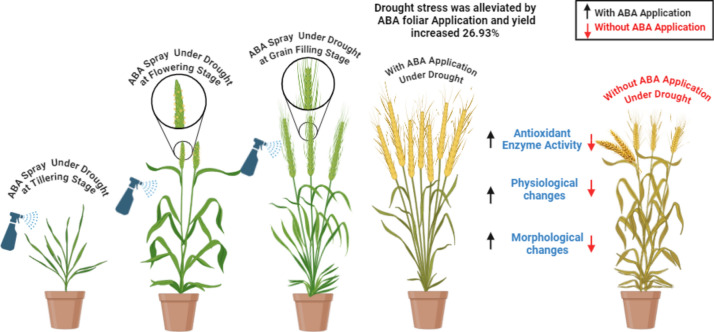
Abstract
Wheat is an important staple crop not only in Pakistan but all over the globe. Although the area dedicated to wheat cultivation expands annually, the quantity of wheat harvested is declining due to various biotic and abiotic factors. Global wheat production and output have suffered as a result of the drought, which is largely driven by a lack of water and environmental factors. Organic fertilizers have been shown to reduce the severity of drought. The current research was conducted in semi-arid climates to mitigate the negative effects of drought on wheat during its critical tillering (DTS), flowering (DFS), and grain filling (DGFS) stages through the application of three different abscisic acid treatments: ABA0 (0 mgL−1) control, ABA1 (100 mgL−1) and ABA2 (200 mgL−1). Wheat growth and yield characteristics were severely harmed by drought stress across all critical development stages, with the DGFS stage being particularly vulnerable and leading to a considerable loss in yield. Plant height was increased by 24.25%, the number of fertile tillers by 25.66%, spike length by 17.24%, the number of spikelets per spike by 16.68%, grain count per spike by 11.98%, thousand-grain weight by 14.34%, grain yield by 26.93% and biological yield by 14.55% when abscisic acid (ABA) was applied instead of the control treatment. Moreover, ABA2 increased the more physiological indices (water use efficiency (36.12%), stomatal conductance (44.23%), chlorophyll a (24.5%), chlorophyll b (29.8%), transpiration rate (23.03%), photosynthetic rate (24.84%), electrolyte leakage (− 38.76%) hydrogen peroxide (− 18.09%) superoxide dismutase (15.3%), catalase (20.8%), peroxidase (− 18.09%), and malondialdehyde (− 13.7%)) of drought-stressed wheat as compared to other treatments. In the case of N, P, and K contents in grain were maximally improved with the application of ABA2. Through the use of principal component analysis, we were able to correlate our results across scales and provide an explanation for the observed effects of ABA on wheat growth and production under arid conditions. Overall, ABA application at a rate of 200 mgL−1 is an effective technique to boost wheat grain output by mitigating the negative effects of drought stress.
Keywords: Abscisic acid, Wheat, Drought, Antioxidants, Water use efficiency
Subject terms: Biochemistry, Plant sciences
Introduction
Reduced farmland, shifting weather patterns, less predictable precipitation, higher costs for fertilizers and pesticides, and people leaving rural regions in droves are just some of the factors working against agriculture’s expansion. For this reason, new strategies must be used in crop production to boost agricultural output1,2. Increasing food production by 50% by 2050 is essential if the globe is to keep up with its fast-expanding population. Rather than increasing the size of the wheat fields, look at different ways to get the highest possible yield3. Water shortage is a major problem that has far-reaching consequences for agricultural output. More than half of the world's wheat crop region has also been negatively affected by persistent droughts4–6.
One of the most damaging abiotic stresses, drought is responsible for the vast majority of worldwide yield losses7. Drought stress is most harmful to wheat at its later phases of growth when it is referred to as terminal drought. Conditions of low atmospheric and soil humidity combined with high air temperature lead to drought because of an imbalance between evaporation and transpiration8,9. Tolerant plants have a huge hurdle when trying to strengthen their resistance to oxidative stress by adopting new and better methods10. Several factors, such as the spell and timing of the stress, the phenology and genotype of the plant, biotic and abiotic indicators11,12, the activation of photosynthesis mechanisms13,14 and numerous membrane-bounded transporters as well as respiration activity influence a plant’s response to drought15,16.
Stimulating plant growth and development using chemical molecules known as plant growth regulators (PGRs) is a common practice17,18. In addition, Kumar et al.19 stated that stress-induced PGRs are present, and their control of morphogenesis (root development), meiosis (fruit ripening), oogenesis (embryogenesis), lifespan, water intake, and sensitivity to biotic and abiotic stress have all been linked to stress plants. PGRs such as abscisic acid is also present naturally in plants, although in very small amounts, and can improve and suppress plant growth while allowing for complete regulation of the plant's biochemistry, physiology, and anatomy20. ABA, a hormone present in all kingdoms, has been shown to regulate several physiological responses21. It controls a wide variety of physiologic processes in vascular plants, such as dormancy of seeds19, cambium activity22, organ genesis23, and fruit ripening24. However, it has a role in controlling the physiological reactions to environmental stresses such as heat, salt, and dehydration25–27.
There are a wide variety of environmental factors, including drought, salt, and cold, that may naturally threaten that plant's response by producing the hormone abscisic acid (ABA), demonstrating its high agronomic potential28. Drought was considered an interesting topic of research, with the processes underlying ABA activity on both the molecular and physiological levels and their findings have already been summarised29–31. Exogenous ABA has been found to decrease reactive oxygen species (ROS), however, the evidence supporting this concept is still mixed. Many plant species have reported higher endogenous ABA levels in response to drought stress32. Build-up via upregulating the activity or expression levels of several antioxidative enzymes33. For instance, in plants of the kiwifruit family that are under stress due to drought, exogenous ABA treatment significantly increases peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD)34. Cotinus, while under drought stress, has a decreased concentration of ABA increase in CAT activity, although it has a dramatic effect on SOD and POD activity in this species35,36.
Furthermore, the external treatment of ABA during periods of water stress expedited the buildup of osmolytes. It enhanced the hydration level of grains, leading to increased grain weight in vulnerable wheat cultivars37. In addition, the application of ABA to pre-soak seeds resulted in a considerable increase in the activity of antioxidant enzymes, including SOD, POD, CAT, ascorbate peroxidase (APX), and glutathione reductase (GR), in maize seedlings38. Similarly, it was shown that the plants treated with ABA had a greater relative water content (RWC) compared to the control plants when exposed to drought stress. The number38. Travaglia et al.39,40 found that applying ABA directly to the leaves of wheat during the reproductive stage effectively reduced the negative impact of drought. Research has shown that ABA is a crucial ingredient in mitigating oxidative damage by stimulating the antioxidant defence system in maize seedlings experiencing water restriction41,42.
Numerous research studies have highlighted the positive impact of abscisic acid on promoting wheat growth. Nonetheless, information is scarce regarding the enzymatic activities and other physiological mechanisms of wheat in response to drought stress at different growth stages of wheat. Therefore, this novel study was performed with the following aims: the influence of ABA on growth and physiological indicators during critical growth stages of wheat under drought circumstances, the efficacy of ABA through foliar spray, and assess it as a potential approach to mitigate the negative consequences of drought on the crucial growth stages (tillering, flowering, and grain-filling) of wheat. We forecast that the foliar application of ABA will boost wheat's growth, water-related metrics, physiological characteristics, and productivity in dry environments.
Materials and methods
Experimental layout and crop husbandry
This pot experiment was conducted at the experimental station of The Islamia University of Bahawalpur, Pakistan (29° 23′ 60.00′′ N, 71° 40′ 59.99′′ E) with a factorial arrangement of the complete randomized design (CRD). There were 3 treatments of different concentration of foliar application of ABA (ABA0 = 0 mgL−1, ABA1 = 100 mgL−1, and ABA2 = 200 mgL−1) was applied on each development stages (tillering (DTS), flowering (DFS), and grain filling (DGFS) phases). While, drought was enforced on specific one stage in each pot according to the treatment with three replications. Furthermore, full irrigation was used as a control (CK) and plants in the control treatment were sprayed with the same amount of deionized water43.
In this experiment, used Galaxy 2013 wheat cultivar selected after the screening of 5 different verities such as Punjab 2011, Faisalabad 2008, Lasani 2008, 8200 and Galaxy 2013. Moreover, this verity is also recommended in this semi-arid region by Regional Agricultural Research Institute (RARI) Bahawalpur 44. On November 10th, 2019, wheat seeds were sown in the 36 pots (26 × 29 cm; three for each application) filled with six kg of soil and seeds were purchased from RARI Bahawalpur. Fertilizers 0.52 g N, 0.35 g P, and 0.23 g K were added in each pot according to the recommended dose of N, P, and K (160–115–75 kg/ha) in wheat. The soils physiochemical composition is shown in Table 1. In the event of inclement weather, a transparent plastic cover was put over the wire enclosure to shield the plants within. Up to the point of complete emergence, all containers were watered uniformly. After 20 days of seeding, a total of four plants were kept in each container by removing the excess. A translucent plastic canopy was placed over the wire-house to protect the crop from rainfall when needed. All the containers were watered equally until the plants fully emerged.
Table 1.
Physicochemical characteristics of the soil.
| Parameters | Contents |
|---|---|
| Sand | 53.5% |
| Clay | 14.5% |
| Silt | 32% |
| Ph | 7.36 |
| Texture | Sandy-loam soil |
| Ammoniac N (mg g−1) | 1.48 |
| Electric conductivity (dSm−1) | 2.63 |
| Available potassium (ppm) | 108 |
| Available phosphorus (ppm) | 6.23 |
| Organic matter (%) | 0.88 |
ABA application and drought imposition
In this experiment used three different concentrations (ABA0 = 0 mgL−1, ABA1 = 100 mgL−1, and ABA2 = 200 mgL−1) of foliar ABA during three DFS, DTS, and DGFS observed development stages of wheat. Since the drought started, every pot has been watered in the same manner. Then, throughout the flowering (DFS), tillering (DTS), and grain filling (DGFS) phases, under drought we kept the water holding capacity (WHC) of each pot at 35% during 40–50 days after sowing (DAS) for DTS, 80–90 DAS for DFS, and 100–110 DAS for DGFS, whereas the control was kept at 85% WHC using tensiometer44–47.
Growth and yield parameters
The growth, yield, and various attributes of wheat were measured, including spike length (cm), number of grains per spike, number of spikelets per spike, number of fertile tillers, 1000-grain weight (g), plant height (cm), organic yield per plant (g), grain yield per plant (g), and harvest index. These measurements were obtained using established procedures and protocols. The yield of wheat plants was obtained by physically harvesting them at the end of their life cycle. Similarly, to measure the height of the plants, three plants were randomly chosen from each pot and their height was measured from the surface of the soil to the spikelet using a ruler calibrated in millimetres and centimetres. To measure the length of the spike and the number of grains per spike, use a centimetre scale to measure the distance from the base to the terminal spikelet of three randomly chosen plants. Additionally, count the number of grains in ten randomly selected clusters of seeds. In addition, the digital balance was used to measure the 1000-grain weight, taking advantage of its precision of 0.01 g. The following formula was used to determine the harvest index (HI):
Determination of physiological parameters
In accordance with the method employed by Khalilzadeh et al.48, 0.5 g of recently harvested leaf tissue was slowly mixed with 10 mL of acetone (80%) to determine the chlorophyll levels. The mixture was then subjected to centrifugation at a speed of 400 rpm for a duration of 10 min. The spectrophotometer recorded the absorbance at wavelengths of 645 nm, 663 nm, and 470 nm. The chlorophyll contents were acquired using the following method:
Raza et al.47 defined a mathematical equation for determining the water usage efficiency (WUE, g pot−1 mm−1) as follows:
Automatic porometer MK-3 Delta-T Devices, BC, England, was used to measure the transpiration rate and stomatal conductance (SC). It was measured from 5 leaves per pot between 11:00 and 13:00 h, when atomosphre was cleared and stomata were fully opened and photosynthetic activity were high49.
Determination of electrolyte leakage and hydrogen peroxide concentration
The shoot samples were vertically inserted into tubes to measure electrolyte leakage percentage and heated in distilled water at 32 °C for 2 h. After this step, the electrical conductivity (EC) of the solution was determined as EC1. Subsequently, the solution was heated at a constant temperature of 121 °C for 20 min. EL was calculated using a previously defined equation 50.
To determine the concentration of hydrogen peroxide (H2O2), a 50 mg leaf tissue was mixed with 3.0 mL of phosphate buffer solution and centrifuged at 6000g for 30 min at 4 °C. Then an upper layer of the centrifuges sample was mixed with 1.0 mL of (0.1%) titanium sulphate by centrifuging at 6000g for 20 min at 4 °C. Specifically, the absorbance was measured at 410 nm. The extinction coefficient for H2O2 was found to be 0.28 mol−1 cmss−1.
Estimation of antioxidant enzymes
The activity of superoxide dismutase (SOD) was measured using the procedure outlined by Giannopolitis and Ries51. 2 µM riboflavin was added to a 3 mL reaction mixture containing 50 mM phosphate buffer (pH 7.8), 13 mM methionine, 75 µM nitro blue tetrazolium, 0.1 µM EDTA, and 0–100 µl of enzyme extract51. The tubes were shaken and lighted with a 15-Watt fluorescent bulb. The reaction was allowed to proceed for a duration of 10 min. Subsequently, the light source was turned off and the absorbance was measured at a wavelength of 560 nm. A single unit of SOD activity was determined as the quantity of enzyme needed to induce a 50% decrease in the rate of nitroblue tetrazolium chloride reduction.
An approach for determining CAT activity was suggested by Hwang et al.52, which involves monitoring the rate of H2O2 breakdown at 240 nm. Modifications were made to the guaiacol oxidation procedure reported by Maechlay and Chance53 to determine POD activity. The reaction mix included 50 mM potassium phosphate buffer (pH 6.1), 1% guaiacol, 0.4% H2O2, and extracted enzyme, and it took up a total volume of 3 ml. At 470 nm, an increase in absorbance (E = 25.5 mM−1 cm−1) was seen owing to oxidation of guaiacol54. MDA contents were estimated as described by Wu et al.55.
Grain quality parameters
The levels of nitrogen (N), phosphorus (P), and potassium (K) were examined to evaluate the quality of the grain. The estimation of total nitrogen from wheat seeds was conducted using the Micro Kjeldahl's technique, as described by Piper56. To assess the P content in wheat seed, the vanado-phosphate molybdate yellow colour technique proposed by Kitson and Mellon57 was used on a di-acid extract. To assess the K level in wheat seed, a flame photometer with a di-acid extract method, as reported by Piper56, was used.
Statistical analysis
The analysis of variance (ANOVA) was calculated using STATISTIX software (version 8.1) on the present data with the least significant difference (Tukey’s HSD) at the 5% probability level to compare the means of the variables58. A biplot graph was created in Origin Pro 9.1 to visualize the principal component analysis (PCA) findings (Fig. 1).
Fig. 1.
Graphical abstract about role of foliar application of Abscisic Acid under drought stress at different growth stages of wheat.
Results
Plant height and spike length
The imposition of drought stress caused a significant reduction in plant height by 34.9%, 32.1%, and 24.3%, and spike length by 38.6%, 33.1%, and 26.6% at the DTS, DFS, and DGFS, respectively as compared to CK (no drought at any stage). However, the ABA applications (ABA1 and ABA2) mitigated the impact of drought stress, leading to an increase in plant height and spike length by 18.7%, 24.2%, and 13.5%, 17.2%, respectively as compared to control treatment ABA0 (Table 2).
Table 2.
The effect of abscisic acid on plant height (PH, cm), spike length (SL, cm), number of spikelets per spike (NSPS), number of grains per spike (NGPS), number of fertile tiller (NFT) of wheat under different drought episodes.
| Treatments | PH | SL | NSPS | NGPS | NFT |
|---|---|---|---|---|---|
| CK (Control, No Drought at any Stage) | |||||
| ABA0 (0 mgL−1) | 52.23 ± 1.88 b | 9.50 ± 0.81 abc | 14.13 ± 0.97 abc | 35.13 ± 0.97 ab | 6.73 ± 0.33 abc |
| ABA1 (100 mgL−1) | 58.10 ± 1.09 a | 10.65 ± 0.73 ab | 15.34 ± 1.15ab | 37.00 ± 0.94 a | 7.53 ± 0.39 ab |
| ABA2 (200 mgL−1) | 61.23 ± 1.02 a | 11.13 ± 0.64 a | 16.62 ± 0.86 a | 39.23 ± 1.07 a | 8.10 ± 0.33 a |
| DTS (Drought at tillering) | |||||
| ABA0 (0 mgL−1) | 36.53 ± 0.97 ef | 6.93 ± 0.97 c | 11.43 ± 0.94 abc | 28.83 ± 0.86 cde | 5.03 ± 0.34 cde |
| ABA1 (100 mgL−1) | 43.01 ± 0.95 cd | 7.71 ± 0.70 bc | 12.81 ± 0.95 abc | 30.81 ± 0.95 bcde | 5.91 ± 0.38 abcde |
| ABA2 (200 mgL−1) | 47.62 ± 1.03 bc | 7.92 ± 0.65 bc | 14.22 ± 1.03 abc | 34.02 ± 1.03 abc | 7.02 ± 0.45 abc |
| DFS (Drought at flowering) | |||||
| ABA0 (0 mgL−1) | 32.40 ± 1.14 f. | 6.80 ± 0.63 c | 10.40 ± 1.05 bc | 27.20 ± 1.05 de | 4.41 ± 0.48 de |
| ABA1 (100 mgL−1) | 47.30 ± 1.05 bc | 8.00 ± 0.49 bc | 11.00 ± 0.97 bc | 29.30 ± 0.98 cde | 5.44 ± 0.45 bcde |
| ABA2 (200 mgL−1) | 50.10 ± 1.35 b | 8.70 ± 0.82 abc | 12.30 ± 0.91 abc | 31.10 ± 0.88 bcd | 6.12 ± 0.40 abcd |
| DGFS (Drought at grain filling) | |||||
| ABA0 | 38.40 ± 1.06 de | 7.10 ± 0.62 c | 9.40 ± 0.85 c | 25.60 ± 0.99 e | 3.80 ± 0.46 e |
| ABA1 | 47.90 ± 0.72 bc | 8.70 ± 0.64 abc | 9.80 ± 0.98 c | 26.70 ± 1.00 de | 4.83 ± 0.43 cde |
| ABA2 | 51.70 ± 0.66 | 8.90 ± 0.72 abc | 11.30 ± 0.95 bc | 28.30 ± 1.01 de | 5.60 ± 0.49 bcde |
| HSD (p < 0.05) | 5.33 | 3.04 | 5.25 | 5.33 | 2.29 |
| Significance | |||||
| Drought | *** | ** | *** | *** | ** |
| ABA | *** | NS | ** | *** | *** |
| Drought × ABA | ** | NS | NS | NS | *** |
ABA0, ABA1 and ABA2 treatments indicates 0 mgL−1 (control), 100 mgL−1, and 200 mgL−1 of ABA respectively. Ck, DTS, DFS, and DGFS treatments indicates no drought at any stage, drought at tillering, drought at flowering and grain filling stages respectively. There were no significant differences at the 5% probability level among the means that share the same letter case. NS = non-significant, * = significant at p ≤ 0.05, ** = significant at p ≤ 0.01 and *** = significant at p ≤ 0.001.
Significant values are in bold.
Yield attributes
The drought conditions at specific growth stages (DTS, DFS, and DGFS) lowered the spikelet’s numbers per spike (19.8, 36.7, and 51.1%), the number of fertile tillers (24.6, 40.5, and 57.4%), the grain number per spike (18.8, 27.1, and 38.1%), the 1000-grain weight (12.7, 25.4, and 35.5%), the grain yield (13.0, 31.1, and 40.4%), the biological yield (17.1, 39.0, and 76.5%), and the harvest index (3.3, 4.9, and 20.6%) as compared to control (CK) when no drought imposed at any stages. Under both drought and control conditions, ABA demonstrated a positive effect, mitigating the adverse effects of drought and enhancing the values of the aforementioned parameters. Notably, a foliar application of ABA2 at 200 mgL−1 resulted in a significantly higher grain yield (26.9%) compared to the application of ABA1 at 100 mgL−1 and ABA0 control treatment (Table 3). Moreover, ABA foliar treatment effectively reduced the negative impact of drought and simultaneously enhanced the potential of various yield-related characteristics of wheat, including NSPS, NFT, NGPS, 1000Gwt, GY, BY, and HI (Tables 2, 3).
Table 3.
The effect of ABA on 1000-grain weight (1000 Gwt, g), grain yield per plant (GY, g), biological yield per plant (BY, g), harvesting index (HI, %) of wheat under different drought episodes.
| Treatments | 1000 Gwt | GY | BY | HI |
|---|---|---|---|---|
| CK (Control, No Drought at any Stage) | ||||
| ABA0 | 30.23 ± 0.97 abc | 6.67 ± 0.48 ab | 15.20 ± 0.80 a | 42.73 ± 1.14 f. |
| ABA1 | 32.41 ± 0.95 ab | 7.73 ± 0.45 a | 15.43 ± 0.92 a | 50.51 ± 1.21 de |
| ABA2 | 34.87 ± 0.78 a | 8.23 ± 0.50 a | 16.20 ± 0.97 a | 51.08 ± 1.23 de |
| DTS (Drought at tillering) | ||||
| ABA0 | 27.00 ± 0.89 bcd | 5.73 ± 0.39 abc | 12.33 ± 0.89 abcd | 46.52 ± 1.04 ef |
| ABA1 | 29.32 ± 0.96 bc | 6.83 ± 0.57 ab | 13.43 ± 0.92 abc | 50.98 ± 1.46 de |
| ABA2 | 30.12 ± 1.03 abc | 7.48 ± 0.54 a | 14.40 ± 0.91 ab | 51.99 ± 1.29 cd |
| DFS (Drought at flowering) | ||||
| ABA0 | 23.40 ± 1.06 cd | 4.31 ± 0.29 bc | 9.70 ± 0.57 abcd | 44.25 ± 1.21 f. |
| ABA1 | 26.70 ± 1.00 cd | 6.10 ± 0.48 abc | 11.70 ± 0.76 abcd | 52.12 ± 1.09 cd |
| ABA2 | 27.53 ± 1.02 cd | 6.83 ± 0.53 ab | 12.30 ± 0.90 abcd | 55.64 ± 1.32 c |
| DGFS (Drought at grain filling) | ||||
| ABA0 | 21.34 ± 0.96 e | 4.13 ± 0.42 c | 7.70 ± 0.98 cd | 54.04 ± 1.17 cd |
| ABA1 | 24.30 ± 0.80 de | 5.73 ± 0.49 abc | 8.81 ± 0.92 d | 65.77 ± 1.24 a |
| ABA2 | 26.50 ± 0.95 de | 6.07 ± 0.53 abc | 10.13 ± 0.75 d | 59.93 ± 1.61 b |
| HSD (p < 0.05) | 4.99 | 2.57 | 4.64 | 4.23 |
| Significance | ||||
| Drought | ** | *** | *** | *** |
| ABA | *** | *** | ** | *** |
| Drought × ABA | *** | NS | NS | ** |
ABA0, ABA1 and ABA2 treatments indicates 0 mgL−1 (control), 100 mgL−1, and 200 mgL−1 of ABA respectively. Ck, DTS, DFS, and DGFS treatments indicates no drought at any stage, drought at tillering, drought at flowering and grain filling stages respectively. There were no significant differences at the 5% probability level among the means that share the same letter case. NS = non-significant, * = significant at p ≤ 0.05, ** = significant at p ≤ 0.01 and *** = significant at p ≤ 0.001.
Significant values are in bold.
Chlorophyll concentrations
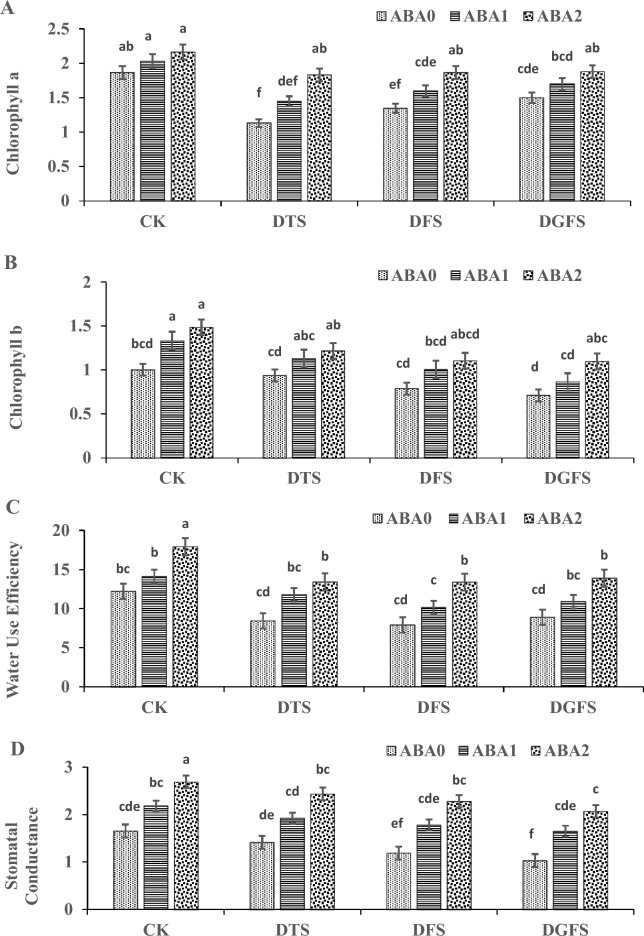
The application of ABA had a significant impact on the chlorophyll content (a and b) of wheat leaves under drought as shown in Fig. 2. Compared to the control (ABA0) with 100 and 200 mgL−1 concentrations of ABA (ABA1 and ABA2) significantly enhanced the chlorophyll content a and b by 14.0%, 25.7%, and 10.9%, 23.1%, respectively. Moreover, drought stress led to reductions in Chlorophyll a and b levels by 37.3%, 26.0%, and 19.4%, and 16.3%, 31.9%, and 43.0%, respectively, at DTS, DFS, and DGFS stages of wheat as compared to CK when no drought was imposed at any growth stages. Notably, under drought, the application of ABA2 resulted in more pronounced effects on chlorophyll content.
Fig. 2.
Illustrates the impact of ABA application on stomatal conductance (m mol−2 s−1), water use efficiency (%), and Chlorophyll a and b (mg g−1 FW) contents at critical growth stages of wheat under drought stress. The labels CK, DGFS, DFS, and DTS represent the control as no drought at any stage, drought at grain filling, flowering, and tillering stages, respectively. ABA0, ABA1 and ABA2 indicates control, 100mgL−1 and 200 mgL−1 ABA application respectively. The error bars in the figure indicate the standard error (n = 3).
Water use efficiency and stomatal conductance
ABA applications (ABA1 and ABA2) significantly reduced the drought impact by 29.8% and 44.2%, respectively, as compared to the control (ABA0). The application of ABA at different development stages resulted in increased efficiency of wheat water consumption under drought (Fig. 2). Water use efficiency (WUE) decreased by 31.1%, 40.8%, and 32.1% under drought at DTS, DFS, and DGFS, respectively, in comparison to the control (CK) when no drought was imposed at any stages. However, the application of ABA (ABA1 and ABA2) significantly mitigated the impact of drought, reducing the WUE decline by 20.2% and 36.1%, respectively, compared to the non-ABA treated plants. Additionally, stomatal conductance (m mol m−2 s−1) was reduced by 13.0%, 24.3%, and 37.4% under drought stress at DTS, DFS, and DGFS, respectively, in comparison to the control treatment (CK). Nevertheless, ABA1 and ABA2 applications significantly alleviated the drought impact, reducing the stomatal conductance decline by 29.8% and 44.2%, respectively, compared to the control treatment ABA0.
Gas exchange attributes
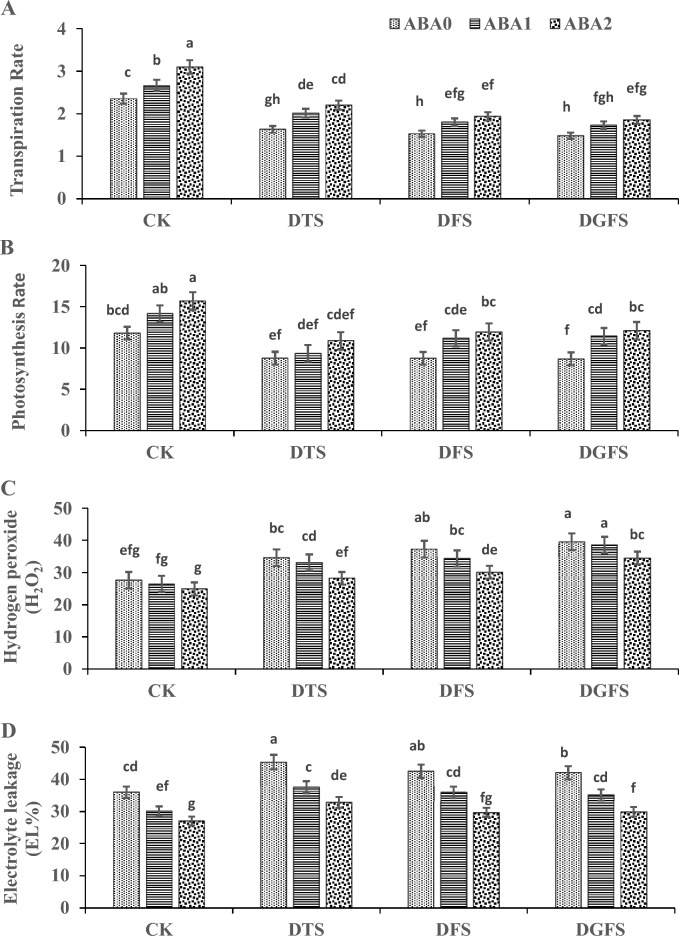
Drought stress caused a significant reduction in transpiration (38.9%, 54.3%, and 60.2%) and photosynthesis activities (43.7%, 30.8%, and 29.4%) at DTS, DFS, and DGFS, respectively, compared to the control (CK) when no drought was imposed at any stage. ABA1 and ABA2 applications ominously increased transpiration and photosynthesis by 14.7%, 23.0%, and 17.5%, 24.9%, respectively, in comparison to the control ABA0 treatment when no application of ABA under drought (Fig. 3A,B).
Fig. 3.
Illustrates the impact of ABA application on Photosynthetic rate (μmol CO2 m−2 S−1), transpiration rate (mmol H2O m−2 S−1), Hydrogen peroxide (μM g−1 FW) and Electrolyte leakage (%) under drought stress at critical growth stages of wheat. The labels CK, DGFS, DFS, and DTS represent the control as no drought at any stage, drought at grain filling, flowering, and tillering stages, respectively. ABA0, ABA1 and ABA2 indicates control, 100mgL-1 and 200mgL-1 ABA application respectively. The error bars in the figure indicate the standard error (n = 3).
Antioxidant enzymatic activities
Under drought stress, H2O2 and EL percentage were elevated by 17.6%, 22.4%, and 29.8% for H2O2 and 19.5%, 13.8%, and 12.9% for EL, at the DTS, DFS, and DGFS stages of wheat, respectively, as compared to control when no drought was imposed at any stage (Fig. 3C,D). However, the application of ABA1 and ABA2 (Fig. 3C,D) significantly reduced the levels of H2O2 (− 4.9% and − 18.0%) and EL (− 19.5% and − 38.7%) in the leaves under drought stress as compared to control treatment (ABA0).
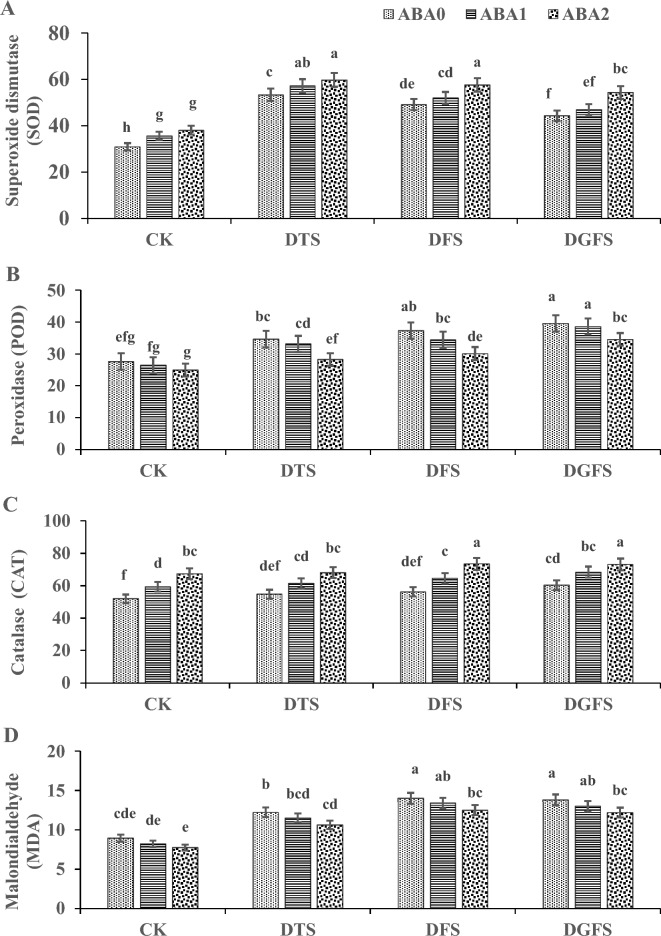
The application of ABA under drought conditions had a significant impact on the antioxidant enzymatic activities of wheat leaves, as demonstrated in Fig. 4. Drought stress influence the enzymatic activities of SOD, CAT, POD, and MDA as compare to control treatments (CK and ABA0). However, the addition of ABA1 and ABA2 during the DTS, DFS, and DGFS stages substantially modulated these activities when compared with ABA0. SOD and CAT activities (Fig. 4A,C) were decreased by 38.5%, 34.0%, 28.0%, and 3.1%, 8.1%, 11.4%, respectively, when compared to CK, no drought was imposed. Moreover, ABA1 and ABA2 applications significantly increased SOD and CAT activities by 7.3%, 15.3%, and 12.0%, 20.8%, respectively, in comparison to the control (ABA0) under drought conditions.
Fig. 4.
Illustrates the impact of ABA application on Superoxide dismutase (μmol min−1 mg−1 protein), Peroxidase (μmol min−1 mg−1 protein), Catalase (μmol min−1 mg−1 protein) and Malondialdehyde (μmol min−1 mg−1 protein) activities under drought stress at critical growth stages of wheat. The labels CK, DTS, DFS, and DGFS represent the control as no drought at any stage, drought at tillering, flowering, and grain-filling stages, respectively. ABA0, ABA1 and ABA2 indicates control, 100 mgL−1 and 200 mgL−1 ABA application respectively. The error bars in the figure indicate the standard error (n = 3).
While, drought at DTS, DFS, and DGFS boosted the POD and MDA levels by 17.6%, 22.4%, 29.8%, and 27.6%, 37.6%, 36.2%, respectively, as compared to CK. But, application of ABA1 and ABA2 significantly diminished the POD and MDA activities under drought by − 4.9%, − 18.0%, and − 6.1%, − 13.7%, respectively, in comparison to the control treatment ABA0 (Fig. 4B,D). Furthermore, ABA2 (200 mgL−1) application was more significant results than ABA1 (100 mgL−1) under drought, for antioxidant enzymatic activities.
Nutrient percentage in grains
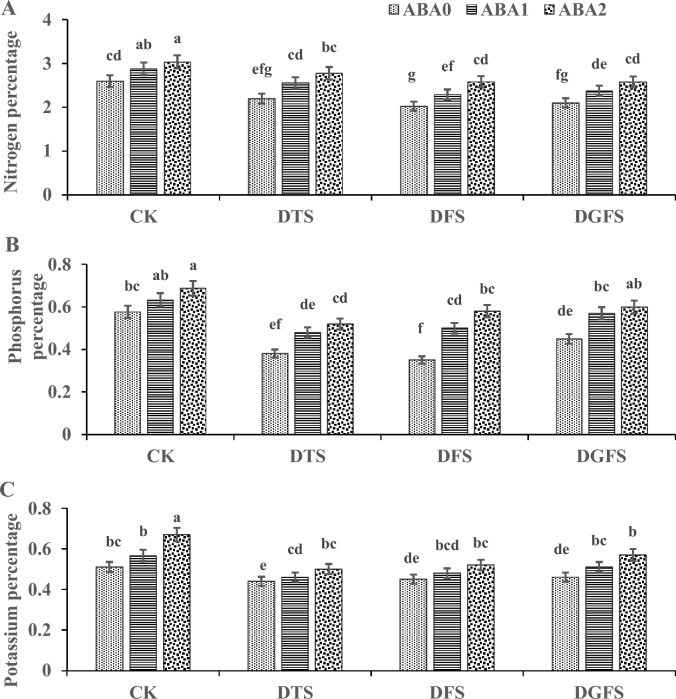
The results regarding the percentage of N, P, and K in wheat grains enhanced with the application of ABA under drought episodes are shown in Fig. 5A–C. Drought stress at critical growth stages (DTS, DFS, and DGFS) reduced the percentage of nitrogen (− 12.9, − 23.2, and − 20.7%), phosphorus (− 37.4, − 32.6, and − 17.1%), and potassium (− 24.7, − 20.6, and − 13.4%) respectively, as compared to CK. Moreover, ABA1 and ABA2 significantly enhanced the N (11.6% and 18.7%), P (19.5% and 26.3%), and K (7.7% and 17.6%) respectively when compared with no application of ABA0 under drought.
Fig. 5.
Illustrates the impact of ABA application on Nitrogen (%), Phosphorus (%), and Potassium (%) affected under drought stress at critical growth stages of wheat. The labels CK, DTS, DFS, and DGFS represent the control as no drought at any stage, drought at tillering, flowering, and grain filling stages, respectively. ABA0, ABA1 and ABA2 indicates control, 100 mgL−1and 200 mgL−1ABA application respectively. The error bars in the figure indicate the standard error (n = 3).
Principal component analysis
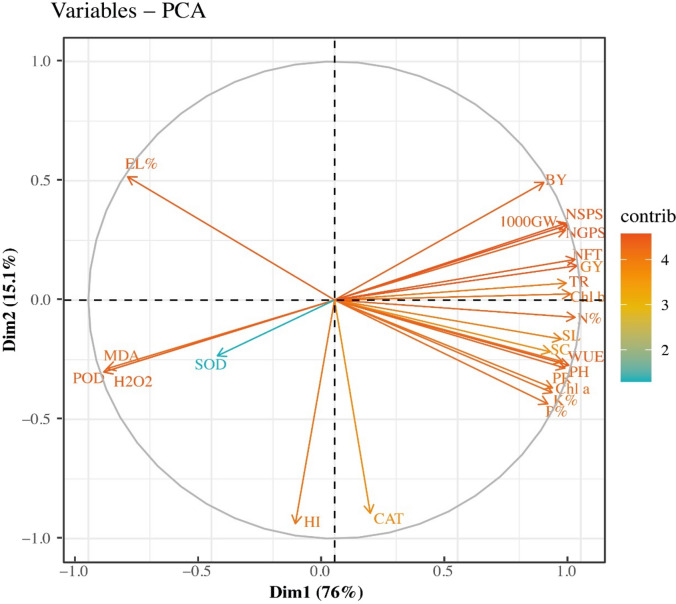
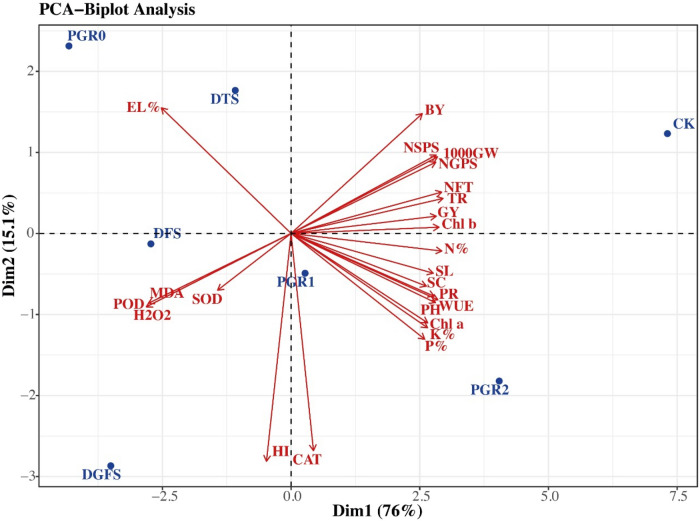
Principal component analysis (PCA) has been widely employed to analyze the variations and associations among different growth, physiological, morphological, biochemical, yield, and quality-linked attributes of wheat under the application of nutrient44,59. In this study, we also conducted PCA to evaluate the variability and associations among agronomic traits and the distribution of treatments (see Figs. 6 and 7). In Fig. 6, agronomic traits which are denoted with different vectors were used and spread among the different clades. In Fig. 7, both agronomic traits and treatments were used and a biplot was created. The cumulative variance of the first two PCs was 91.1% of the total variance (Fig. 6, 7). The first two PCs (PC1 = 76%, and PC2 = 15.1%) were significant. All the studied parameters were the major contributing factors in PC1 except EL%, MDA, POD, SOD, H2O2, HI, and CAT. Similarly, in PC2, SOD was the main factor and showed the highest loading values (Fig. 6). Projection of traits showed that MDA, H2O2, and POD were strongly related to each other. Similarly, in the PCA-biplot, the treatments scattered away from each other and indicated clear differences among them (Fig. 7). Based on PCA results, these parameters can be utilized in future breeding programs.
Fig. 6.
Principal component analysis among the recorded variables during this study. Plant height (PH, cm), spike length (SL, cm), number of grains per spike (NGPS), number of spikelets per spike (NSPS), number of fertile tillers (NFT), 1000 grain weight (GW, g), grain yield per plant (GY, g), biological yield per plant (BY, g), harvesting index (HI), chlorophyll a (Chl a), chlorophyll b (Chl b), photosynthesis rate (PR), transpiration rate (Tr), water use efficiency (WUE), stomatal conductance (SC), hydrogen peroxide (H2O2), malondialdehyde (MDA), superoxide dismutase (SOD), peroxidase (POD), electro leakage percentage (EL%), nitrogen (N%), phosphorus (P%), and potassium (K%).
Fig. 7.
Principal component analysis PCA-biplot among the recorded variables and treatments during this study. Plant height (PH, cm), spike length (SL, cm), number of grains per spike (NGPS), number of spikelets per spike (NSPS), number of fertile tillers (NFT), 1000 grain weight (1000 GW, g), grain yield per pot (GY, g), biological yield per pot (BY, g), Harvesting Index (HI), chlorophyll a (Chl a), chlorophyll b (Chl b), photosynthesis rate (PR), transpiration rate (Tr), water use efficiency (WUE), stomatal conductance (SC), hydrogen peroxide (H2O2), malondialdehyde (MDA), superoxide dismutase (SOD), peroxidase (POD), electro leakage percentage (EL%), nitrogen (N%), phosphorus (P%), and potassium (K%). Application of ABA 0 mg/L (PGR0), ABA 100 mg/L (PGR1), and ABA 200 mg/L (PGR2) under drought stress condition at different growth stages Control (CK), Drought at tillering stage (DTS), drought at flowering stage (DFS), and drought at grain filling stage (DGFS).
Discussion
The main goal of this study was to identify the most effective doses of ABA for foliar application, aiming to expedite wheat growth and alleviate the adverse impacts of drought (Fig. 1). Notably, substantial improvements in growth attributes were observed when applying a dosage of ABA 200 mgL−1 under stressful conditions. Reduced wheat harvests may be attributed to drought stress, a global growth limitation60. Low water availability might stunt a plants growth61. Wheat plant development is drastically altered by ABA treatments. Hussain et al.62 found that water stress significantly affected cell division. The disruption of protoplasmic processes caused by diminished turgidity and dehydration leads to less cell division and shorter plants as a result. Plant growth regulators are very useful here since they increase plant height. Drought stress, at any stage of the development cycle, reduced the plants height63. Possible hormonal changes during drought stress might have a significant impact on plant height64. Our result also in line with the previous researches and showed that the height of wheat was increased by ABA1 and ABA2 application as compared to the control under stress.
Plants with longer spikes are more likely to produce a greater number of spikelets and a better production44. Lack of water in many plant functions, as described by Fahad et al.65 reduces the number of spike lengths. According to Iqbal66, using ABA improve the nutritional availability, which in turn results in longer spikes. Specifically, proline synthesis is increased by ABA under dry circumstances, which in turn increases spike length and enhances plant metabolic processes67. Same pattern observed in our study that ABA treatment increased spike numbers under drought conditions when compared with control.
Reduced water availability in the plant as a result of drought stress reduces the number of fertile tillers. When plants have less access to water, their metabolic activities slow down, resulting in fewer tillers68. The number of tillers is reduced by 14.6% during the tillering stage, 20.4% during the blooming stage, and 27.4% during the grain-filling stage when drought is applied. In the face of drought, ABA showed its greatest effectiveness in increasing the number of tillers. According to Sabagh et al.69, the capacity of ABA to enhance plant development is context-dependent.
The economic yield is very sensitive to the quantity of spikelets per spike. A decrease in spike length as a consequence of drought stress leads to a drop in spikelet density61. Drought during wheat grain-filling phase resulted in a decrease in spikelet production. The outcomes of ABA are equally noteworthy. Wheat plants with ABA2 application had the most spikelets per spike. He et al.28 found that plant hormones boost plant vegetative development by inducing the synthesis of chemicals that mitigate the negative effects of drought. According to research by Raza et al.71 grains per spike were significantly impacted by drought stress at any growth stage. The quantity of grains produced per spike shortens and the length of the spike under drought stress.
According to Tripathi et al.72, ABA may stimulate more robust plant development. Plant growth regulators boost proline production, which benefits plant health. In comparison to the control, seed priming with ABA1 and ABA2 increased grain yield. Zulfiqar et al.10 found that drought stress reduces grain weight, due to stunted development and reduced nutrient absorption by plants under water deficit. The molecule Indole-3-acetic acid is produced by ABA, and proline synthesis also aids in raising the drought threshold73. Plants that produce growth-regulating chemicals have better metabolic activities and a greater sink capacity, both of which contribute to a heavier 1000-grain yield. ABA1 and ABA2 in the current study had positively increase 1000-grain weight.
Plants under drought stress saw a drop in biological output at the tillering stage, flowering stage, and the grain-filling stage. Drought-related reductions in grain weight and plant height reduced biological yield44,62. ABA promotes optimal plant development74. ABA aids in enhancing nutrient absorption, which in turn imparts physiological changes in plant development, which in turn leads to a greater biological yield. According to Tripathi et al.72 research, the capacity of various ABA applications to stimulate plant development varies. Same trend was seen in this research for biological yield when ABA1 and ABA2 were applied.
Grain yield was reduced due to drought stress in every drought stages. At the anthesis stage, Zulfiqar et al.10 found that drought stress was most damaging to grain output. Water scarcity stress also reduces grain weight and yield by disrupting the absorption of nutrients. Under drought circumstances, the grain yield of ABA1 and ABA2 was raised as compared to the control treatment. These results have similar pattern with the previous studies.
As the temperature and humidity were both regulated, water use efficiency improved. When comparing tillering, blooming, and grain-filling drought, the control treatment had greater WUE. According to Lu et al.75 WUE improved when their interconnected units were used to their fullest extent. The use of ABA in wheat crops has been shown to increase both plant function and plant WUE. ABA1 and ABA2 foliar application, respectively, resulted higher WUE compared to the control when tested under drought circumstances which were matched with previous studies.
The chlorophyll level of a leaf is crucial to the plants ability to make nourishment. When leaf area is reduced, chlorophyll concentration is also reduced76. In addition, researchers44,71 found that drought stress reduced the plant's leaf area (LA), and a drop in LA resulted in a decrease in chlorophyll concentration. According to research by Raza et al.60 production of ROS that are harmful to chloroplasts increases in response to drought stress. ABA administration resulted in enhanced metabolic processes, decreased ROS levels, and increased cell proliferation. When ABA1 and ABA2 applied, the chlorophyll content increased, compared to the control in a drought experiment.
Furthermore, oxidative stress can detrimentally affect a plants biological activity through lipid peroxidation and nucleic acid damage77,78. It is essential to strike a balance between ROS generation and breakdown for optimal plant development78. For this reason, plants are vulnerable to oxidative stress since they cannot detoxify ROS79,80. Our findings revealed that during drought stress, plant EL, H2O2 concentration, and POD activity all increased, whereas SOD and CAT activities decreased. In addition, ABA role in maintaining and restoring wheat plant cell membranes contributed considerably to a reduction in ROS. According to Wang et al.27 when plants are subjected to biotic and abiotic stress, the electric transport chain becomes unbalanced, leading to an increase in the formation of ROS, which are harmful to cells. Following this, the plants immune system protects the cells by using anti-oxidant enzymes including SOD, CAT, and POD81.
These findings align with previous research indicating that olive cultivars with elevated levels of antioxidant enzymes have greater resistance to drought82 and same results for rapeseed83 brassica napus84. Furthermore, the heightened SOD activity seen after the application of ABA indicates the effective functioning of the antioxidant system inducer in protecting plants from oxidative damage. ABA enhances the activities of SOD, glutathione reductase, and catalase, hence reducing the detrimental effects of drought on plants13,25,27.
N, P, and K is the most important and abundant nutrient in plants, playing a vital role in growth and development59. The application of ABA significantly increased the N, P, and K contents in wheat grains under drought conditions60. This enhancement can be attributed to multiple mechanisms: ABA acts as a phytohormone that helps plants respond to drought by reducing water loss and promoting stress tolerance85. It also facilitates the uptake and transport of essential nutrients from the soil to the plant, particularly crucial when nutrient availability is limited86. Furthermore, Trapeznikov et al.87 stated that ABA promotes root development and nutrient remobilization, ensuring optimal nutrient levels even under stress.
Lastly, ABA induces the expression of genes related to stress tolerance and nutrient utilization as reported by Wei et al.88. In our research, major N-uptake was seen during the grain filling stage, while the lowest was shown under control conditions without drought stress. N absorption during the grain filling stage was greater than the control treatment. The highest values for P-uptake were observed when ABA2 was applied. The correlation between N uptake and K-uptake is consistent. Correlation analysis revealed that ABA effectively enhances SC, WUE, and chlorophyll content, and mitigates the adverse effects of drought stress.
Conclusion
In the present study, water deficiency had a significant impact on both wheat growth and yield. However, the foliar application of ABA at different wheat growth stages was shown to be effective in improving wheat germination, growth, physiological, water-related characteristics, and yield, even under drought conditions. Specifically, we observed that drought stress during the grain-filling stage resulted in the greatest reduction in grain production. Importantly, we found that ABA application at a concentration of 200 mg/L was the most effective in mitigating the negative effects of drought in wheat. Our findings suggest that the use of ABA could be a valuable tool for enhancing wheat crop yields in dry agricultural systems. Further research is needed to evaluate the effectiveness of such applications under field conditions.
Acknowledgements
This manuscript is part of the Ph.D. thesis (Bilal Zulfiqar) submitted to HEC for an award of degree and not published anywhere else. The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R483), King Saud University, Riyadh, Saudi Arabia.
Author contributions
Conceptualization, B.Z., M.U.A., M.F.S., R.I. and M.A.S.R.; Data curation, M.U.H., A.A.A.G., J.A., M.S.E.; Formal analysis, B.A.; Investigation, B.Z., M.U.A., R.I. and M.A.S.R.; Methodology, B.Z., M.U.A., R.I., M.A.S.R.; Software, B.A.; Supervision, M.A.S.R. and M.F.S.; Writing—original draft, B.Z., M.U.A., R.I., M.T., M.R., A.A.A.G. and M.A.S.R.; Writing—review & editing, All authors. visualization, R.I., B.Z., J.A., A.A.A.G. and B.A.; project administration, B.A. M.A.S.R. and R.I.; funding acquisition, M.R., and R.I. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. Thanks to RTO project KarboIzotopas and LAMMC for promotion of collaboration.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The wheat seeds (Galaxy 2013) were purchased from the Regional Agricultural Research Institute (RARI) Bahawalpur, Pakistan. All the experiments were performed under relevant guidelines and regulations".
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Aown Sammar Raza, Email: aown.sammar@iub.edu.pk.
Muhammad Rizwan, Email: m.rizwan@uni-bonn.de.
Rashid Iqbal, Email: rashid.iqbal@iub.edu.pk.
References
- 1.Hassan, S. T., Xia, E., Huang, J., Khan, N. H. & Iqbal, K. Natural resources, globalization, and economic growth: evidence from Pakistan. Environ. Sci. Pollut. Res.26, 15527–15534 (2019). 10.1007/s11356-019-04890-z [DOI] [PubMed] [Google Scholar]
- 2.Go, P. Economic survey of Pakistan. Preprint at (2019).
- 3.Araus, J. L., Slafer, G. A., Royo, C. & Serret, M. D. Breeding for yield potential and stress adaptation in cereals. CRC Crit. Rev. Plant Sci.27, 377–412 (2008). 10.1080/07352680802467736 [DOI] [Google Scholar]
- 4.Khan, I. et al. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant172, 696–706 (2021). 10.1111/ppl.13237 [DOI] [PubMed] [Google Scholar]
- 5.Jabborova, D. et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep.11, 1–9 (2021). 10.1038/s41598-021-01337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Huqail, A. A. et al. Efficacy of priming wheat (Triticum aestivum) seeds with a benzothiazine derivative to improve drought stress tolerance. Funct. Plant Biol.50, 915–931 (2023). 10.1071/FP22140 [DOI] [PubMed] [Google Scholar]
- 7.Rizwan, M. et al. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res.22, 15416–15431 (2015). 10.1007/s11356-015-5305-x [DOI] [PubMed] [Google Scholar]
- 8.Lipiec, J., Doussan, C., Nosalewicz, A. & Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys.27, 463–477 (2013). 10.2478/intag-2013-0017 [DOI] [Google Scholar]
- 9.Kakar, H. A. et al. Seed priming modulates physiological and agronomic attributes of maize (Zea mays L.) under induced polyethylene glycol osmotic stress. ACS Omega8, 22788–22808 (2023). 10.1021/acsomega.3c01715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zulfiqar, B. et al. Biochar enhances wheat crop productivity by mitigating the effects of drought: Insights into physiological and antioxidant defense mechanisms. PLoS ONE17, e0267819 (2022). 10.1371/journal.pone.0267819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aghaie, P. & Tafreshi, S. A. H. Central role of 70-kDa heat shock protein in adaptation of plants to drought stress. Cell Stress Chaperones25, 1071–1081 (2020). 10.1007/s12192-020-01144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan, N. et al. Water conservation and plant survival strategies of rhizobacteria under drought stress. Agronomy10, 1683 (2020). 10.3390/agronomy10111683 [DOI] [Google Scholar]
- 13.Nawaz, F. et al. Pretreatment with selenium and zinc modulates physiological indices and antioxidant machinery to improve drought tolerance in maize (Zea mays L.). S. Afr. J. Bot.138, 209–216 (2021). 10.1016/j.sajb.2020.12.016 [DOI] [Google Scholar]
- 14.Naz, R. et al. Interactive effects of hydrogen sulphide and silicon enhance drought and heat tolerance by modulating hormones, antioxidant defence enzymes and redox status in barley (Hordeum vulgare L.). Plant Biol.24, 684–696. 10.1111/plb.13374 (2021). 10.1111/plb.13374 [DOI] [PubMed] [Google Scholar]
- 15.Wang, D. et al. Genome-wide characterization of OFP family genes in wheat (Triticum aestivum L.) reveals that TaOPF29a-A promotes drought tolerance. Biomed. Res. Int.2020, 9708324 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali, J. et al. Biochemical response of okra (Abelmoschus esculentus L.) to Selenium (Se) under drought stress. Sustainability15, 5694 (2023). 10.3390/su15075694 [DOI] [Google Scholar]
- 17.Zaid, A., Mohammad, F. & Siddique, K. H. M. Salicylic acid priming regulates stomatal conductance, trichome density and improves cadmium stress tolerance in Mentha arvensis L. Front. Plant Sci.13, 895427 (2022). 10.3389/fpls.2022.895427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nawaz, F. et al. Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: A critical review. Environ. Sci. Pollut. Res.24, 15959–15975 (2017). 10.1007/s11356-017-9163-6 [DOI] [PubMed] [Google Scholar]
- 19.Kumar, S. et al. Abscisic acid: Metabolism, transport, crosstalk with other plant growth regulators, and its role in heavy metal stress mitigation. Front Plant Sci.13, 972856 (2022). 10.3389/fpls.2022.972856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawar, K. et al. Nitrification inhibitor and plant growth regulators improve wheat yield and nitrogen use efficiency. J. Plant Growth Regul.41, 216–226 (2022). 10.1007/s00344-020-10295-x [DOI] [Google Scholar]
- 21.Noein, B. & Soleymani, A. Corn (Zea mays L.) physiology and yield affected by plant growth regulators under drought stress. J. Plant Growth Regul.41, 672–681 (2022). 10.1007/s00344-021-10332-3 [DOI] [Google Scholar]
- 22.Ding, Q., Zeng, J. & He, X.-Q. MiR169 and its target PagHAP2-6 regulated by ABA are involved in poplar cambium dormancy. J. Plant Physiol.198, 1–9 (2016). 10.1016/j.jplph.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 23.Preciado, J., Begcy, K. & Liu, T. The Arabidopsis HDZIP class II transcription factor ABA INSENSITIVE TO GROWTH 1 functions in leaf development. J. Exp. Bot.73, 1978–1991 (2022). 10.1093/jxb/erab523 [DOI] [PubMed] [Google Scholar]
- 24.Wu, S. et al. Metabolomic and transcriptomic analyses reveal new insights into the role of abscisic acid in modulating mango fruit ripening. Hortic. Res.9, uhac102 (2022). 10.1093/hr/uhac102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iqbal, N. et al. Nitric oxide and abscisic acid mediate heat stress tolerance through regulation of osmolytes and antioxidants to protect photosynthesis and growth in wheat plants. Antioxidants11, 372 (2022). 10.3390/antiox11020372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Y. et al. Salt and ABA Response ERF1 improves seed germination and salt tolerance by repressing ABA signaling in rice. Plant Physiol.189, 1110–1127 (2022). 10.1093/plphys/kiac125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, B. et al. TaFDL2-1A confers drought stress tolerance by promoting ABA biosynthesis, ABA responses, and ROS scavenging in transgenic wheat. Plant J.112, 722–737 (2022). 10.1111/tpj.15975 [DOI] [PubMed] [Google Scholar]
- 28.He, J. et al. Exogenous ABA induces osmotic adjustment, improves leaf water relations and water use efficiency, but not yield in soybean under water stress. Agronomy9, 395 (2019). 10.3390/agronomy9070395 [DOI] [Google Scholar]
- 29.Saradadevi, R., Palta, J. A. & Siddique, K. H. M. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front Plant Sci.8, 1251 (2017). 10.3389/fpls.2017.01251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bano, A., Ullah, F. & Nosheen, A. Role of abscisic acid and drought stress on the activities of antioxidant enzymes in wheat. Plant Soil Environ.58, 181–185 (2012). 10.17221/210/2011-PSE [DOI] [Google Scholar]
- 31.Zhou, Y. et al. A novel ABA functional analogue B2 enhances drought tolerance in wheat. Sci. Rep.9, 1–9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustafa, F. et al. Validation of gosat and oco-2 against in situ aircraft measurements and comparison with carbontracker and geos-chem over Qinhuangdao, China. Remote Sens. (Basel)13, 1–15 (2021).36817948 [Google Scholar]
- 33.Tanotra, S., Zhawar, V. K. & Sharma, S. Regulation of antioxidant enzymes and invertases by hydrogen peroxide and nitric oxide under ABA and water-deficit stress in wheat. Agricult. Res.8, 441–451 (2019). 10.1007/s40003-019-00399-6 [DOI] [Google Scholar]
- 34.Xu, Z., Wang, J., Zhen, W., Sun, T. & Hu, X. Abscisic acid alleviates harmful effect of saline–alkaline stress on tomato seedlings. Plant Physiol. Biochem.175, 58–67 (2022). 10.1016/j.plaphy.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 35.Yan, M. et al. The involvement of abscisic acid in hydrogen gas-enhanced drought resistance in tomato seedlings. Sci. Hortic.292, 110631 (2022). 10.1016/j.scienta.2021.110631 [DOI] [Google Scholar]
- 36.Kong, H. et al. Role of abscisic acid in modulating drought acclimation, agronomic characteristics and β-N-oxalyl-L-α, β-diaminopropionic acid (β-ODAP) accumulation in grass pea (Lathyrus sativus L.). J. Sci. Food Agric.102, 2553–2562 (2022). 10.1002/jsfa.11597 [DOI] [PubMed] [Google Scholar]
- 37.Nayyar, H. & Walia, D. P. Genotypic variation in wheat in response to water stress and abscisic acid-induced accumulation of osmolytes in developing grains. J. Agron. Crop Sci.190, 39–45 (2004). 10.1046/j.0931-2250.2003.00072.x [DOI] [Google Scholar]
- 38.Jiang, M. & Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot.53, 2401–2410 (2002). 10.1093/jxb/erf090 [DOI] [PubMed] [Google Scholar]
- 39.Travaglia, C. et al. Exogenous ABA increases yield in field-grown wheat with moderate water restriction. J. Plant Growth Regul.29, 366–374 (2010). 10.1007/s00344-010-9147-y [DOI] [Google Scholar]
- 40.Travaglia, C., Cohen, A. C., Reinoso, H., Castillo, C. & Bottini, R. Exogenous abscisic acid increases carbohydrate accumulation and redistribution to the grains in wheat grown under field conditions of soil water restriction. J. Plant Growth Regul.26, 285–289 (2007). 10.1007/s00344-007-9018-3 [DOI] [Google Scholar]
- 41.Jiang, M. & Zhang, J. Role of abscisic acid in water stress-induced antioxidant defense in leaves of maize seedlings. Free Radic. Res.36, 1001–1015 (2002). 10.1080/1071576021000006563 [DOI] [PubMed] [Google Scholar]
- 42.Wei, L. et al. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci.6, 458 (2015). 10.3389/fpls.2015.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, X. et al. Improving winter wheat performance by foliar spray of ABA and FA under water deficit conditions. J. Plant Growth Regul.35, 83–96 (2016). 10.1007/s00344-015-9509-6 [DOI] [Google Scholar]
- 44.Raza, M. A. S. et al. Morpho-physiological and biochemical response of wheat to various treatments of silicon nano-particles under drought stress conditions. Sci. Rep.13, 2700 (2023). 10.1038/s41598-023-29784-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussain, M., Shabir, G., Farooq, M., Jabran, K. & Farooq, S. Developmental and phenological responses of wheat to sowing dates. Pak. J. Agric. Sci49, 459–468 (2012). [Google Scholar]
- 46.Aslam, M. A. et al. Can growing degree days and photoperiod predict spring wheat phenology?. Front. Environ Sci.5, 57 (2017). 10.3389/fenvs.2017.00057 [DOI] [Google Scholar]
- 47.Raza, M. A. S. et al. Cu-nanoparticles enhance the sustainable growth and yield of drought-subjected wheat through physiological progress. Sci. Rep.14, 14254 (2024). 10.1038/s41598-024-62680-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khalilzadeh, R., Seyed Sharifi, R. & Jalilian, J. Antioxidant status and physiological responses of wheat (Triticum aestivum L.) to cycocel application and bio fertilizers under water limitation condition. J. Plant Interact.11, 130–137 (2016). 10.1080/17429145.2016.1221150 [DOI] [Google Scholar]
- 49.Morinaga, K. & Sykes, S. R. Effect of salt and water stress on fruit quality, physiological responses, macro-and micro-element contents in leaves of Satsuma mandarin trees under greenhouse conditions. Jpn. Agricult. Res. Q. JARQ35, 53–58 (2001). 10.6090/jarq.35.53 [DOI] [Google Scholar]
- 50.Dionisio-Sese, M. L. & Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci.135, 1–9 (1998). 10.1016/S0168-9452(98)00025-9 [DOI] [Google Scholar]
- 51.Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol.59, 315–318 (1977). 10.1104/pp.59.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang, S.-Y., Lin, H.-W., Chern, R.-H., Lo, H. F. & Li, L. Reduced susceptibility to waterlogging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul.27, 167–172 (1999). 10.1023/A:1006100508910 [DOI] [Google Scholar]
- 53.Maechlay, A. C. & Chance, B. The assay of catalase and peroxidase. Methods of biochemical analysis 357–424 (Interscience Inc., 1954). [DOI] [PubMed] [Google Scholar]
- 54.Zhang, G., Tanakamaru, K., Abe, J. & Morita, S. Influence of waterlogging on some anti-oxidative enzymatic activities of two barley genotypes differing in anoxia tolerance. Acta Physiol. Plant29, 171–176 (2007). 10.1007/s11738-006-0022-1 [DOI] [Google Scholar]
- 55.Wu, F., Zhang, G. & Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot.50, 67–78 (2003). 10.1016/S0098-8472(02)00113-2 [DOI] [Google Scholar]
- 56.Piper, C. S. Soil and plant analysis (Scientific Publishers, 2019). [Google Scholar]
- 57.Kitson, R. E. & Mellon, M. G. Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Ind. Eng. Chem. Analyt. Ed.16, 379–383 (1944). 10.1021/i560130a017 [DOI] [Google Scholar]
- 58.Steel, R. Analysis of variance I: The one-way classification. Princ. Proc. Stat. Biometr. Approach 139–203 (1997).
- 59.Muhammad, F. et al. Ameliorating drought effects in wheat using an exclusive or Co-applied rhizobacteria and ZnO nanoparticles. Biol. (Basel)11, 1564 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raza, M. A. S. et al. Integrating biochar, rhizobacteria and silicon for strenuous productivity of drought stressed wheat. Commun. Soil Sci. Plant Anal.52, 338–352 (2021). 10.1080/00103624.2020.1853149 [DOI] [Google Scholar]
- 61.Haider, I. et al. Potential effects of biochar application on mitigating the drought stress implications on wheat (Triticum aestivum L.) under various growth stages. J. Saudi Chem. Soc.24, 974–981 (2020). 10.1016/j.jscs.2020.10.005 [DOI] [Google Scholar]
- 62.Hussain, I. et al. Alleviating effects of salicylic acid spray on stage-based growth and antioxidative defense system in two drought-stressed rice (Oryza sativa L.) cultivars. Turk. J. Agricult. For.47, 79–99 (2023). 10.55730/1300-011X.3066 [DOI] [Google Scholar]
- 63.Wahab, A. et al. Plants’ physio-biochemical and phyto-hormonal responses to alleviate the adverse effects of drought stress: A comprehensive review. Plants11, 1620 (2022). 10.3390/plants11131620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao, T.-J. et al. Regulating the drought-responsive element (DRE)-mediated signaling pathway by synergic functions of trans-active and trans-inactive DRE binding factors in Brassica napus. J. Biol. Chem.281, 10752–10759 (2006). 10.1074/jbc.M510535200 [DOI] [PubMed] [Google Scholar]
- 65.Fahad, S. et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci.8, 1147 (2017). 10.3389/fpls.2017.01147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iqbal, M. T. Utilization of biochar in improving yield of wheat in Bangladesh. Bulg. J. Soil Sci.2, 53–74 (2017). [Google Scholar]
- 67.Raheem, A., Shaposhnikov, A., Belimov, A. A., Dodd, I. C. & Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci.64, 574–587 (2018). 10.1080/03650340.2017.1362105 [DOI] [Google Scholar]
- 68.Wang, J.-Y., Xiong, Y.-C., Li, F.-M., Siddique, K. H. M. & Turner, N. C. Effects of drought stress on morphophysiological traits, biochemical characteristics, yield, and yield components in different ploidy wheat: A meta-analysis. Adv. Agron.143, 139–173 (2017). 10.1016/bs.agron.2017.01.002 [DOI] [Google Scholar]
- 69.Sabagh, A. E. L. et al. Potential role of plant growth regulators in administering crucial processes against abiotic stresses. Front. Agron.3, 648694 (2021). 10.3389/fagro.2021.648694 [DOI] [Google Scholar]
- 70.Wang, X. et al. Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul.75, 677–687 (2015). 10.1007/s10725-014-9969-x [DOI] [Google Scholar]
- 71.Aown, M. et al. Foliar application of potassium under water deficit conditions improved the growth and yield of wheat (Triticum aestivum L.). J. Anim. Plant Sci.22, 431–437 (2012). [Google Scholar]
- 72.Tripathi, D. K., Yadav, S. R., Mochida, K. & Tran, L.-S.P. Plant growth regulators: True managers of plant life. Plant Cell Physiol.63, 1757–1760 (2022). 10.1093/pcp/pcac170 [DOI] [PubMed] [Google Scholar]
- 73.Zhang, Y., Li, Y., Hassan, M. J., Li, Z. & Peng, Y. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol.20, 1–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Onyemaobi, O. et al. Reproductive stage drought tolerance in wheat: Importance of stomatal conductance and plant growth regulators. Genes (Basel)12, 1742 (2021). 10.3390/genes12111742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu, C., Ji, W., Hou, M., Ma, T. & Mao, J. Evaluation of efficiency and resilience of agricultural water resources system in the Yellow River Basin, China. Agric. Water Manag.266, 107605 (2022). 10.1016/j.agwat.2022.107605 [DOI] [Google Scholar]
- 76.Iqbal, R. et al. Foliar applied iron and zinc improves the growth, physiological and yield related traits of wheat under drought. Int. J. Biosci.14, 376–387 (2019). 10.12692/ijb/14.3.376-387 [DOI] [Google Scholar]
- 77.Feng, J. et al. Oxidative stress and lipid peroxidation: prospective associations between ferroptosis and delayed wound healing in diabetic ulcers. Front. Cell Dev. Biol.10, 898657 (2022). 10.3389/fcell.2022.898657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vuković, R. et al. Physiological, biochemical and molecular response of different winter wheat varieties under drought stress at germination and seedling growth stage. Antioxidants11, 693 (2022). 10.3390/antiox11040693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rizwan, M. et al. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf.130, 43–53 (2016). 10.1016/j.ecoenv.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 80.Rizwan, M. et al. Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res.23, 2230–2248 (2016). 10.1007/s11356-015-5697-7 [DOI] [PubMed] [Google Scholar]
- 81.Vijayaraghavareddy, P. et al. Production and scavenging of reactive oxygen species confer to differential sensitivity of rice and wheat to drought stress. Crop Environ.1, 15–23 (2022). 10.1016/j.crope.2022.03.010 [DOI] [Google Scholar]
- 82.Gholami, R., Fahadi Hoveizeh, N., Zahedi, S. M., Gholami, H. & Carillo, P. Effect of three water-regimes on morpho-physiological, biochemical and yield responses of local and foreign olive cultivars under field conditions. BMC Plant Biol.22, 477 (2022). 10.1186/s12870-022-03855-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Batool, M. et al. Rapeseed morpho-physio-biochemical responses to drought stress induced by PEG-6000. Agronomy12, 579 (2022). 10.3390/agronomy12030579 [DOI] [Google Scholar]
- 84.Batool, M. et al. Drought stress in Brassica napus: effects, tolerance mechanisms, and management strategies. J. Plant Growth Regul. 1–25 (2022).
- 85.Raza, M. A. S. et al. Drought ameliorating effect of exogenous applied cytokinin in wheat. Pak. J. Agric. Sci.57 (2020).
- 86.Kuromori, T., Seo, M. & Shinozaki, K. ABA transport and plant water stress responses. Trends Plant Sci.23, 513–522 (2018). 10.1016/j.tplants.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 87.Trapeznikov, V. K., Ivanov, I. I. & Kudoyarova, G. R. Effect of heterogeneous distribution of nutrients on root growth, ABA content and drought resistance of wheat plants. Plant Soil252, 207–214 (2003). 10.1023/A:1024734310214 [DOI] [Google Scholar]
- 88.Wei, L. et al. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front Plant Sci.6, 458 (2015). 10.3389/fpls.2015.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.