: > 6.15 U/L. Hyperuricemia was defined as SUA concentration of > 7.0 mg/dL (416.4 µmol/L) in men or > 6.0 mg/dL (356.9 µmol/L) in women30. Physical activity was classified into three groups: inadequate (comfortable office work and housework), medium (walking, swimming), and adequate (carrying, lifting, jogging, and/or sports). The smoking status was divided into two categories: never smokers and current smokers.
Statistical analysis
Statistical data analyses were conducted using IBM SPSS statistics (version 23), Chicago, IL, USA. We have plotted Q-Q plot and histogram to check the normality of the data set. The data is presented as mean ± SD. The baseline characteristics of the volunteers in the gender group were compared using an independent sample t-test, while one-way ANOVA was used for the XO quartiles. To differentiate the proportions of the categorical variables, a Chi-square test was applied. The association between XO activity and MetS was assessed using multivariate logistic regression models. MetS was categorized as either "yes" (presence) or "no" (absence). In the regression analysis, MetS (yes) was considered the dependent variable, while XO served as the independent variable. Reference category was XO level Q1. Three models were used in the regression analysis. Model 1 was adjusted for age (years) and gender (male and female). Model 2 was adjusted for model 1 and SUA (mg/dL). Model 3 was adjusted for variables in model 2 and physical activity (low vs medium/adequate), and smoking (yes vs no). A p-value of less than 0.05 was considered statistically significant.
Results
Participant’s characteristics in the MetS and non-MetS group
Table 1 presents the baseline characteristics of the participants in the MetS and non-MetS groups. Out of the total 601 participants, 271 individuals (198 male and 73 female) were diagnosed with MetS based on the diagnostic criteria. The MetS group had significantly higher mean values of age, WC, HC, BMI, SBP and DBP as compared to the non-MetS group (p < 0.001 for all cases). Additionally, the mean level of XO was significantly higher in the MetS group (5.34 ± 3.39 U/L) in contrast to the non-MetS group (3.86 ± 2.90 U/L) (p < 0.001). There was also a significant difference in the mean level of other biochemical parameters such as FBG, lipid profile (TG, TC, HDL-C, LDL-C), and SUA (at least p < 0.05 for all cases). However, no significant difference was found between the MetS and non-MetS groups for smoking and physical activity.
Table 1.
Baseline characteristics of the participants based on the presence of MetS.
| Non-MetS | MetS | P-value | |
|---|---|---|---|
| N | 330 | 271 | |
| Gender, m/f | 244/86 | 198/73 | – |
| Age (years) | 36.89 ± 13.59 | 42.76 ± 12.38 | 0.000 |
| WC (cm) | 82.57 ± 9.81 | 88.42 ± 11.54 | 0.000 |
| HC (cm) | 91.22 ± 8.34 | 93.33 ± 8.68 | 0.009 |
| BMI (kg/m2) | 23.72 ± 3.63 | 25.62 ± 3.62 | 0.000 |
| SBP (mmHg) | 119.30 ± 12.48 | 134.48 ± 14.77 | 0.000 |
| DBP (mmHg) | 77.16 ± 8.12 | 88.07 ± 8.83 | 0.000 |
| FBG (mmol/L) | 6.11 ± 2.92 | 8.06 ± 4.17 | 0.000 |
| TG (mg/dL) | 151.94 ± 92.50 | 225.34 ± 122.98 | 0.000 |
| TC (mg/dL) | 182.16 ± 53.80 | 223.95 ± 85.21 | 0.000 |
| HDL-C (mg/dL) | 35.00 ± 12.31 | 31.70 ± 11.76 | 0.003 |
| LDL-C (mg/dL) | 117.72 ± 48.58 | 151.61 ± 78.60 | 0.000 |
| XO (U/L) | 3.86 ± 2.90 | 5.34 ± 3.39 | 0.000 |
| SUA (mg/dL) | 6.09 ± 1.92 | 5.69 ± 1.93 | 0.014 |
| Hyperuricemia (%) | 29.2 | 25.6 | 0.327 |
| Diabetes (%) | 24.5 | 53.1 | 0.000 |
| Hypertension (%) | 19.1 | 64.6 | 0.000 |
| Physical activity (%) | |||
| Low | 20.9 | 24.2 | 0.402 |
| Moderate/adequate | 79.1 | 75.8 | 0.470 |
| Smoking (%) | |||
| Yes | 23.0 | 18.8 | 0.208 |
| No | 77.0 | 81.2 | 0.247 |
MetS: Metabolic Syndrome. Values are presented as mean ± SD. P-values for continuous and categorical variables are obtained from independent sample t-test and chi-square test, respectively.
Prevalence of MetS and its components by gender
The prevalence of MetS and its components are displayed in Table 2 and Fig. 1. Overall, 45.1% of the subjects had MetS, with 44.8% in males and 45.9% in females. The prevalence of MetS components such as increased WC, elevated SBP, and TG levels was significantly higher in males than in females (p < 0.05 for all cases). Conversely, the levels of elevated FBG and HDL-C were significantly higher in females than in males (p < 0.01 for both cases). The prevalence of hyperuricemia was 27.6%, with a significant difference between males (30.5%) and females (19.5%) (p < 0.01). There was no significant difference in the prevalence of MetS between males (44.8%) and females (45.9%). Males were more likely to engage either in moderate or adequate physical activity than females (p < 0.01). Only male subjects were habitual smokers.
Table 2.
Baseline characteristics of the participants according to the sex groups.
| Total | Female | Male | P-value | |
|---|---|---|---|---|
| N | 601 | 159 | 442 | – |
| Age (year) | 39.44 ± 13.37 | 39.24 ± 13.87 | 39.51 ± 13.20 | 0.833 |
| WC (cm) | 84.99 ± 10.96 | 82.44 ± 10.79 | 86.08 ± 10.88 | 0.001 |
| HC (cm) | 92.13 ± 8.54 | 91.52 ± 8.75 | 92.39 ± 8.45 | 0.323 |
| BMI (kg/m2) | 24.55 ± 3.74 | 24.43 ± 4.46 | 24.59 ± 3.45 | 0.637 |
| SBP (mmHg) | 126.16 ± 15.51 | 123.92 ± 17.57 | 126.96 ± 14.64 | 0.034 |
| DBP (mmHg) | 82.09 ± 10.04 | 88.93 ± 10.68 | 82.50 ± 9.78 | 0.091 |
| FBG (mmol/L) | 6.99 ± 3.66 | 7.77 ± 4.07 | 6.71 ± 3.48 | 0.002 |
| TG (mg/dL) | 187.31 ± 114.21 | 171.09 ± 124.68 | 193.11 ± 109.81 | 0.046 |
| TC (mg/dL) | 201.42 ± 73.02 | 215.34 ± 86.71 | 197.14 ± 67.81 | 0.019 |
| HDL-C (mg/dL) | 33.48 ± 12.16 | 36.61 ± 10.06 | 32.52 ± 12.59 | 0.002 |
| LDL-C (mg/dL) | 133.34 ± 66.31 | 151.01 ± 83.71 | 127.90 ± 59.02 | 0.001 |
| XO (U/L) | 4.62 ± 3.24 | 6.17 ± 3.77 | 4.00 ± 2.77 | 0.000 |
| SUA (mg/dL) | 5.91 ± 1.94 | 4.82 ± 1.92 | 6.29 ± 1.79 | 0.034 |
| MetS (%) | 45.1 | 45.9 | 44.8 | 0.853 |
| Hyperuricemia (%) | 27.6 | 19.5 | 30.5 | 0.008 |
| Diabetes (%) | 37.5 | 53.8 | 31.7 | 0.000 |
| Hypertension (%) | 39.7 | 40.3 | 39.5 | 0.860 |
| Physical activity (%) | ||||
| Low | 23.3 | 31.6 | 18.3 | 0.002 |
| Moderate/adequate | 77.7 | 68.4 | 81.7 | 0.003 |
| Smoking (%) | ||||
| Yes | 21.1 | 0.0 | 28.7 | 0.000 |
| No | 78.9 | 100.0 | 71.3 | 0.000 |
MetS: Metabolic Syndrome. Values are presented as mean ± SD. P-values for continuous and categorical variables are obtained from independent sample t-test and chi-square test, respectively.
Fig. 1.
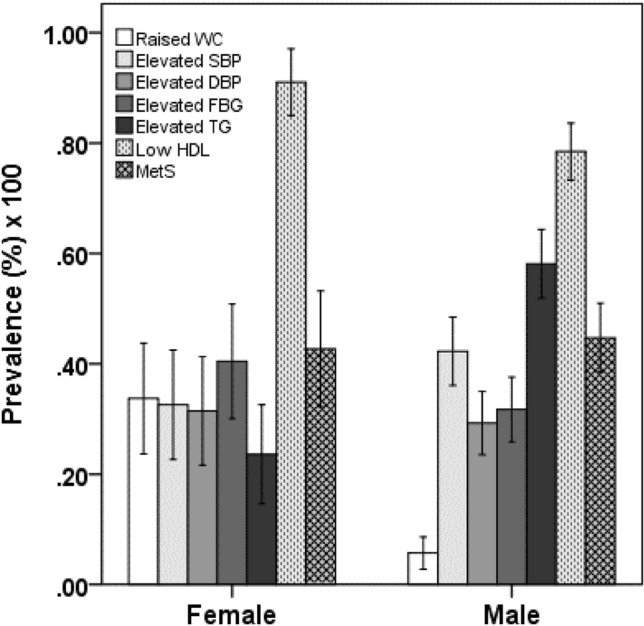
Prevalence of MetS and its components in the gender group. P < 0.001 when the prevalence of raised WC, elevated FBG and elevated TG is compared between the sex groups. P < 0.05 when the prevalence of low HDL-C is compared between the sex groups. P-values are obtained from chi-square test.
Prevalence of MetS and its components across the XO quartiles
The levels of XO were categorized into four groups, based on quartiles. The first group, Q1, had levels of ≤ 2.23 U/L, the second group, Q2, had levels ranging from 2.24 to 3.72 U/L, the third group, Q3, had levels ranging from 3.73 to 6.15 U/L, and the fourth group, Q4, had levels greater than 6.15 U/L (as shown in Table 3). The prevalence of MetS increased significantly across the quartiles of XO, with percentages of 38.6%, 39%, 61.1%, and 67.1% (p < 0.001). There was also a significant increasing trend observed for elevated SBP and FBG across the quartiles of XO. However, there was a significant decreasing trend observed for low HDL-C across the quartiles of XO (p < 0.05).
Table 3.
Prevalence of MetS and its components in the XO quartile groups.
| Parameters | Serum XO | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P-value | |
| Raised WC (%) | 11.6 | 23.2 | 12.7 | 15.8 | 0.335 |
| Elevated SBP (%) | 31.7 | 39.0 | 65.5 | 61.0 | 0.000 |
| Elevated DBP (%) | 42.7 | 43.9 | 46.4 | 46.3 | 0.952 |
| Elevated FBG (%) | 24.1 | 40.2 | 60.7 | 73.2 | 0.000 |
| Elevated TG (%) | 55.4 | 45.6 | 62.5 | 65.8 | 0.074 |
| Low HDL-C (%) | 92.8 | 79.1 | 73.0 | 85.1 | 0.021 |
| MetS (%) | 38.6 | 39.0 | 61.1 | 67.1 | 0.000 |
XO quartiles, Q1: ≤ 2.23 U/L, Q2: 2.24–3.72 U/L, Q3: 3.73–6.15 U/L, Q4: > 6.15 U/L. P-values are obtained from chi-square test.
Association of XO with the prevalence of MetS and its components
Multivariate logistic regression was applied to investigate the relationship between XO and MetS, with XO as the independent variable and MetS as the dependent variable. The results of the regression analysis are presented in Table 4. In all four models, there was a positive and significant association between XO and MetS (at least p < 0.05 for all cases). We also examined the relationship between XO and individual components of MetS (Table 5). After adjusting for age and sex, a significant association was found between XO and three MetS components, namely high blood pressure (p < 0.01), hyperglycemia (p < 0.01), and low HDL-C (p < 0.05).
Table 4.
logistic regression analysis to assess the relationship between XO and MetS.
| OR (95% CI) | P-value for trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Model 1 | 1 | 0.880 (0.448–1.729) | 2.129 (1.082–4.188) | 2.565 (1.082–5.216) | < 0.05 |
| Model 2 | 1 | 0.890 (0.443–1.788) | 2.063 (1.027–4.145) | 2.881 (1.374–6.039) | < 0.05 |
| Model 3 | 1 | 0.863 (0.428–1.740) | 2.052 (1.020–4.126) | 2.893 (1.377–6.075) | < 0.05 |
XO quartiles, Q1: ≤ 2.23 U/L, Q2: 2.24–3.72 U/L, Q3: 3.73–6.15 U/L, Q4: > 6.15 U/L. Dependent variable is MetS (yes) and independent variable is XO (U/L). Reference category is XO level Q1. Model 1: adjusted for age (years) and gender (male and female). Model 2: model 1 + SUA (mg/dL). Model 3: model 2 + physical activity (low vs medium/adequate) and smoking (Yes vs No). OR, odds ratio; CI, confidence interval.
Table 5.
Age and sex-adjusted multivariate logistic regression analysis to assess the relationship between XO and the components of MetS.
| OR (95% CI) | P-value for trend | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Abdominal obesity | 1 | 1.662 (0.466–5.921) | 0.673 (0.181–2.506) | 0.405 (0.110–1.495) | > 0.05 |
| High blood pressure | 1 | 1.164 (0.585–2.314) | 3.314 (1.652–6.647) | 3.001 (1.463–6.158) | < 0.01 |
| Hyperglycemia | 1 | 1.003 (0.478–2.105) | 2.812 (1.329–5.949) | 3.839 (1.730–8.520) | < 0.01 |
| High TG | 1 | 0.618 (0.299–1.276) | 1.204 (0.588–2.465) | 1.731 (0.806–3.718) | > 0.05 |
| Low HDL-C | 1 | 0.422 (0.140–1.269) | 0.333 (0.112–0.993) | 0.663 (0.193–2.274) | < 0.05 |
XO quartiles, Q1: ≤ 2.23 U/L, Q2: 2.24–3.72 U/L, Q3: 3.73–6.15 U/L, Q4: > 6.15 U/L. The dependent variable is MetS components (yes) and the independent variable is XO (U/L). Reference category is XO level Q1. The model is adjusted for age (years) and sex (male and female). OR, odds ratio; CI, confidence interval.
Discussion
The present study evaluated the potential relationship between XO activity and MetS in a cohort of adult individuals in Bangladesh. Previous studies have demonstrated a possible link between XO and hypertension and cardiovascular disease, yet there is currently a scarcity of information pertaining to the relationship between SUA and MetS in the adult population at large. Our investigation shows a significant association between XO activity and MetS, as well as its individual components, among Bangladeshi adults.
Our study found that females have significantly higher serum XO activity than males. The participants with MetS had significantly higher serum XO activity than those without MetS. We also observed an increased prevalence of MetS and its components across the XO quartiles. Regression analysis showed a significant association between serum XO activity and MetS and its components, including elevated blood pressure, high blood glucose, and low HDL-C. Our findings align with some previous studies conducted on general individuals and type 2 diabetes patients17,19,31,32. A study conducted in Brazil included 17 participants, consisting of 9 healthy individuals and 8 individuals with MetS17. The study showed that the MetS group had higher XO activity compared to the healthy control group and XO activity was positively correlated with SOD, hs-CRP, WC, and BMI. Similarly, a study in a general population group in Japan (n = 627) reported a positive correlation between plasma XOR activity and various factors such as BMI, WC, blood pressure, and liver enzyme31. Another study showed that participants (n = 98) with type 2 diabetes had a positive correlation between XOR and TG and a negative correlation with HDL-C19. The study also indicated that XOR activity was positively associated with BMI, liver enzymes, plasma insulin, and insulin resistance. XO activity was also found to be independently associated with albuminuria in patients with type 2 diabetes33 and MetS34 in other population groups. In a further study conducted in Japan, plasma XO activity was found to be correlated with indices of insulin resistance and liver dysfunction in participants (n = 60) with type 2 diabetes mellitus and MetS32. It is worth mentioning that most of the previous studies had a small number of participants and a history of diabetes, which limits their generalizability. Additionally, the studies did not establish a direct association between XO and MetS. In our study, we included a moderate number of participants, including healthy, hypertensive, and diabetic subjects, and our findings suggest an independent association of XO activity with MetS.
The two main mechanisms that cause metabolic derangement are insulin resistance and an abundance of circulating free fatty acids35. Adipose tissue produces pro-inflammatory cytokines that cause insulin resistance, lipolysis, and liver production of pro-thrombotic factors35. This abnormal production leads to an inflammatory chronic state, resulting in endothelial dysfunction and a pro-thrombotic state (reviewed in Ref.36). High levels of free fatty acids and impaired insulin activity can lead to hyperglycemia and insulin resistance (reviewed in Refs.37,38. Insulin resistance may indue hypertension due to increased renal sodium reabsorption and the secretion of angiotensinogen, leptin, and resistin by adipose tissue39. Insulin resistance also impairs endothelial function by reducing nitric oxide-dependent vasodilation (reviewed in Ref.37). MetS and hyperinsulinemia are associated with inflammation and oxidative stress, which can lead to high levels of oxidized LDL and increase the risk of atherosclerosis40,41. Hyperinsulinemia can also cause urate reabsorption, leading to hyperuricemia, commonly observed in MetS (reviewed in Refs.42,43).
As discussed above, XOR activity in human blood is positively correlated with insulin resistance, BMI, and liver dysfunctions. In a study, allopurinol treatment has been found to improve insulin resistance and lower SUA and systemic inflammation in subjects with asymptomatic hyperuricemia44. Women who have Polycystic Ovary Syndrome (PCOS) have a higher likelihood of developing MetS. For women with PCOS (n = 45), high serum XO activity was shown to be correlated with high serum levels of C-reactive protein, fasting glycemia, fasting insulinemia, and insulin resistance index45. These findings imply that XOR activity may play a role in the development of these metabolic disorders.
The role of XOR in MetS has been indirectly demonstrated in animal models and clinical studies. Inhibiting XOR with allopurinol in a mouse model of MetS has been found to reduce inflammation, insulin resistance, and increase adiponectin production46. In a rat model with MetS, allopurinol's XO inhibition reversed hypertension and proteinuria without changing body weight gain, hyperinsulinemia, or fasting hyperglycemia47. XO inhibition also reversed kidney factors linked to hypertension and proteinuria, including TGF-β, collagen deposition, and angiotensin II and receptor expression47. Studies demonstrated that elevated XOR activity may raise blood pressure through multiple mechanisms including (1) the endothelial dysfunction caused by ROS, RNS and uric acid-derived free radicals, (2) the remodeling of arterioles, (3) the up-regulation of the renin/angiotensin pathway, and (4) the inflammatory reactions (reviewed in Refs.16,48). Increased XOR activity leads to oxidative stress, impairing NO-mediated vasodilation, whereas, inhibition of XOR by allopurinol, has been found to improve vascular function and myocardial function in some clinical trials. This suggests a direct link between XOR-generated ROS and endothelial dysfunction, which can lead to MetS49.
There are certain limitations to our study. First, the sample size was relatively small. Second, our data was collected in a cross-sectional manner, which makes it difficult to establish a causal relationship between XO activity and MetS. Thirdly, participants with type 2 diabetes had taken some medications that could have influenced metabolic markers. For example, one study showed that taking metformin decreased plasma XO activity and thiobarbituric acid reactive substances50, while another study found no statistical difference between plasma XO activities and taking metformin or not31. While we took into account some potential confounding factors, there may be others that we did not consider. For example, we did not have access to data on renal function tests, which can also impact XO activity. Therefore, we should be cautious when extrapolating our findings to other populations. Although there are some limitations, our findings can serve as a valuable reference point for future studies.
Conclusions
The present study indicates that the prevalence of MetS and its components increases with higher levels of XO across the quartile groups. Elevated levels of XO were significantly associated with MetS and its components, including elevated blood pressure, blood glucose, and low HDL-C levels. The measurements of XO activity in blood may provide clues about the risk of developing MetS. However, further studies are necessary to examine the role of XO in the pathogenesis of MetS.
Acknowledgements
The authors would like to express their gratitude towards all the volunteers who have actively cooperated in the study. We are also grateful to the SUST research centre for their support of this study with an grant (LS/2018/2/07).
Author contributions
N.A., played a major role in the conception and design of the study, data analysis and interpretation and wrote the manuscript. A.T., N. I., and N.Z.S., contributed to sample collection and laboratory experiments. F.I. helped in the result analysis and revision of the manuscript draft. All authors read the manuscript and approved the final version.
Data availability
All data generated or analysed during this study are included in this published article
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hille, R. & Nishino, T. Xanthine oxidase and xanthine dehydrogenase. FASEB J.9, 995–1003 (1995). 10.1096/fasebj.9.11.7649415 [DOI] [PubMed] [Google Scholar]
- 2.Chung, H. Y. et al. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. AGE20, 127–140 (1997). 10.1007/s11357-997-0012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron, A. J., Shaw, J. E. & Zimmet, P. Z. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol. Metab. Clin. N. Am.33, 351–375 (2004). 10.1016/j.ecl.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 4.Pacher, P. A. L., Nivorozhkin, A. & Szabó, C. Therapeutic effects of xanthine oxidase inhibitors: Renaissance half a century after the discovery of allopurinol. Pharmacol. Rev.58, 87–114 (2006). 10.1124/pr.58.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry, C. E. & Hare, J. M. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J. Physiol.555, 589–606 (2004). 10.1113/jphysiol.2003.055913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley, E. E. Dispelling dogma and misconceptions regarding the most pharmacologically targetable source of reactive species in inflammatory disease, xanthine oxidoreductase. Arch. Toxicol.89, 1193–1207 (2015). 10.1007/s00204-015-1523-8 [DOI] [PubMed] [Google Scholar]
- 7.Touyz, R. M. & Briones, A. M. Reactive oxygen species and vascular biology: Implications in human hypertension. Hypertens. Res.34, 5–14 (2011). 10.1038/hr.2010.201 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki, H. et al. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl. Acad. Sci.95, 4754–4759 (1998). 10.1073/pnas.95.8.4754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiekermann, S. et al. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: Relation to endothelium-dependent vasodilation. Circulation107, 1383–1389 (2003). 10.1161/01.CIR.0000056762.69302.46 [DOI] [PubMed] [Google Scholar]
- 10.Otaki, Y. et al. Association of plasma xanthine oxidoreductase activity with severity and clinical outcome in patients with chronic heart failure. Int. J. Cardiol.228, 151–157 (2017). 10.1016/j.ijcard.2016.11.077 [DOI] [PubMed] [Google Scholar]
- 11.Alberti, K. G. M. M. et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation120, 1640–1645 (2009). 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 12.Cleeman, J. I., Grundy, S. M., Becker, D. & Clark, L. Expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP III). JAMA285, 2486–2497 (2001). 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 13.Fang, J. & Alderman, M. H. Serum uric acid and cardiovascular mortality: The NHANES I epidemiologic follow-up study, 1971–1992. Jama283, 2404–2410 (2000). 10.1001/jama.283.18.2404 [DOI] [PubMed] [Google Scholar]
- 14.Levine, W., Dyer, A. R., Shekelle, R. B., Schoenberger, J. A. & Stamler, J. Serum uric acid and 11.5-year mortality of middle-aged women: Findings of the Chicago Heart Association Detection Project in Industry. J. Clin. Epidemiol.42, 257–267 (1989). 10.1016/0895-4356(89)90061-9 [DOI] [PubMed] [Google Scholar]
- 15.Gagliardi, A. C., Miname, M. H. & Santos, R. D. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis202, 11–17 (2009). 10.1016/j.atherosclerosis.2008.05.022 [DOI] [PubMed] [Google Scholar]
- 16.Pasalic, D., Marinkovic, N. & Feher-Turkovic, L. Uric acid as one of the important factors in multifactorial disorders–facts and controversies. Biochem. Medica22, 63–75 (2012). 10.11613/BM.2012.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feoli, A. M. P., Macagnan, F. E., Piovesan, C. H., Bodanese, L. C. & Siqueira, I. R. Xanthine oxidase activity is associated with risk factors for cardiovascular disease and inflammatory and oxidative status markers in metabolic syndrome: Effects of a single exercise session. Oxid. Med. Cell. Longev.2014, 1–8 (2014). 10.1155/2014/587083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, X. et al. Elevated serum xanthine oxidase activity is associated with the development of type 2 diabetes: A prospective cohort study. Diabetes Care41, 884–890 (2018). 10.2337/dc17-1434 [DOI] [PubMed] [Google Scholar]
- 19.Okuyama, T. et al. Association of the plasma xanthine oxidoreductase activity with the metabolic parameters and vascular complications in patients with type 2 diabetes. Sci. Rep.11, 3768 (2021). 10.1038/s41598-021-83234-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan, M. et al. Assessment of the relationship between serum xanthine oxidase levels and type 2 diabetes: A cross-sectional study. Sci. Rep.12, 20816 (2022). 10.1038/s41598-022-25413-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miah, R. et al. Association of serum xanthine oxidase levels with hypertension: A study on Bangladeshi adults. Sci. Rep.12, 21727 (2022). 10.1038/s41598-022-26341-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klisic, A. et al. Body mass index is independently associated with xanthine oxidase activity in overweight/obese population. Eat. Weight Disord. Stud. Anorex. Bulim. Obes.25, 9–15 (2020). 10.1007/s40519-018-0490-5 [DOI] [PubMed] [Google Scholar]
- 23.Ali, N., Kathak, R. R., Fariha, K. A., Taher, A. & Islam, F. Prevalence of dyslipidemia and its associated factors among university academic staff and students in Bangladesh. BMC Cardiovasc. Disord.23, 366 (2023). 10.1186/s12872-023-03399-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali, N., Samadder, M., Mahmud, F. & Islam, F. Association between liver enzymes and metabolic syndrome: A study in Bangladeshi adults. Expert Rev. Endocrinol. Metab.18, 541–547 (2023). 10.1080/17446651.2023.2272867 [DOI] [PubMed] [Google Scholar]
- 25.Ali, N., Samadder, M., Shourove, J. H., Taher, A. & Islam, F. Prevalence and factors associated with metabolic syndrome in university students and academic staff in Bangladesh. Sci. Rep.13, 19912 (2023). 10.1038/s41598-023-46943-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali, N. et al. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci. Rep.10, 7841 (2020). 10.1038/s41598-020-64884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali, N. et al. The relationship between serum uric acid and lipid profile in Bangladeshi adults. BMC Cardiovasc. Disord.19, 42 (2019). 10.1186/s12872-019-1026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali, N. et al. The prevalence of general obesity, abdominal obesity, and hypertension and its related risk factors among young adult students in Bangladesh. J. Clin. Hypertens. Greenwich Conn.24, 1339–1349 (2022). 10.1111/jch.14560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali, N., Mohanto, N. C., Nurunnabi, S. M., Haque, T. & Islam, F. Prevalence and risk factors of general and abdominal obesity and hypertension in rural and urban residents in Bangladesh: A cross-sectional study. BMC Public Health22, 1707 (2022). 10.1186/s12889-022-14087-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali, N. et al. Prevalence of hyperuricemia and the relationship between serum uric acid and obesity: A study on Bangladeshi adults. PloS One13, e0206850 (2018). 10.1371/journal.pone.0206850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuhashi, M. et al. Plasma xanthine oxidoreductase activity as a novel biomarker of metabolic disorders in a general population. Circ. J.82, 1892–1899 (2018). 10.1253/circj.CJ-18-0082 [DOI] [PubMed] [Google Scholar]
- 32.Sunagawa, S. et al. Activity of xanthine oxidase in plasma correlates with indices of insulin resistance and liver dysfunction in patients with type 2 diabetes mellitus and metabolic syndrome: A pilot exploratory study. J. Diabetes Investig.10, 94–103 (2019). 10.1111/jdi.12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klisic, A. et al. Xanthine oxidase and uric acid as independent predictors of albuminuria in patients with diabetes mellitus type 2. Clin. Exp. Med.18, 283–290 (2018). 10.1007/s10238-017-0483-0 [DOI] [PubMed] [Google Scholar]
- 34.Klisic, A. et al. Nitric oxide products are not associated with metabolic syndrome. J. Med. Biochem.38, 361–367 (2019). 10.2478/jomb-2018-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battelli, M. G., Bortolotti, M., Polito, L. & Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta BBA Mol. Basis Dis.1864, 2557–2565 (2018). 10.1016/j.bbadis.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 36.Fuentes, E., Fuentes, F., Vilahur, G., Badimon, L. & Palomo, I. Mechanisms of chronic state of inflammation as mediators that link obese adipose tissue and metabolic syndrome. Mediators Inflamm.2013, 1–11 (2013). 10.1155/2013/136584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet365, 1415–1428 (2005). 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 38.Moller, D. E. & Kaufman, K. D. Metabolic syndrome: A clinical and molecular perspective. Annu. Rev. Med.56, 45–62 (2005). 10.1146/annurev.med.56.082103.104751 [DOI] [PubMed] [Google Scholar]
- 39.Wu, Y. et al. PTEN phosphorylation and nuclear export mediate free fatty acid-induced oxidative stress. Antioxid. Redox Signal.20, 1382–1395 (2014). 10.1089/ars.2013.5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holvoet, P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh. K. Acad. Geneeskd. Belg.70, 193–219 (2008). [PubMed] [Google Scholar]
- 41.Mahjoub, S. & Masrour-Roudsari, J. Role of oxidative stress in pathogenesis of metabolic syndrome. Casp. J. Intern. Med.3, 386 (2012). [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson, R. J. et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease?. Hypertension41, 1183–1190 (2003). 10.1161/01.HYP.0000069700.62727.C5 [DOI] [PubMed] [Google Scholar]
- 43.Rizzo, M. et al. Uric acid metabolism in pre-hypertension and the metabolic syndrome. Curr. Vasc. Pharmacol.12, 572–585 (2014). 10.2174/1570161111999131205160756 [DOI] [PubMed] [Google Scholar]
- 44.Takir, M. et al. Lowering uric acid with allopurinol improves insulin resistance and systemic inflammation in asymptomatic hyperuricemia. J. Investig. Med.63, 924–929 (2015). 10.1097/JIM.0000000000000242 [DOI] [PubMed] [Google Scholar]
- 45.Isık, H. et al. Is Xanthine oxidase activity in polycystic ovary syndrome associated with inflammatory and cardiovascular risk factors?. J. Reprod. Immunol.116, 98–103 (2016). 10.1016/j.jri.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 46.Baldwin, W. et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes60, 1258–1269 (2011). 10.2337/db10-0916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Bassossy, H. M. & Shaltout, H. A. Allopurinol alleviates hypertension and proteinuria in high fructose, high salt and high fat induced model of metabolic syndrome. Transl. Res.165, 621–630 (2015). 10.1016/j.trsl.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 48.Battelli, M. G., Bolognesi, A. & Polito, L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme. Biochim. Biophys. Acta BBA Mol. Basis Dis.1842, 1502–1517 (2014). 10.1016/j.bbadis.2014.05.022 [DOI] [PubMed] [Google Scholar]
- 49.Erdei, N. et al. High-fat diet-induced reduction in nitric oxide-dependent arteriolar dilation in rats: Role of xanthine oxidase-derived superoxide anion. Am. J. Physiol.-Heart Circ. Physiol.291, H2107–H2115 (2006). 10.1152/ajpheart.00389.2006 [DOI] [PubMed] [Google Scholar]
- 50.Vorbach, C., Scriven, A. & Capecchi, M. R. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet enveloping and secretion: Gene sharing in the lactating mammary gland. Genes Dev.16, 3223–3235 (2002). 10.1101/gad.1032702 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article


