Abstract
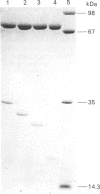
Two forms of pig kidney calpain II were isolated, both of which appeared to contain an intact 80 kDa large subunit, but which showed specific proteolytic degradation at the N-terminal end of the 30 kDa small subunit. The structure of each of these molecules was investigated by amino acid sequence analysis. The forms corresponded to molecules with small subunits starting at residue 38 (degraded calpain A) and at residue 62 (degraded calpain B) of the complete sequence. These molecules were tested for their ability to interact with phosphatidylinositol and with carbohydrate (agarose gel-filtration media). Calpain and degraded calpain A, but not degraded calpain B, would interact with phosphatidylinositol. Thus the sequence (G)17TAMRILG (residues 38-61) is essential for the interaction. Neither calpain nor the degraded forms of the enzyme showed specific interaction with carbohydrate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Coolican S. A., Haiech J., Hathaway D. R. The role of subunit autolysis in activation of smooth muscle Ca2+-dependent proteases. J Biol Chem. 1986 Mar 25;261(9):4170–4176. [PubMed] [Google Scholar]
- Coolican S. A., Hathaway D. R. Effect of L-alpha-phosphatidylinositol on a vascular smooth muscle Ca2+-dependent protease. Reduction of the Ca2+ requirement for autolysis. J Biol Chem. 1984 Oct 10;259(19):11627–11630. [PubMed] [Google Scholar]
- Crawford C., Willis A. C., Gagnon J. The effects of autolysis on the structure of chicken calpain II. Biochem J. 1987 Dec 1;248(2):579–588. doi: 10.1042/bj2480579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino G. N., Huff C. A., Croall D. E. Autoproteolysis of the small subunit of calcium-dependent protease II activates and regulates protease activity. J Biol Chem. 1986 Sep 15;261(26):12047–12052. [PubMed] [Google Scholar]
- Garret C., Cottin P., Dufourcq J., Ducastaing A. Evidence for a Ca2+-independent association between calpain II and phospholipid vesicles. FEBS Lett. 1988 Jan 25;227(2):209–214. doi: 10.1016/0014-5793(88)80900-1. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Walker J. H., Boustead C., Taylor W. Annexins--new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep. 1987 Apr;7(4):289–298. doi: 10.1007/BF01121450. [DOI] [PubMed] [Google Scholar]
- Imajoh S., Kawasaki H., Suzuki K. Limited autolysis of calcium-activated neutral protease (CANP): reduction of the Ca2+-requirement is due to the NH2-terminal processing of the large subunit. J Biochem. 1986 Sep;100(3):633–642. doi: 10.1093/oxfordjournals.jbchem.a121755. [DOI] [PubMed] [Google Scholar]
- Imajoh S., Kawasaki H., Suzuki K. The amino-terminal hydrophobic region of the small subunit of calcium-activated neutral protease (CANP) is essential for its activation by phosphatidylinositol. J Biochem. 1986 Apr;99(4):1281–1284. doi: 10.1093/oxfordjournals.jbchem.a135593. [DOI] [PubMed] [Google Scholar]
- Jain M. K., Zakim D. The spontaneous incorporation of proteins into preformed bilayers. Biochim Biophys Acta. 1987 Apr 27;906(1):33–68. doi: 10.1016/0304-4157(87)90004-9. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Peptides with affinity for membranes. Annu Rev Biophys Biophys Chem. 1987;16:561–581. doi: 10.1146/annurev.bb.16.060187.003021. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Kuboki M., Ishii H., Kazama M. Procalpain is activated on the plasma membrane and the calpain acts on the membrane. Biochim Biophys Acta. 1987 Jul 6;929(2):164–172. doi: 10.1016/0167-4889(87)90172-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Ohno S., Emori Y., Imajoh S., Kawasaki H., Kisaragi M., Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984 Dec 6;312(5994):566–570. doi: 10.1038/312566a0. [DOI] [PubMed] [Google Scholar]
- Parkes C., Kembhavi A. A., Barrett A. J. Calpain inhibition by peptide epoxides. Biochem J. 1985 Sep 1;230(2):509–516. doi: 10.1042/bj2300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Sparatore B., Salamino F., Michetti M., Sacco O., Horecker B. L. Binding to erythrocyte membrane is the physiological mechanism for activation of Ca2+-dependent neutral proteinase. Biochem Biophys Res Commun. 1985 Apr 16;128(1):331–338. doi: 10.1016/0006-291x(85)91683-3. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Sparatore B., Salamino F., Michetti M., Sacco O., Horecker B. L. Role of phospholipids in the activation of the Ca2+-dependent neutral proteinase of human erythrocytes. Biochem Biophys Res Commun. 1985 Jun 14;129(2):389–395. doi: 10.1016/0006-291x(85)90163-9. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Sparatore B., Salamino F., Michetti M., Sacco O., Melloni E. Reversible activation of human neutrophil calpain promoted by interaction with plasma membranes. Biochem Int. 1985 Jul;11(1):35–44. [PubMed] [Google Scholar]
- Sakihama T., Kakidani H., Zenita K., Yumoto N., Kikuchi T., Sasaki T., Kannagi R., Nakanishi S., Ohmori M., Takio K. A putative Ca2+-binding protein: structure of the light subunit of porcine calpain elucidated by molecular cloning and protein sequence analysis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6075–6079. doi: 10.1073/pnas.82.18.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek G. T., Feijen A., Hage W. J., van Rotterdam W., de Laat S. W. The role of hydrophobic interactions in the phospholipid-dependent activation of protein kinase C. Biochem J. 1988 Oct 15;255(2):629–637. [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Imajoh S., Emori Y., Kawasaki H., Minami Y., Ohno S. Calcium-activated neutral protease and its endogenous inhibitor. Activation at the cell membrane and biological function. FEBS Lett. 1987 Aug 17;220(2):271–277. doi: 10.1016/0014-5793(87)80828-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman U. J., Schlaepfer W. W. Calcium-activated neutral proteases (calpains) are carbohydrate binding proteins. J Biol Chem. 1988 Aug 25;263(24):11609–11612. [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]