Abstract
We investigated factors affecting the community composition of lignicolous myxomycetes in dead wood with white and brown rot through summer and autumn surveys in a subalpine forest in Central Japan. In both seasons, wood had decayed to a softer state under brown rot than under white rot. The pH of wood with white rot was nearly neutral, while wood with brown rot was weakly acidic. Wood pH was lower in summer than in autumn. Forty-two myxomycetes taxa in 19 genera were identified in 302 fruiting-body colonies; white rot yielded 31 taxa and brown rot 24 taxa. Species diversity was higher on wood with white rot than on wood with brown rot. The effect of wood hardness on species composition depended on season. Several species exhibited a preference for one of the rot types. The substrate conditions associated with brown rot limit myxomycetes species diversity.
Keywords: brown rot, seasonality, white rot, wood pH, wood hardness
In myxomycetes, a group of fungus-like protists, the reproductive stage comprises an amoeba and the developmental stage comprises spore-containing fruiting bodies (Novozhilov et al., 2017). Myxomycetes typically inhabit coarse woody debris and prey on decomposers such as bacteria and fungi (Clark & Haskins, 2015; Madelin, 1984), and their nutritional preferences may vary between species (Hoppe & Kutschera, 2015). Wood-inhabiting (lignicolous) myxomycetes are associated with the state of decay of dead wood (Novozhilov et al., 2017; Takahashi & Hada, 2009; Xavier de Lima & de Holanda Cavalcanti, 2015), but the factors influencing their association with white and brown rot remain to be clarified.
Fungi decompose the lignocellulose in wood, although the process differs depending on the type of wood rot (Fukasawa, 2013). This difference may impact lignicolous myxomycete community composition (Fukasawa et al., 2015). The relationships between the amount of white and brown rot present in the forest and myxomycete community dynamics remain incompletely understood. To address this gap in knowledge, we examined the responses of myxomycete communities to the state of wood decay and substrate pH associated with brown and white wood rot in a subalpine coniferous forest of Japan.
The survey site was located in the subalpine forest of the Yatsugatake in Nagano Prefecture, Central Japan, beside Shirakoma-ike (2,132 m altitude, Sakuho-machi Minamisaku-gun, 36.04752121° N, 138. 36402719° E). The forest, comprising old-growth conifers, is dominated by Tsuga diversifolia Mast. and a mixture of Abies veitchii Lindley and Abies mariesii Mast. During our survey, large deadwood logs were abundant on the forest floor (Fig. 1A). The predicted mean annual temperature was 2.3 °C and mean annual precipitation >1,271.5 mm for 1999-2020, according to the neighboring Japanese Meteorological Agency station (Haramura; 35.5801200° N, 138.1301200° E, 1,017 m asl).
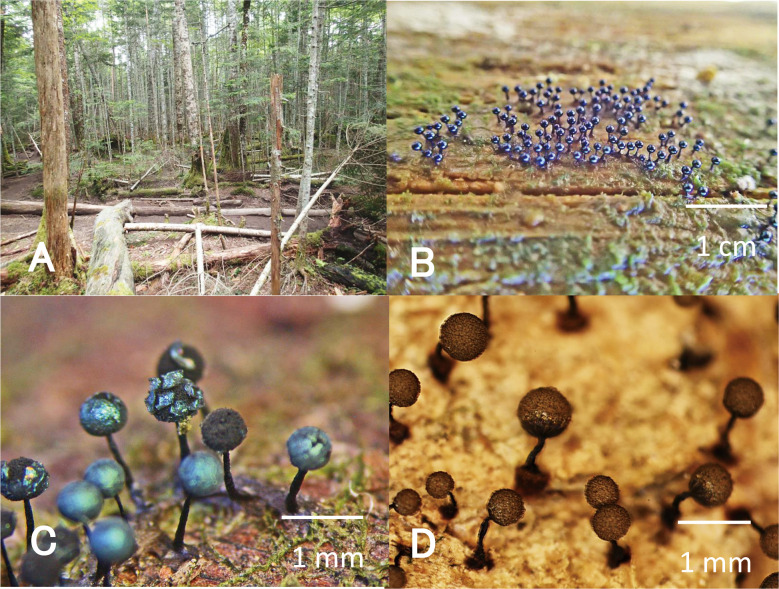
Fig. 1 - Survey site in a subalpine coniferous forest on Mt. Yatugatake, and myxomycete fruiting bodies. A: Fallen logs on the forest floor. B: Colony of myxomycete fruiting bodies on a portion of a log exhibiting white rot. C: Fruiting bodies of Lamproderma columbinum. D: Fruiting bodies of Cribraria macrocarpa.
The surveys were performed over 3y during two field seasons (summer and autumn), on Jul 23, 2017; Jul 25, 2018; Oct 28, 2018; and Oct 28, 2019, at Shirakoma-ike. The process of data collection was as follows. First, we located portions of logs exhibiting myxomycete fruiting bodies (Fig. 1B). We identified the rot type in those portions based on macroscopic features. White rot is distinguished by the separation of the wood into fibers, whereas brown rot is distinguished by cracks along the length and breadth of the rotten wood (Araya, 1993). We then measured wood hardness (depth, in mm) using a soil hardness tester (No. 351; Fujiwara Scientific, Nishigahara Kita-ku, Tokyo, Japan), and pH using a hand-held pH meter (Shinwa code 72724, measurement range of 4.0-7.0), following Fukasawa et al. (2015). Surveys were conducted on ca. 200 fallen logs along a trekking path on the forest floor (ca. 800 m × 30 m) using the naked eye and a magnifying glass. During each survey, searching continued until ca. 100 colonies of fruiting bodies were located and recorded. The average wood hardness and substrate pH were calculated for each species and for white rot and brown rot separately.
For each myxomycete species, the number of colonies was recorded for each season and wood rot type. Myxomycete fruiting bodies were partly sampled and glued to specimen boxes then identified to species level via microscopic observation in the laboratory. Myxomycete nomenclature was according to Yamamoto (1998), and the most recent published scientific names were used (Lado, 2005-2022). Both species and varieties were considered when assessing species richness. Voucher specimens were deposited in the Tottori Prefectural Museum herbarium (TRPM).
To describe the myxomycete assemblage, abundance (colony count) was compared by season and rot type. Survey accuracy was estimated using the index of exactitude, as [observed number of species (Sobs) in each assemblage] / [estimated number of species (Sest)] × 100; Sest was calculated using the Chao1 method (Chao, 1984). Myxomycete assemblage species diversity was calculated using the Shannon-Wiener index (Hʹ) (Shannon & Weaver, 1963) and the equitability (Jʹ) index (Pielou, 1996), as in previous studies (Stephenson, 1989). These indices were estimated using PAST (Hammer et al., 2001; https://past.en.lo4d.com/windows). Species composition was calculated using the relative abundance of each species (as %), as [number of colonies for a given species] / [total colonies] × 100. Species preference for each rot type was determined using an independent sample t-test in ESUMI Excel Statistics 5.0 (ESUMI Co. Ltd., Tokyo, Japan).
In total, 302 myxomycete colonies were identified, 222 on white rot and 80 on brown rot. Considering wood with myxomycete colonies, in terms of wood hardness, the depth for white rot was 21.0 ± 7.0 mm in summer and 20.6 ± 7.8 mm depth in autumn; for brown rot, the depth was 18.4 ± 7.1 mm in summer and 16.0 ± 8.0 mm in autumn (Table 1). The wood was decayed to a significantly softer state under brown rot than under white rot in both seasons (p < 0.05). Overall, wood pH ranged from 4.4 to 7.0, and differed seasonally; mean pH was significantly lower in summer (pH = 5.9) than in autumn (pH = 6.7) (t-test, p < 0.01, Table 1). The rot types were significantly influenced by the pH: wood with brown rot was more acidic (pH 5.4 ± 0.5 in summer, 6.4 ± 0.3 in autumn) than wood with white rot (pH 6.2 ± 0.4 in summer, 6.8 ± 0.2 in autumn) (p < 0.01).
Table 1. Number of colonies, wood hardness, and pH at which myxomycete fruiting colony occurred on a portion of log according to the season and decay type. Hardness and pH are indicated by the mean value with its standard deviation (*p <0.05, **p < 0.01).
| White rot | Brown rot | Total | ||
| Summer | ||||
| Colonies | 135 | 55 | 190 | |
| Hardness | 21.0 ± 7.0 | 18.4 *± 7.1 | 20.2 ± 7.1 | |
| pH | 6.2 ± 0.4 | 5.4 ** ± 0.5 | 5.9 ± 0.6 ** | |
| Autumn | ||||
| Colonies | 87 | 25 | 112 | |
| Hardness | 20.6 ± 7.8 | 16.0 * ± 8.0 | 19.6 ± 8.0 | |
| pH | 6.8 ± 0.2 | 6.4 ** ± 0.3 | 6.7 ± 0.3 | |
In contrast, considering wood without myxomycete colonies in summer, wood with white rot exhibited hardness of 14.9 ± 6.5 mm depth and pH of 6.2 ± 0.4 (n = 66), while wood with brown rot exhibited hardness of 14.7 ± 6.9 mm depth and pH of 5.2 ± 0.5 (n = 71); the two rot types did not differ significantly in wood hardness, whereas brown rot exhibited significantly lower pH than white rot (p < 0.01). These findings indicate that rot type influenced wood pH, which was nearly neutral for wood with white rot, whereas wood with brown rot exhibited weak acidity.
Accuracy for the entire survey was 85.7%, based on the Chao1-estimated number of species (Table 2). In total, 42 myxomycete taxa belonging to 19 genera were identified. In summer, 27 taxa in 12 genera were identified in 190 colonies of fruiting bodies; in autumn, 16 taxa in 10 genera were identified in 112 colonies (Table 3). The species fruited seasonally, with some fruiting in summer and some in autumn, except for one taxon, Lycogala epidendrum (L.) Fr. The dominant species in summer was Ceratiomyxa fruticulosa (O.F. Müll.) T. Macbr., followed (in decreasing order of relative abundance) by L. epidendrum, Stemonitis axifera (Bull.) T. Macbr., Cribraria cancellata (Batsch) Nann.-Bremek., Stemonitopsis hyperopta (Meyl.) Nann.-Bremek., and Physarum viride (Bull.) Pers., all of which exhibited 10 colonies or more (Table 3). In autumn, two species―Lamproderma columbinum (Pers.) Rostaf. (Fig. 1C) and Cribraria macrocarpa Schrad. (Fig. 1D)―were prominent. The remaining species typically exhibited fewer than 10 colonies each.
Table 2. Accuracy of assessments and myxomycete species diversity in rot types. The numbers in brackets of taxa indicate the predicted species by Chao 1. Species diversity is indicated by the Shannon & Weaver index (H') and the numbers in brackets are the equitability index (J').
| White | Brown | Total | ||
| Summer | ||||
| Taxa | 18 (20) | 19 (23) | 27 (28) | |
| Accuracy (%) | 90 | 82.6 | 96.4 | |
| Autumn | ||||
| Taxa | 14 (17) | 5 (6) | 16 (24) | |
| Accuracy (%) | 82.3 | 83.3 | 66.7 | |
| Total | Taxa | 31 (38) | 24 (30) | 42 (49) |
| Accuracy (%) | 81.5 | 80 | 85.7 | |
| Species diversity | 2.82 (0.82) | 2.75 (0.87) | 3.05 (0.82) | |
Table 3. Myxomycete taxa and the number of colonies occurring on coniferous decay wood, classified based on wood rot types in summer and autumn.
| Taxaa | White rot | Brown rot | Total colonies | |||||||
| Abbreviationb | Colonies | Hardness | pH | Colonies | Hardness | pH | ||||
| Summer | ||||||||||
| Ceratiomyxa fruticulosa (O. F. Müell.) T. Macbr. | Cefu | 34 * | 18 | 6.1 | 6 | 21 | 5.5 | 40 | ||
| Lycogala epidendrum (L.) Fr. | Lyep-S | 26 * | 21 | 6.0 | 4 | 25 | 6.0 | 30 | ||
| Stemonitis axifera (Bull.) T. Macbr. | Stax | 14 | 22 | 6.1 | 7 | 16 | 5.1 | 21 | ||
| Stemonitopsis hyperopta (Meyl.) Nann.-Bremek. | Sthy | 14 | 20 | 6.1 | 3 | 23 | 5.1 | 17 | ||
| Cribraria cancellata (Batsch) Nann-.Bremek. | Crca | 3 | 16 | 5.9 | 10 ** | 15 | 5.3 | 13 | ||
| Physarum viride (Bull.) Pers. | Phvi | 10 | 29 | 6.4 | 1 | 34 | 6.2 | 11 | ||
| Enerthenema papillatum (Pers.) Rostaf. | Enpa | 7 | 25 | 6.4 | 7 | |||||
| Stemonitopsis aequalis (Peck) Y. Yamam. | Stae | 5 | 24 | 6.5 | 1 | 25 | 5.0 | 6 | ||
| Stemonitis smithii T. Macbr. | Stsm | 4 | 22 | 6.3 | 1 | 16 | 5.2 | 5 | ||
| Stemonitopsis gracilis (G. Lister) Nann.-Bremek. | Stgr | 4 | 19 | 5.8 | 4 | |||||
| Cribraria piriformis var. notabilis Rex | Crpyn | 4 ** | 14 | 5.2 | 4 | |||||
| Arcyria cinerea (Bull.) Pers. | Arci | 3 | 19 | 6.3 | 3 | |||||
| Stemonitis virginiensis Rex | Stvi | 3 | 23 | 6.0 | 3 | |||||
| Comatricha nigra (Pers. Ex J. F. Gmel.) J. SchrÖt | Coni | 1 | 22 | 5.8 | 2 | 15 | 5.5 | 3 | ||
| Stemonitis pallida Wingate | Stpa | 3 ** | 21 | 5.4 | 3 | |||||
| Stemonitopsis amoena (Nann.-Bremek.) Nann.-Bremek. | Stam | 3 ** | 20 | 5.5 | 3 | |||||
| Arcyria obvelata (Oeder) Onsberg | 2 | 26 | 6.5 | 2 | ||||||
| Lindbladia tubulina Fr. | 2 | 10 | 6.4 | 2 | ||||||
| Fuligo septica (L.) Wiggers | 1 | 12 | 6.2 | 1 | 27 | 5.8 | 2 | |||
| Arcyria virescens G. Lister | 2 | 10 | 5.0 | 2 | ||||||
| Stemonitopsis reticulata var. similis (G. Lister) Nann.-Bremek. & Y. Yamam. | 2 | 21 | 5.3 | 2 | ||||||
| Stemonitopsis typhina var. similis (G. Lister) Nann.-Bremek. & Y. Yamam. | 2 | 25 | 4.9 | 2 | ||||||
| Hemitrichia calyculata (Speg.) M. L. Farr | 1 | 25 | 6.4 | 1 | ||||||
| Stemonitis flavogenita E. Jahn | 1 | 32 | 6.5 | 1 | ||||||
| Comatricha pulchella (C. Bab.) Rostaf. | 1 | 15 | 5.2 | 1 | ||||||
| Cribraria intricata Schrad. | 1 | 3 | 6.6 | 1 | ||||||
| Cribraria vulgaris Schrad. | 1 | 8 | 4.8 | 1 | ||||||
| Autumn | ||||||||||
| Lamproderma columbinum (Pers.) Rostaf. | Laco | 33 * | 22 | 6.8 | 3 | 21 | 6.1 | 36 | ||
| Cribraria macrocarpa Schrad. | Crma | 13 | 15 | 6.7 | 18 ** | 15 | 6.4 | 31 | ||
| Lycogala epidendrum (L.) Fr. | Lyep-A | 8 | 21 | 6.7 | 8 | |||||
| Trichia decipiens (Pers.) T. Macbr. | Trde | 6 | 19 | 6.7 | 2 | 30 | 6.9 | 8 | ||
| Elaeomyxa cerifera (G. Lister) Hagelst. | Elce | 6 | 24 | 6.9 | 6 | |||||
| Trichia erecta Rex | Trel | 5 | 24 | 7.0 | 5 | |||||
| Tubifera ferruginosa (Batsch) J. F. Gmel. | Tufe | 4 | 16 | 6.9 | 4 | |||||
| Colloderma oculatum (Lippert) G. Lister | Cooc | 3 | 27 | 6.8 | 3 | |||||
| Physarum atroviolaceum G. Moreno, Y. Yamam. & A. Castillo | Phat | 3 | 30 | 6.9 | 3 | |||||
| Diderma ochraceum Hoffm. | 2 | 8 | 6.9 | 2 | ||||||
| Cribraria vulgaris var. oregana (H. C. Gilbert) Nann.-Bremek. & Lado | 1 | 10 | 6.8 | 1 | ||||||
| Diderma aurantiacum Y. Yamam. & Nann.-Bremek. | 1 | 15 | 6.6 | 1 | ||||||
| Lepidoderma tigrinum (Pers.) Rostaf. | 1 | 17 | 6.9 | 1 | ||||||
| Trichia botrytis (J.F. Gmel.) Pers. | 1 | 25 | 7.0 | 1 | ||||||
| Cribraria filiformis Nowotny & H. Neubert | 1 | 4 | 6.7 | 1 | ||||||
| Cribraria purpurea Schrad. | 1 | 7 | 6.8 | 1 | ||||||
| Total number of colonies | 222 | 80 | 302 | |||||||
| Number of species | 32 | 24 | 42 | |||||||
a The taxa were arranged by the number of total colonies in each season.
b Name abbreviations of species occurred at three or more colonies
*p < 0.05, **p < 0.01
Table 3 presents the average wood hardness and pH for the species. Most of the species were widely distributed on moderately decayed wood with a hardness depth range of 13-27 mm. However, two species [Physarum atroviolaceum G. Moreno, Y. Yamam. & A. Castillo and P. viride (Bull.) Pers.] showed preferences for hard wood with depth ≥ 29 mm. Cribraria and Lindbladia were the only species present on well-decayed wood (depth < 13 mm).
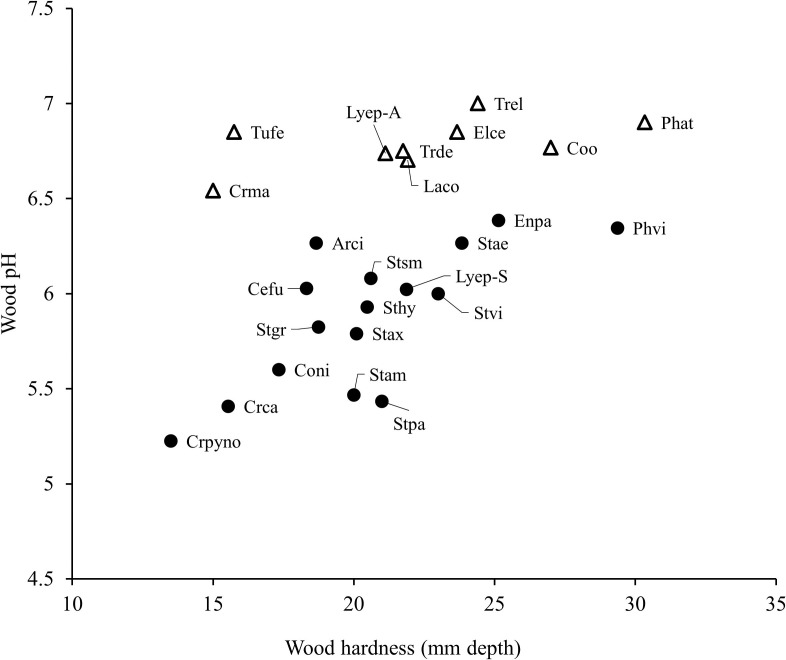
Figure 2 presents the pH and wood hardness of the 25 taxa exhibiting at least three colonies. Wood pH was 5.2-7.0 for most of the myxomycetes species; the autumn-fruiting myxomycetes species preferred wood with pH > 6.4, whereas summer-fruiting species preferred wood with pH < 6.4. In summer, L. epidendrum occurred at a hardness of 20.0 mm depth and pH of 5.9; in autumn, it occurred at 21.1 mm depth and pH of 6.7. In summary, myxomycetes substrate usage (and substrate chemistry) varied between the seasons.
Fig. 2 - Preferences of dominant myxomycete species for microhabitats on decaying wood in summer and autumn. The state of wood decay is indicated by the wood hardness and pH. The name codes for respective taxa, hardness values, and pH are shown in Table 3. ●, summer; ∆, autumn.
Substrate condition, which depends on the type of wood rot, affected the myxomycete assemblage. Wood with white rot exhibited many colonies, hosting 31 taxa, whereas brown rot hosted 24 taxa. White rot exhibited higher species diversity (H′ = 2.82, J′ = 0.82) than brown rot (H′ = 2.75, J′ = 0.87) (Table 2). Thirteen taxa were present on both rot types (Table 3), 18 only on white rot, and 11 only on brown rot. Three species―C. fruticulosa, L. epidendrum, and L. columbinum―were significantly biased toward white rot, while C. macrocarpa was prominently biased toward brown rot.
Three species exhibited seasonal selectivity for the specific rot type with a particular substrate pH. Lamproderma columbinum exhibited a preference for wood with white rot in autumn (independent sample t-test, p < 0.05), with a hardness depth of 21.9 mm and pH of 6.7 (Table 3). Two species of Cribraria preferred wood with brown rot (independent sample t-test, p < 0.01): C. pyriformis var. notabilis Rex occurred on average on wood with 14 mm depth and pH of 5.2 in summer, and C. macrocarpa on wood with 15 mm depth and pH of 6.0 in autumn. Stemonitis pallida Wingate and Stemonitopsis amoena (Nann.-Bremek.) Nann.-Bremek. exhibited a preference for brown rot (Table 3).
Brown rot accounted for 26% of the subalpine coniferous forest samples that we collected, although brown rot occurs on only a small proportion of pine logs in Japan (Fukasawa, 2015). Brown rot fungi, however, are more abundant than white rot fungi on Norway spruce logs (Rajala et al., 2011) and occur widely on well decayed Picea abies logs (Bütler et al., 2007). The present forest survey enabled us to examine myxomycete species diversity on brown rot in the subalpine coniferous forest.
The wood decay process involves two phases, the first involving white rot, and the second brown rot (Fukasawa et al., 2014). Thus, in this study, the wood with brown rot had decayed further and was softer than the wood with white rot. Under white rot, wood pH increases. In contrast, brown rot leads to acidification (Fukasawa et al., 2015), because the fungi responsible for brown rot produce oxalic acid as they degrade wood (Espejo & Agosin, 1991). Low substrate pH may limit the growth and development of several myxomycete species, limiting zygote formation (Shinnick et al., 1978), plasmodium formation (Collins & Tang, 1973), and sporulation (Gray, 1939, 1953). Our findings indicate that few species were suited to an acidic substrate and that species diversity was lower on wood with brown rot. Fukasawa et al. (2015) reported that lignin-rich logs with brown rot hosted Cribraria tenella and Cribraria intricata, which prefer a lower pH. Several Cribraria species have been reported on acidic decaying wood; these include Cribraria cancellata (mean pH 5.0) and Cribraria tenella (pH 5.1), as well as other species such as Arcyria cinerea (Bull.) Pers. (pH 5.2), L. epidendrum (pH 5.4), S. axifera (pH 5.4), and C. fruticulosa (pH 5.7) (Xavier de Lima & de Holanda Cavalcanti, 2015). Vlasenko et al. (2018) suggested that a preference for acidic substrates on decaying wood restricts the community composition to Cribraria and Comatricha species. These reports suggest that the myxomycete species assemblage is associated with wood rot type via effects of substrate pH.
The type of wood rot influences bacterial activity (Jurgensen et al., 1989) as well as the dominant bacterial taxa (Haq et al., 2022). This potentially affects the food source for the trophic stages of myxomycetes, indirectly enhancing the growth of their fruiting bodies. Acidity restricts the growth of most bacteria (Lauber et al., 2009). This is potentially a key driver of the reduced myxomycetes species diversity observed in this study under the lower pH of wood with brown rot.
This study described associations of myxomycetes community composition with wood hardness, acidity, and decay types. The growth of myxomycetes species on decaying wood is known to be affected by the decay state. Myxomycetes may play an important role in the decomposition of woody debris in forest ecosystems. Most myxomycetes exhibit spatial and temporal niche separation in woody debris, as do species of Ceratiomyxa (Rojas et al., 2008), potentially interacting with other organisms (Dudka & Romanenko, 2006) and slowing the decay of woody debris by preying on decomposers (Yoshida, 2015). The findings suggest potential directions for future research to clarify the mechanisms and ecological aspects of the functions of myxomycetes in forests.
Declarations of interest
The authors declare no conflicts of interest. The survey undertaken complies with the current laws of Japan.
Acknowledgments
This research was supported in part by a grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan (no. 17H00447). We are grateful to Naka Shinetsu at the local forest management office of the Ministry of Agriculture, Forestry and Fisheries for providing permission for the forest survey. We thank Miss Kyōko Tateishi for the photograph of myxomycete fruiting bodies.
References
- Araya, K.(1993). Relationship between the decay types of dead wood and occurrence of lucanid beetles (Coleoptera: Lucanidae). Applied Entomology and Zoology, 28, 27-33. https://doi.org/10.1303/aez.28.27 [Google Scholar]
- Bütler, R., Patty, L., Le Bayon, R.-C., Guenat, C., &Schlaepfer, R.(2007). Log decay of Picea abies in the Swiss Jura Mountains of central Europe. Forest Ecology and Management, 242, 791-799. https://doi.org/10.1016/j.foreco.2007.02.017 [Google Scholar]
- Chao, A.(1984). Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics, 11, 265-270.https://www.jstor.org/stable/4615964 [Google Scholar]
- Clark, J., &Haskins E. F.(2015). Myxomycete plasmodial biology: A review. Mycosphere, 6, 643-658. https://doi.org/10.5943/mycosphere/6/6/1 [Google Scholar]
- Collins, O. R., &Tang, H.(1973). Physarum polycephalum: pH and plasmodium formation. Mycologia, 65, 232-236. https://doi.org/10.1080/00275514.1973.12019428 [Google Scholar]
- Dudka, I. O., &Romanenko, K. O.(2006). Co-existence and interaction between myxomycetes and other organisms in shared niches. Acta Mycologica, 41, 99-112. https://doi.org/10.5586/am.2006.014 [Google Scholar]
- Espejo, E., &Agosin, E.(1991). Production and degradation of oxalic acid by brown rot fungi. Applied and Environmental Microbiology, 57, 1980-1986. https://doi.org/10.1128/aem.57.7.1980-1986.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa, Y.(2013). The influences of wood decay fungi and their decay types on communities of diverse organisms developed on coarse woody debris [In Japanese with English abstract]. Japanese Journal of Ecology, 63, 311-325. [Google Scholar]
- Fukasawa, Y.(2015). The geographical gradient of pine log decomposition in Japan. Forest Ecology and Management, 349, 29-35. https://doi.org/10.1016/j.foreco.2015.04.010 [Google Scholar]
- Fukasawa, Y., Katsumata, S., Mori, A. S., Osono, T., &Takeda, H.(2014). Accumulation and decay dynamics of coarse woody debris in a Japanese old-growth subalpine coniferous forest. Ecological Research, 29, 257-269. https://doi.org/10.1007/s11284-013-1120-3 [Google Scholar]
- Fukasawa, Y., Takahashi, K., Arikawa, T., Hattori, T., &Maekawa, N.(2015). Fungal wood decomposer activities influence community structures of myxomycetes and bryophytes on coarse woody debris. Fungal Ecology, 14, 44-52. https://doi.org/10.1016/j.funeco.2014.11.003 [Google Scholar]
- Gray, W. D.(1939). The relation of pH and temperature to the fruiting of Physarum polycephalum. American Journal of Botany, 26, 709-714. https://doi.org/10.2307/2437020 [Google Scholar]
- Gray, W. D.(1953). Further studies on the fruiting of Physarum polycephalum. Mycologia, 45, 817-824. https://doi.org/10.1080/00275514.1953.12024318 [Google Scholar]
- Hammer, Ø., Harper, D. A. T., &Ryan, P. D.(2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 4. https://palaeo-electronica.org/2001_1/past/past.pdf [Google Scholar]
- Haq, I. U., Hillmann, B., Moran, M., Willard, S., Knights, D., Fixen, K. R., &Schilling, J. S.(2022). Bacterial communities associated with wood rot fungi that use distinct decomposition mechanisms. ISME Communications, 2, 1-9. https://doi.org/10.1038/s43705-022-00108-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe, T., &Kutschera, U.(2015). Species-specific cell mobility of bacteria-feeding myxoamoebae in plasmodial slime molds. Plant Signaling & Behavior, 10, e1074368. http://dx.doi.org/10.1080/15592324.2015.1074368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgensen, M. F., Larsen, M. J., Wolosiewicz, M., &Harvey, A. E.(1989). A comparison of dinitrogen fixation rates in wood litter decayed by white-rot and brown-rot fungi. Plant and Soil, 115, 117-122. https://doi.org/10.1007/BF02220701 [Google Scholar]
- Lado, C.(2005-2022). An online nomenclatural information system of Eumycetozoa. Real Jardín Botánico, CSIC. Madrid, Spain. Retrieved Aug 1, 2022, from https://eumycetozoa.com
- Lauber, C. L., Hamady, M., Knight, R., &Fierer, N.(2009). Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Applied and Environmental Microbiology, 75, 5111-5120. https://doi.org/10.1128/AEM.00335-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelin, M. F.(1984). Myxomycete data of ecological significance. Transactions of the British Mycological Society, 83, 1-19. https://doi.org/10.1016/S0007-1536(84)80240-5 [Google Scholar]
- Novozhilov, Y. K., Rollins, A., &Schnittler, M.(2017). Ecology and distribution of Myxomycetes. In: S. L. Stephenson & C. A. Rojas (Eds.), Myxomycetes: biology, systematics, biogeography and ecology (pp. 253-297). Academic Press. [Google Scholar]
- Pielou, E. C.(1996). The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13, 131-144. https://doi.org/10.1016/0022-5193(66)90013-0 [Google Scholar]
- Rajala, T., Peltoniemi, M., Hantula, J., Makipaa, R., &Pennanen, T.(2011). RNA reveals a succession of active fungi during the decay of Norway spruce logs. Fungal Ecology, 4, 437-448. https://doi.10.1016/j.funeco.2011.05.005 [Google Scholar]
- Rojas, C., Schnittler, M., Biffi, D., &Stephenson, S. L.(2008). Microhabitat and niche separation in species of Ceratiomyxa. Mycologia, 100, 843-850. https://doi.org/10.3852/07-197 [DOI] [PubMed] [Google Scholar]
- Shannon, C. E., &Weaver, W.(1963). The Mathematical Theory of Communication (first published in 1949). Urbana: University of Illinois Press. [Google Scholar]
- Shinnick, T. M., Pallota, D. J., Jones-Brown, Y. V. R., Youngman, P. J., &Holt, C. E.(1978). A gene, imz, affecting the pH sensitivity of zygote formation in Physarum polycephalum. Current Microbiology, 1, 163-166. https://doi.org/10.1007/BF02601670 [DOI] [PubMed] [Google Scholar]
- Stephenson, S. L.(1989). Distribution and ecology of myxomycetes in temperate forests. Ⅱ. Patterns of occurrence on bark surface of living trees, leaf litter, and dung. Mycologia, 81, 608-621. https://doi.org/10.2307/3760136 [Google Scholar]
- Takahashi, K., &Hada, Y.(2009). Distribution of Myxomycetes on coarse woody debris of Pinus densiflora at different decay stages in secondary forests of western Japan. Mycoscience, 50, 253-260. https://doi.org/10.1007/S10267-008-0479-4 [Google Scholar]
- Vlasenko, A. V., Novozhilov, Y. K., Schnittler, M., Vlasenko, V. A., &Tomoshevich, M. A.(2018). Pattern of substrate preferences of free-living Protists (Myxomycetes) on decaying wood. Contemporary Problems of Ecology, 11, 494-502. https://doi.org/10.1134/S1995425518050104 [Google Scholar]
- Xavier de Lima, V., &de Holanda Cavalcanti, L.(2015). Ecology of lignicolous myxomycetes in Brazilian Atlantic rain forest. Mycological Progress, 14, 92. http://dx.doi.org/10.1007/s11557-015-1115-2 [Google Scholar]
- Yamamoto, Y.(1998). The myxomycetes biota of Japan [In Japanese]. Toyo Shorin. [Google Scholar]
- Yoshida, Y.(2015). Diversity of wood decay phenomena in nature [In Japanese with English abstract]. Journal of Applied Glycoscience, 5, 200-203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lado, C.(2005-2022). An online nomenclatural information system of Eumycetozoa. Real Jardín Botánico, CSIC. Madrid, Spain. Retrieved Aug 1, 2022, from https://eumycetozoa.com