Abstract
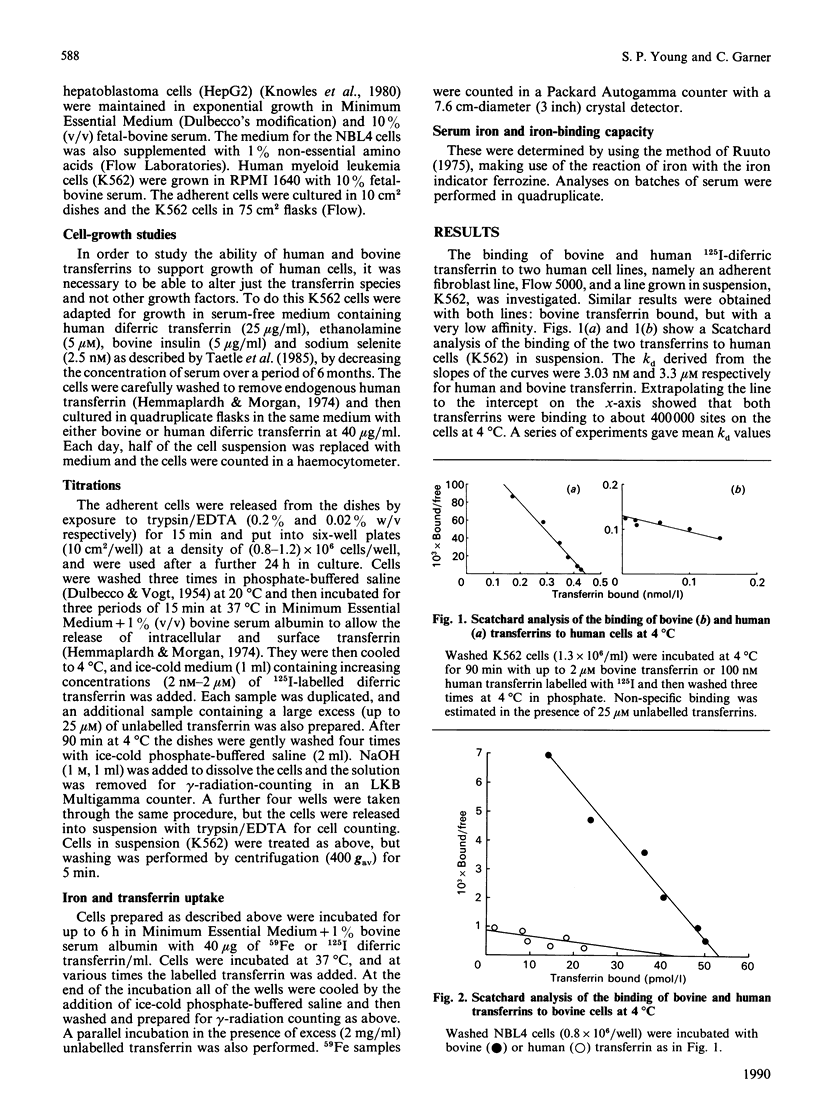
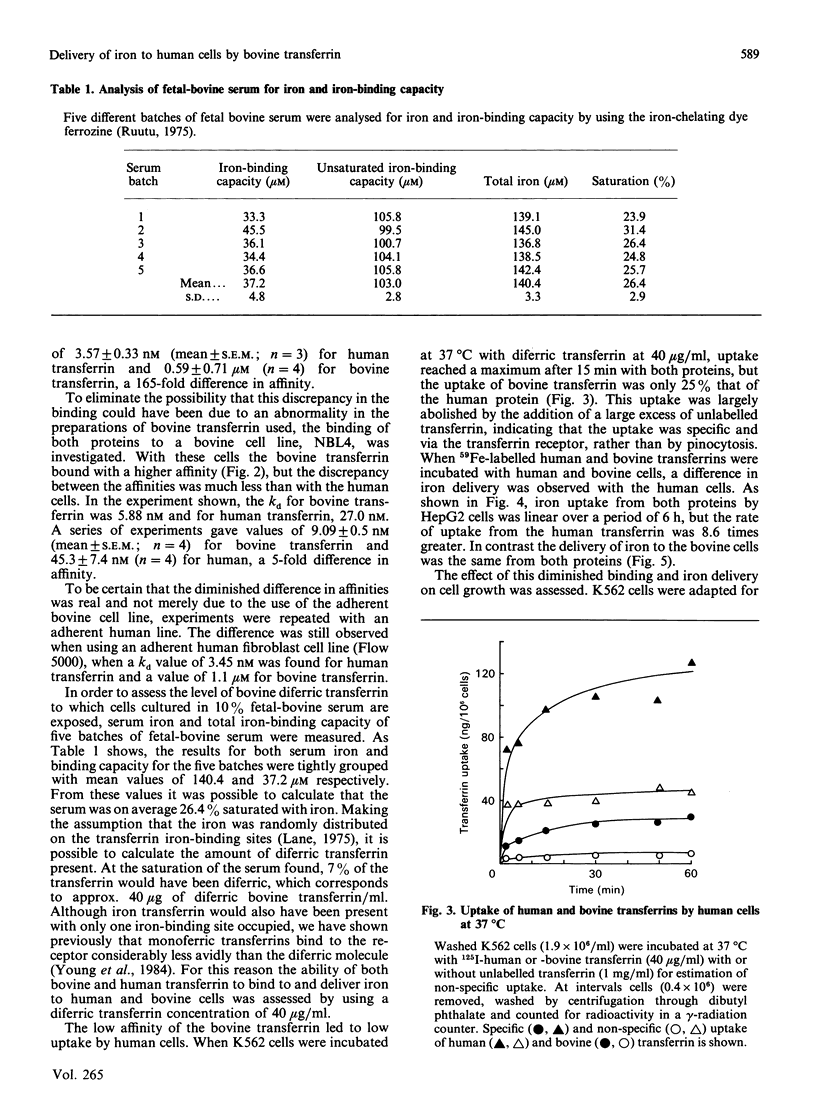
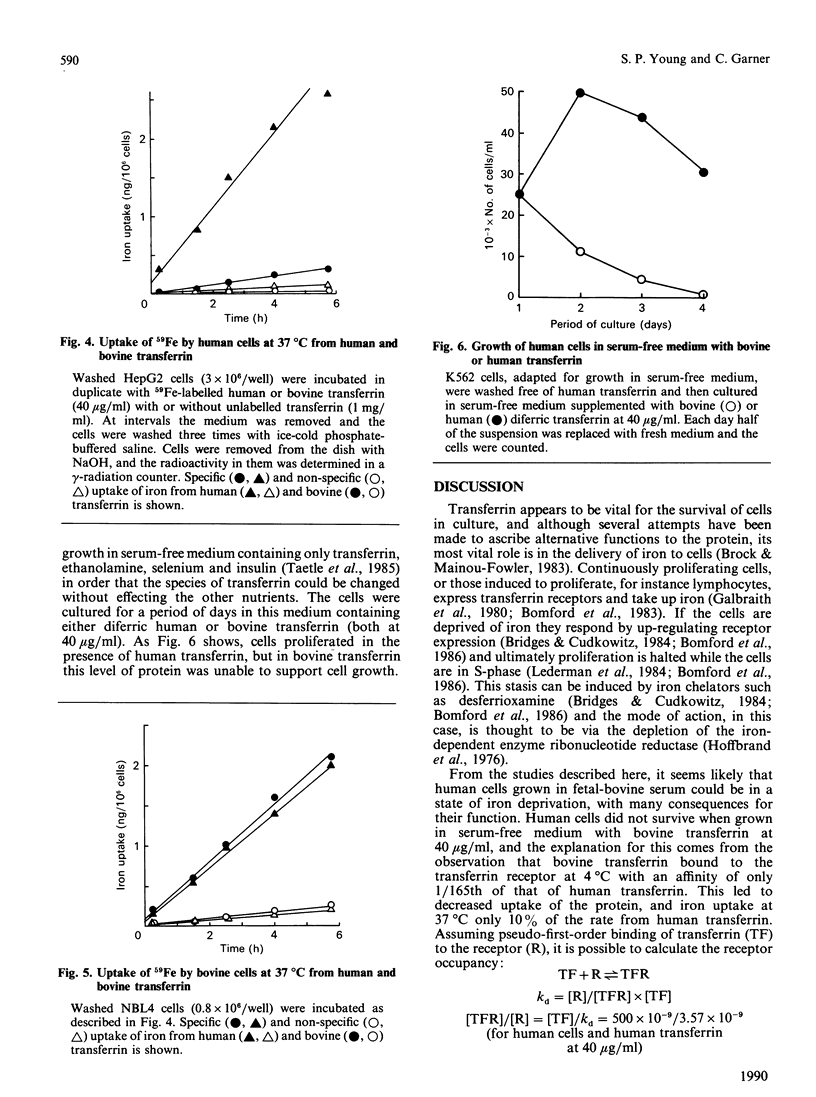
Following suggestions that transferrin present in fetal-bovine serum, a common supplement used in tissue-culture media, may not bind well to human cells, we have isolated the protein and investigated its interaction with both human and bovine cells. Bovine transferrin bound to a human cell line, K562, at 4 degrees C with a kd of 590 nM, whereas human transferrin bound with a kd of 3.57 nM, a 165-fold difference. With a bovine cell line, NBL4, bovine transferrin bound with the higher affinity, kd 9.09 nM, whereas human transferrin bound with a kd of 41.7 nM, only a 5-fold difference. These values were reflected in an 8.6-fold difference in the rate of iron delivery by the two proteins to human cells, whereas delivery to bovine cells was the same. Nevertheless, the bovine transferrin was taken up by the human cells by a specific receptor-mediated process. Human cells cultured in bovine diferric transferrin at 40 micrograms/ml, the concentration expected in the presence of 10% fetal-bovine serum, failed to thrive, whereas cells cultured in the presence of human transferrin proliferated normally. These results suggest that growth of human cells in bovine serum could give rise to a cellular iron deficiency, which may in turn lead to the selection of clones of cells adapted for survival with less iron. This has important consequences for the use of such cells as models, since they may have aberrant iron-dependent pathways and perhaps other unknown alterations in cell function.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. L., Chase C. G., Tomasi T. B., Jr Transferrin support of stimulated lymphocytes. In Vitro. 1982 Sep;18(9):766–774. doi: 10.1007/BF02796500. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bomford A., Isaac J., Roberts S., Edwards A., Young S., Williams R. The effect of desferrioxamine on transferrin receptors, the cell cycle and growth rates of human leukaemic cells. Biochem J. 1986 May 15;236(1):243–249. doi: 10.1042/bj2360243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford A., Young S. P., Nouri-Aria K., Williams R. Uptake and release of transferrin and iron by mitogen-stimulated human lymphocytes. Br J Haematol. 1983 Sep;55(1):93–101. doi: 10.1111/j.1365-2141.1983.tb01227.x. [DOI] [PubMed] [Google Scholar]
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Brock J. H. The effect of iron and transferrin on the response of serum-free cultures of mouse lymphocytes to concanavalin A and lipopolysaccharide. Immunology. 1981 Jun;43(2):387–392. [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith G. M., Goust J. M., Mercurio S. M., Galbraith R. M. Transferrin binding by mitogen-activated human peripheral blood lymphocytes. Clin Immunol Immunopathol. 1980 Aug;16(4):387–395. doi: 10.1016/0090-1229(80)90180-4. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Bacon P. A., Strober S. Enhancement of human T-lymphocyte growth by human transferrin in the presence of fetal bovine serum. Cell Immunol. 1987 May;106(2):366–375. doi: 10.1016/0008-8749(87)90179-1. [DOI] [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. The mechanism of iron exchange between synthetic iron chelators and rabbit reticulocytes. Biochim Biophys Acta. 1974 Nov 27;373(1):84–99. doi: 10.1016/0005-2736(74)90108-4. [DOI] [PubMed] [Google Scholar]
- Hoffbrand A. V., Ganeshaguru K., Hooton J. W., Tattersall M. H. Effect of iron deficiency and desferrioxamine on DNA synthesis in human cells. Br J Haematol. 1976 Aug;33(4):517–526. doi: 10.1111/j.1365-2141.1976.tb03570.x. [DOI] [PubMed] [Google Scholar]
- Knowles B. B., Howe C. C., Aden D. P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980 Jul 25;209(4455):497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- Lane R. S. Differences between human Fe1-transferrin molecules. Br J Haematol. 1975 Mar;29(3):511–520. doi: 10.1111/j.1365-2141.1975.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Lederman H. M., Cohen A., Lee J. W., Freedman M. H., Gelfand E. W. Deferoxamine: a reversible S-phase inhibitor of human lymphocyte proliferation. Blood. 1984 Sep;64(3):748–753. [PubMed] [Google Scholar]
- Maeda K., McKenzie H. A., Shaw D. C. Nature of the heterogeneity within genetic variants of bovine serum transferrin. Anim Blood Groups Biochem Genet. 1980;11(2):63–75. doi: 10.1111/j.1365-2052.1980.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Murakami H., Masui H., Sato G. H., Sueoka N., Chow T. P., Kano-Sueoka T. Growth of hybridoma cells in serum-free medium: ethanolamine is an essential component. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1158–1162. doi: 10.1073/pnas.79.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruutu R. Determination of iron and unsaturated iron-binding capacity in serum with ferrozine. Clin Chim Acta. 1975 Jun 2;61(2):229–232. doi: 10.1016/0009-8981(75)90319-8. [DOI] [PubMed] [Google Scholar]
- Sephton R. G., Kraft N. 67Ga and 59Fe uptakes by cultured human lymphoblasts and lymphocytes. Cancer Res. 1978 May;38(5):1213–1216. [PubMed] [Google Scholar]
- Taetle R., Rhyner K., Castagnola J., To D., Mendelsohn J. Role of transferrin, Fe, and transferrin receptors in myeloid leukemia cell growth. Studies with an antitransferrin receptor monoclonal antibody. J Clin Invest. 1985 Mar;75(3):1061–1067. doi: 10.1172/JCI111768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavaler L., Stein B. S., Sussman H. H. Demonstration of the specific binding of bovine transferrin to the human transferrin receptor in K562 cells: evidence for interspecies transferrin internalization. J Cell Physiol. 1986 Jul;128(1):1–8. doi: 10.1002/jcp.1041280102. [DOI] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Young S. P., Bomford A., Williams R. The effect of the iron saturation of transferrin on its binding and uptake by rabbit reticulocytes. Biochem J. 1984 Apr 15;219(2):505–510. doi: 10.1042/bj2190505. [DOI] [PMC free article] [PubMed] [Google Scholar]


