Abstract
Objectives
Patients undergoing a prior failed attempt of chronic total occlusion‐percutaneous coronary intervention (CTO‐PCI) represent a challenging subgroup across all patients undergoing CTO‐PCI. There are limited data on the effects of a prior failed attempt on the outcomes of subsequent CTO‐PCI. We aimed to compare the procedural results and 24‐month outcomes of prior‐failed‐attempt CTO‐PCI with those of initial‐attempt CTO‐PCI.
Methods
Patients who underwent attempted CTO‐PCI between January 2017 and December 2019 were prospectively enrolled. We analyzed the procedural results and 24‐month major adverse cardiac events (MACE) between patients who underwent prior‐failed‐attempt and initial‐attempt CTO‐PCI. MACE was defined as a composite of cardiac death, target vessel‐related myocardial infarction, and ischemia‐driven target vessel revascularization (TVR) during follow‐up.
Results
In total, 484 patients who underwent CTO‐PCI (prior‐failed‐attempt, n = 49; initial‐attempt, n = 435) were enrolled during the study period. After propensity score matching (1:3), 147 patients were included in the initial‐attempt group. The proportion of the Japanese‐CTO (J‐CTO) score ≥2 was higher in the patients who underwent prior failed attempt than in those who underwent initial attempt (77.5% vs. 38.8%, p < 0.001). The retrograde approach was more often adopted in the prior‐failed‐attempt group than in the initial‐attempt group (32.7% vs. 3.4%, [P< 0.001). Successful CTO revascularization rates were significantly lower in the prior‐failed attempt‐group than in the initial attempt group (53.1% vs. 83.3%, P < 0.001). The multivariate analysis revealed that J‐CTO score ≥2 [odds ratio (OR), 0.359; 95% confidence interval (CI), 0.159–0.812; P = 0.014], intravascular ultrasound procedure (OR, 4.640; 95% CI, 1.380–15.603; P = 0.013), and prior failed attempt (OR, 0.285; 95% CI, 0.125–0.648; P = 0.003) were the independent predictors for successful CTO revascularization. There were no significant differences in major procedural complications (2.0% vs. 0.7%, p = 0.438) and MACE rates (4.1% vs. 8.8%, p = 0.438) between the groups, mainly due to the TVR rate (4.1% vs. 8.2%, P = 0.522).
Conclusions
Compared with initial‐attempt CTO‐PCI, prior‐failed‐attempt CTO‐PCI deserves more attention, since it is associated with a lower successful CTO revascularization rate. Prior failed attempt, J‐CTO score ≥2, and IVUS procedure are the determining factors for predicting successful CTO revascularization. There are no significantly different unfavorable outcomes between patients who undergo prior‐failed‐attempt and initial‐attempt CTO‐PCI.
Keywords: chronic total occlusion, coronary artery disease, percutaneous coronary intervention
This study aimed to compare the procedural results and 24‐month outcomes of prior failed attempt CTO‐PCI with those of initial attempt CTO‐PCI. Compared with initial‐attempt CTO‐PCI, prior‐failed‐attempt CTO‐PCI should be paid more attention, which is associated with a lower successful CTO revascularization rate. Prior‐failed‐attempt is the determining factor for predicting successful CTO revascularization. There are no significantly unfavorable outcomes between patients who undergo prior‐failed‐attempt and initial‐attempt CTO‐PCI.
1. INTRODUCTION
The recanalization of chronic total occlusion (CTO) can achieve improved outcomes with a low 1‐year adverse event rate. 1 , 2 , 3 , 4 Despite the introduction of novel devices and advanced techniques, CTO‐percutaneous coronary intervention (PCI) remains a challenging procedure, with variable success rates ranging from 70% to 85% or more, depending on the operator's experience. 5 , 6 Therefore, the number of patients with failed CTO‐PCI is considerable and should not be neglected.
In addition to the complexity of CTO lesions, multiple factors can lead to CTO‐PCI failure, such as insufficient preparation prior to the procedure, excessive procedure time, and contrast volume use as a result of not switching the alternative CTO crossing strategy in time. A prior failed attempt is a key point of the Japanese‐CTO (J‐CTO) scoring system, which is used to predict the likelihood of successful percutaneous recanalization of CTO lesions. 7 There are limited data on the effects of a prior failed attempt on the outcomes of subsequent CTO‐PCI. The present study aimed to compare the procedural results and 24‐month outcomes between CTO‐PCIs with a prior failed attempt and initial‐attempt CTO‐PCIs.
2. METHODS
2.1. Study population
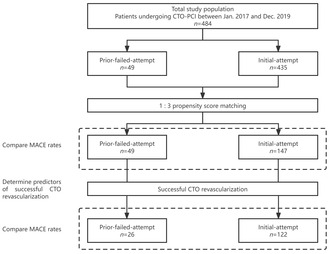
Between January 2017 and December 2019, 484 patients who underwent CTO‐PCI procedures were prospectively enrolled. The patients were divided into two groups based on whether they had a prior failed CTO‐PCI: the initial‐attempt and prior‐failed‐attempt groups (Figure 1). Initial‐attempt CTO‐PCI refers to the first time PCI procedure aimed at the same CTO lesion. Prior‐failed‐attempt CTO‐PCI refers to re‐attempted CTO PCI having previous failure only once, and those with previous failure more than once were excluded. CTO was defined as complete occlusion with an anterograde Thrombolysis in Myocardial Infarction (TIMI) flow grade of 0 in the major epicardial coronary artery for an estimated duration of at least 3 months; this was regardless of the underlying cause (either thrombolysis or stenosis caused by progression of atherosclerosis). The occlusion duration was estimated according to the history of myocardial infarction (MI) in the same target vessel territory, combined with a previous coronary angiogram and changes in electrocardiographic findings. Patients who had received coronary artery bypass grafting or those with side branch occlusion (including the diagonal and marginal branches, as well as post‐descending and post‐lateral arteries) were excluded from this study. CTO‐PCIs were clinically indicated according to the presence of angina or myocardial ischemia. All CTO‐PCIs were electively performed by experienced operators. This study was approved by the Institutional Ethics Committee of the Beijing Hospital. All patients were pretreated with 100–300 mg aspirin, along with a loading dose of 300 mg clopidogrel or 180 mg ticagrelor before CTO‐PCI procedures. Antiplatelet therapy included indefinite aspirin 100 mg/day, clopidogrel 75 mg/day, or ticagrelor 90 mg twice daily for ≥12 months after stent implantation. Finally, clinical baseline characteristics and hospitalization information were recorded.
FIGURE 1.
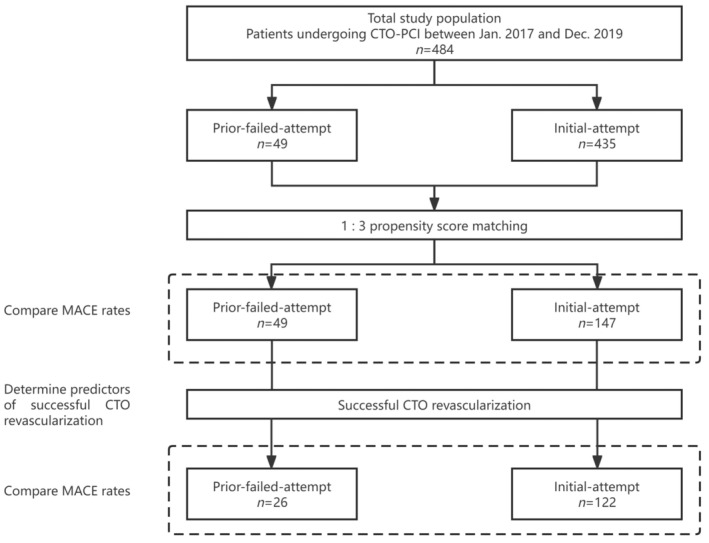
Study flowchart. CTO, chronic total occlusion; PCI, percutaneous coronary intervention.
2.2. Angiographic and interventional procedure variables, and clinical follow‐up
All variables related to angiographic and interventional techniques were analyzed. Multivessel disease (MVD) was defined as ≥75% stenosis in at least two of the major epicardial arteries or their main branch, and left main (LM) disease was defined as ≥50% stenosis of the LM diameter. The syntax score was calculated for each patient to evaluate the severity of coronary artery disease. 8 The J‐CTO score was calculated for each patient undergoing CTO, and a J‐CTO score ≥2 was considered a complicated CTO lesion. 7 An experienced interventionist calculated the syntax and J‐CTO scores. CTO occurring within a previously implanted stent or in occlusive segments within 5 mm proximal or distal to the stent edges was defined as in‐stent restenosis (ISR) CTO. According to the Rentrop classification, the degree of filling of the involved vessel beyond the CTO segment was used to grade the collaterals. 9 The anterograde approach, including antegrade wire escalation (AWE) or antegrade dissection/reentry (ADR), was the first option for crossing CTO lesions. A retrograde approach using retrograde wire escalation (RWE) or retrograde dissection/reentry (RDR) was adopted when the anterograde approach failed. Successful CTO‐PCI was defined as the achievement of <50% residual stenosis in the target CTO lesion with antegrade TIMI flow grade 3 without the occurrence of in‐hospital adverse events, including all‐cause death, tamponade requiring pericardiocentesis, MI, stroke, or repeat target vessel revascularization (TVR) during the index hospitalization. TVR was defined as any repeated percutaneous intervention or surgical bypass of the target vessel. All procedure‐associated adverse events, including procedure‐related death, stroke, periprocedural type 4aMI, major bleeding, coronary perforation with cardiac tamponade requiring intervention, and contrast‐induced nephropathy (increase in serum creatinine level >25% or >0.5 mg/dL at 48 h post‐procedure), were considered major procedural complications. Complete revascularization (CR) was defined as successful treatment of all diseased lesions with ≥75% stenosis in the major epicardial coronary vessels within 30 days of index hospitalization. 10 The primary endpoint was the incidence of major adverse cardiac events (MACE) at the 24‐month follow‐up. MACE was defined as the composite of cardiac death, target vessel‐related MI, and ischemia‐driven TVR.
2.3. Statistical analyses
Continuous variables are presented as mean values ± standard deviations or medians (interquartile ranges), as appropriate. Categorical variables are expressed as numbers (percentages). The chi‐squared or Fisher's exact test was used to compare categorical variables. Student's t‐test or the Mann–Whitney rank‐sum test was used to evaluate the differences among continuous variables according to their distributions.
Propensity scores were calculated using logistic regression, and the clinical baseline and angiographic characteristics were entered into the following model as independent variables: age, hypertension, diabetes mellitus, dyslipidemia, smoking, history of MI, number of diseased vessels, syntax score, target CTO vessel, and Rentrop grade. Propensity scores were used to perform 1:3 nearest‐neighbor matching (one patient with a prior failed attempt CTO‐PCI to three patients with an initial attempt CTO‐PCI). After propensity score matching, the variables between the two groups were compared using Student's t‐test or the Mann–Whitney rank‐sum test. Categorical variables were analyzed using the chi‐squared or Fisher's exact test.
Multivariate analyses were performed using a stepwise multiple logistic regression model to determine the predictors of successful CTO revascularization and are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Variables showing p < 0.10 in univariate analysis or suggested to be related to successful CTO revascularization of interest according to clinical consideration were adopted as candidate predictors for multivariate analysis. The C‐statistic and goodness‐of‐fit with the Hosmer–Lemeshow test were used to determine the model discrimination. Cumulative MACE curves were constructed using the Kaplan–Meier method, and differences between groups were assessed using the log‐rank test. A two‐tailed p < 0.05 was considered statistically significant for all tests. All statistical analyses were performed using the Statistical Package for the Social Sciences version 26.0 (IBM Corp., Armonk, NY, USA).
3. RESULTS
3.1. Baseline clinical characteristics
In total, 484 patients who underwent CTO‐PCI were enrolled during the study period, with 49 (10.1%) having prior‐failed‐attempt CTO‐PCI. The clinical characteristics of the patients are summarized in Table 1. After propensity score matching, the study population included 196 patients whose clinical characteristics matched those of the control participants. There were no significant differences between the prior‐failed‐attempt and initial‐attempt groups in any of the baseline clinical variables, including risk factors for coronary artery disease, left ventricular ejection fraction obtained by echocardiography on admission, and medication.
TABLE 1.
Baseline clinical characteristics for the study patients.
| Total patients | 1:3 Propensity‐matched patients | ||||
|---|---|---|---|---|---|
| Prior‐failed‐attempt | Initial‐attempt | P value | Initial‐attempt | P value | |
| (n = 49) | (n = 435) | (n = 147) | |||
| Age (years) | 60 (52, 66) | 64 (56.5, 71.5) | 0.064 | 62.1 ± 12.11 | 0.964 |
| Male gender | 40 (81.6) | 362 (83.2) | 0.936 | 124 (84.4) | 0.823 |
| Current smoker | 21 (42.9) | 188 (43.3) | 1.000 | 65 (44.2) | 1.000 |
| Hypertension | 33 (67.3) | 309 (71.2) | 0.692 | 100 (68.0) | 1.000 |
| Diabetes mellitus | 19 (38.8) | 211 (48.6) | 0.247 | 65 (44.2) | 0.617 |
| Dyslipidaemia | 35 (71.4) | 275 (63.4) | 0.338 | 101 (68.7) | 0.858 |
| Previous MI | 15 (30.6) | 106 (24.4) | 0.439 | 44 (29.9) | 1.000 |
| Indication of CTO PCI | 0.309 | 0.157 | |||
| Stable angina | 14 (28.6) | 113 (27.9) | 44 (29.9) | ||
| ACS | 15 (48.3) | 235 (54.0) | 88 (59.9) | ||
| Ischemia without symptoms | 0 | 34 (7.8) | 11 (7.5) | ||
| Other | 20 (40.8) | 53 (12.2) | 4 (2.7) | ||
| LDL‐C (mmol/L) | 1.98 (1.45, 2.76) | 2.14 (1.71, 2.69) | 0.102 | 2.07 (1.71, 2.67) | 0.349 |
| eGFR(mL·min−1·1.73−1) | 94.72 (76.38, 107.72) | 93.48 (77.63, 110.33) | 0.922 | 95.39 ± 24.6 | 0.787 |
| LVEF (%) | 57.9 ± 10.5 | 52.2 ± 12.7 | 0.055 | 56.4 ± 12.2 | 0.250 |
| HFrEF | 10 (20.4) | 48 (11.0) | 0.055 | 23 (15.6) | 0.440 |
| Medication | |||||
| Aspirin | 30 (96.8) | 404 (99.3) | 0.255 | 146 (99.3) | 0.438 |
| lopidogrel | 24 (77.4) | 326 (80.3) | 0.878 | 118 (80.3) | 1.000 |
| Ticagrelor | 3 (9.7) | 70 (17.2) | 0.402 | 27 (18.4) | 0.067 |
| Statins | 30 (96.8) | 399 (98.3) | 0.448 | 146 (99.3) | 0.155 |
Note: Values are mean ± SD, n (%), n/N (%), or median (interquartile range).
Abbreviations: ACS, acute coronary syndrome; CTO, chronic total occlusion; eGFR: estimated glomerular filtration rate; HFrEF, heart failure with reduced ejection fraction (EF < 40%); ISR, in‐stent restenosis; LDL‐c, low density lipoprotein cholesterol; LVEF, Left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention.
3.2. Angiographic and procedural characteristics
Detailed angiographic and procedural characteristics of both groups are listed in Table 2. The most common target vessels for CTO were the right coronary (41.3%) and left anterior descending (40.9%) arteries, followed by the circumflex artery (17.8%). The prevalence of MVD and LM disease was similar in both groups and presented a similar distribution in the target CTO vessel. Before propensity score matching, patients who underwent a prior failed attempt had greater syntax scores than those who did not. However, this characteristic did not differ between the two groups after propensity score matching. As expected however, compared with the initial‐attempt group, the prior‐failed‐attempt group had significantly higher proportion of ≥2 J‐CTO score, whether performing propensity score matching or not (77.5% vs. 38.8% and 41.1%, both P < 0.001). The percentages of patients with Rentrop grade 3 and ISR CTO were similar between the two groups.
TABLE 2.
Angiographic, procedural characteristics and 24‐month outcomes for the patients.
| Total population | 1:3 Propensity‐matched patients | ||||
|---|---|---|---|---|---|
| Prior‐failed‐attempt | Initial‐attempt | P value | Initial‐attempt | P value | |
| (n = 49) | (n = 435) | (n = 147) | |||
| Angiographic characteristics | |||||
| Multi‐vessel disease | 43 (87.8) | 388 (89.2) | 0.948 | 126 (85.7) | 0.905 |
| LM disease | 5 (10.2) | 24 (5.5) | 0.199 | 5 (3.4) | 0.125 |
| Target CTO vessel | 0.123 | 0.634 | |||
| LAD | 25 (51) | 173 (39.8) | 75 (51) | ||
| LCX | 4 (8.2) | 82 (18.9) | 7 (4.8) | ||
| RCA | 20 (40.8) | 180 (41.4) | 65 (44.2) | ||
| Syntax score | 19.5 (16.5, 24.5) | 23.25 (17.0, 30.5) | 0.028 | 22.5 (16.0, 28.5) | 0.235 |
| J‐CTO score ≥2 | 31 (77.5) | 175 (41.1) | <0.001 | 57 (38.8) | <0.001 |
| Rentrop grade 3 | 21 (52.5) | 200 (46.9) | 0.612 | 80 (54.4) | 0.62 |
| ISR CTO | 9 (18.4) | 75 (17.2) | 1.000 | 28 (19) | 1.000 |
| Procedural characteristics | |||||
| Femoral artery access | 21 (49.0) | 90 (20.5) | <0.001 | 41 (27.9) | 0.009 |
| Dual injection | 28 (57.1) | 207 (47.6) | 0.204 | 62 (42.2) | 0.069 |
| Retrograde approach | 16 (32.7) | 76 (17.5) | 0.010 | 5 (3.4) | <0.001 |
| IVUS procedure | 11 (22.4) | 64 (14.8) | 0.235 | 28 (19) | 0.757 |
| Contrast volume (mL) | 319.5 ± 121.8 | 244.1 ± 74.0 | 0.192 | 258.6 ± 69.4 | 0.284 |
| Fluoroscopy time (min) | 66.8 ± 33.4 | 41.1 ± 26.4 | 0.067 | 46.7 ± 28.7 | 0.218 |
| Type of intervention | 0.549 | 0.083 | |||
| Stent implantation | 22 (84.6) | 309 (90.4) | 117 (95.9) | ||
| Balloon PTCA | 4 (15.4) | 33 (9.6) | 5 (4.1) | ||
| Successful CTO revascularization | 26 (53.1) | 342 (78.6) | <0.001 | 122 (83.3) | <0.001 |
| Non‐CTO lesion PCI | 9 (18.8) | 167 (38.7) | 0.01 | 55 (37.4) | 0.044 |
| CR | 13 (26.5) | 203 (46.7) | 0.011 | 76 (51.7) | 0.004 |
| Major procedural complications | 1 (2.0) | 10 (2.3) | 1.000 | 1 (0.7) | 0.438 |
| Acute stent thrombosis | 0 (0) | 3 (0.7) | 1.000 | 1 (0.7) | 1.000 |
| Cardiac death | 0 (0) | 4 (0.9) | 1.000 | 0 (0) | — |
| TVR | 2 (4.1) | 48 (11.0) | 0.130 | 12 (8.2) | 0.522 |
| MACE | 2 (4.1) | 56 (12.9) | 0.072 | 13 (8.8) | 0.438 |
| MACE in successful CTO revascularization population | 1/26 (3.8) | 40/342 (11.7) | 0.366 | 10/122 (8.2) | 0.722 |
Note: Values are mean ± SD, n (%), n/N (%), or median (interquartile range).
Abbreviations: CR, complete revascularization; CTO, chronic total occlusion; ISR, in‐stent restenosis; J‐CTO, Japanese‐CTO; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex; LM, left main; MACE, major adverse cardiac event; PCI, percutaneous coronary intervention;RCA, right coronary artery; TVR, target vessel revascularization.
Compared with those who underwent initial attempt, fewer patients who underwent prior failed attempt went through radial artery access, instead of femoral artery access. The retrograde approach was adopted more often in the prior‐failed‐attempt group than in the initial‐attempt group (32.7% vs. 17.5% and 3.4%, P = 0.010 and P < 0.001, respectively). There were no significant differences between the two groups regarding the proportion of cases involving intravascular ultrasound (IVUS) procedures, type of intervention, amount of contrast volume, and fluoroscopy time. Successful CTO revascularization rates were significantly lower in the prior‐failed‐attempt group than in the initial attempt group, even after propensity matching (53.1% vs. 78.6% and 83.3%, respectively; both P < 0.001). More patients in the initial‐attempt group underwent non‐CTO lesion PCI (38.7% and 37.4% vs. 18.8%, P = 0.010 and P = 0.044, respectively) with a higher CR rate (46.7% and 51.7% vs. 26.5%, P = 0.011 and P = 0.004, respectively) than those in the prior‐failed‐attempt group. The incidence of major procedural complications was similar between both groups. No acute stent thrombosis occurred in the prior failed attempt group.
3.3. Predictors of successful chronic total occlusion revascularization in the propensity score matching population by logistic regression analysis
Candidate predictors in the univariate analysis included age, hypertension, diabetes mellitus, dyslipidemia, previous MI, MVD, target CTO vessel, syntax score, J‐CTO score ≥2, Rentrop grade 3, ISR CTO, prior failed attempt, retrograde approach, and IVUS procedure. The final variables entered into the logistic regression model were target CTO vessel, J‐CTO score ≥2, Rentrop grade 3, ISR CTO, retrograde approach, IVUS procedure, and prior failed attempt. Table 3 shows the predictors of successful CTO revascularization in the logistic regression analysis. The multivariate analysis revealed that J‐CTO score ≥2 (OR, 0.359; 95% CI, 0.159–0.812; P = 0.014), IVUS procedure (OR, 4.640; 95% CI, 1.380–15.603; P = 0.013), and prior failed attempt (OR, 0.285; 95% CI, 0.125–0.648; P = 0.003) were independent predictors for successful CTO revascularization.
TABLE 3.
Predictors of successful CTO revascularization in the propensity score‐matching population by logistic regression analysis.
| OR | 95% CI | P value | |
|---|---|---|---|
| Target CTO vessel | 0.749 | 0.504–1.112 | 0.152 |
| J‐CTO score ≥2 | 0.359 | 0.159–0.812 | 0.014 |
| Rentrop grade 3 | 1.571 | 0.731–3.380 | 0.247 |
| ISR CTO | 0.502 | 0.203–1.244 | 0.137 |
| IVUS procedure | 4.640 | 1.380–15.603 | 0.013 |
| Prior‐failed‐attempt | 0.285 | 0.125–0.648 | 0.003 |
| Retrograde approach | 0.450 | 0.150–1.344 | 0.152 |
Abbreviations: CI, confidence interval; CTO, chronic total occlusion; ISR, in‐stent restenosis; IVUS, intravascular ultrasound; J‐CTO, Japan‐CTO; PCI, percutaneous coronary intervention; OR, odds ratio.
3.4. Clinical outcomes
The clinical outcomes at 24‐month follow‐up are shown in Table 2. Cardiac death occurred in only four (0.9%) patients, including two patients with in‐hospital death in the initial‐attempt group. Patients who underwent the initial attempt had an insignificantly higher incidence rate of MACEs than those who underwent prior attempt (12.9% and 8.8% vs. 4.1%, P = 0.072 and P = 0.438, respectively), a finding that was mainly accounted for by TVR rates (11.0% and 8.2% vs. 4.1%, P = 0.130 and P = 0.522, respectively). Similar results could be observed in a population with successful CTO revascularization. Figure 2 shows the curves for the probability of 24‐month MACEs according to whether patients underwent prior‐failed‐attempt CTO‐PCI.
FIGURE 2.
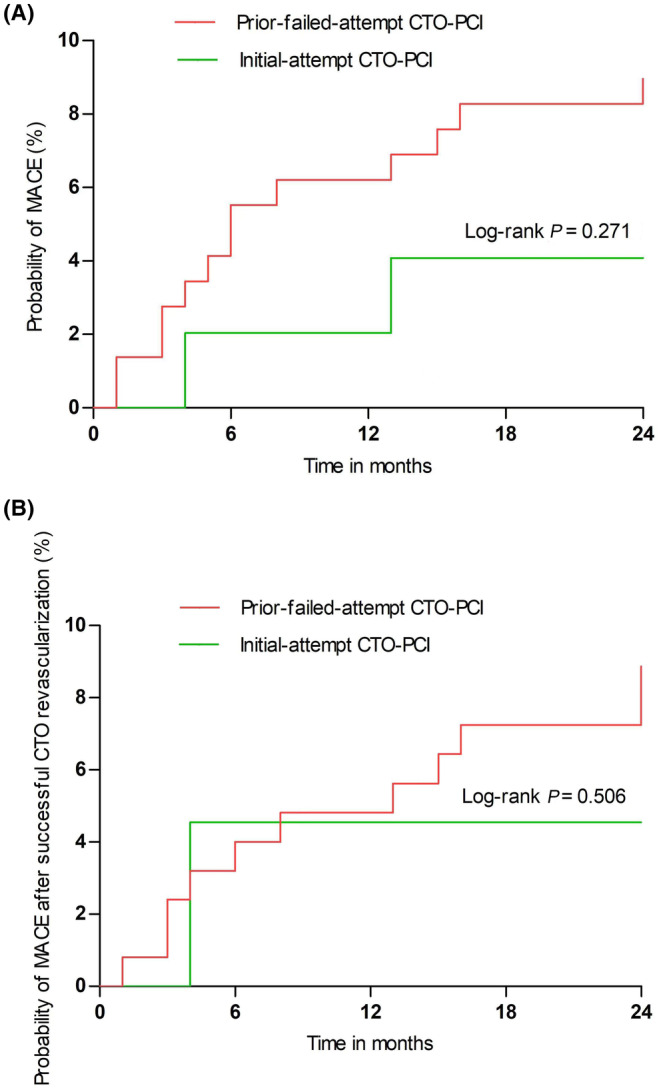
Kaplan–Meier curves for MACEs according to whether prior failed attempt CTO‐PCI was performed. (A) Propensity‐matched population. (B) After successful CTO revascularization in a propensity‐matched population. CTO, chronic total occlusion; MACE, major adverse cardiac events; PCI, percutaneous coronary intervention.
4. DISCUSSION
Considering the aim of the present study, which was to investigate the effects of prior failed attempt on the outcomes of subsequent CTO‐PCI, the main findings of the study were as follows 1 : prior failed attempt accounted for approximately 10% of attempted CTO‐PCI during the study period 2 ; prior failed attempt was associated with a lower successful CTO revascularization rate, J‐CTO score ≥2, IVUS procedure, and prior failed attempt, were independent predictors for successful CTO revascularization; and 3 prior attempt had no significant effects on major procedural complication and MACE rates of subsequent CTO‐PCI, which was mainly accounted for by the TVR rate.
Percutaneous recanalization of CTO lesions remains more challenging despite remarkable progress in dedicated devices and interventionist expertise, with a high success rate of >85%. 11 , 12 Conversely, patients who undergo failed CTO‐PCI constitute a specific subset and should not be neglected. In our study, approximately 10% of the patients underwent prior failed attempt CTO‐PCI, mainly indicated by symptoms of angina or acute coronary syndrome, as described in a previous study. 13 Similar to all patients undergoing CTO‐PCI, for patients with previous CTO‐PCI failure, successful reattempt PCI of CTO is associated with improved clinical outcomes. 14 Therefore, percutaneous recanalization of CTOs is considered reasonable in patients with a prior failed CTO‐PCI.
In our study, prior‐failed‐attempt CTO‐PCI had a lower successful CTO revascularization rate than initial‐attempt CTO‐PCI. Compared with initial‐attempt CTO‐PCI, prior‐failed‐attempt CTO‐PCI appeared even harder, and more complex procedures had to be performed. Previous failure indicated a challenging reattempt CTO recanalization, manifested by a higher J‐CTO score in this subpopulation. In our study, patients who underwent prior‐failed‐attempt CTO‐PCI had a significantly higher proportion of ≥2 J‐CTO score than those who underwent initial‐attempt CTO‐PCI. As a widely used scoring system, the J‐CTO score is frequently used to predict the probability of successful guidewire crossing within 30 min, which reflects the difficulty of percutaneous recanalization of CTO lesions. 7 As one of the key parameters in the scoring system, prior failed attempt was given one point, and a J‐CTO score ≥2 was considered a more difficult lesion. Thus, the re‐attempted CTO lesions are more complex, and the prior‐failed‐attempt CTO‐PCI appears more challenging than the initial‐attempt CTO‐PCI. 15 Our findings validated the effectiveness of the J‐CTO score system and also demonstrated that J‐CTO score ≥2 and prior failed attempt were independent predictors for successful CTO revascularization, consistent with the results reported in previous studies. 7 , 16 Additionally, our study identified the determinative role of IVUS in predicting subsequent CTO‐PCI success. IVUS can be applied to determine the true lumen, help guide wire crossing, and optimize the PCI procedure, especially in heavily calcified CTO segments. 17
The accumulation of operating techniques and expertise in combination with the implementation of the hybrid algorithm in CTO‐PCI has resulted in a sustained improvement in the percutaneous recanalization of CTO lesions in recent years. 2 Accordingly, prior‐failed‐attempt CTO‐PCI inevitably requires complex procedures, including the retrograde approach, longer fluoroscopic time, and longer procedure duration. 15 , 18 In this study, more patients who underwent prior‐failed‐attempt CTO‐PCI underwent femoral artery access, and the retrograde approach was more frequently used in this group, with a trend toward higher contrast volume and longer fluoroscopy time. For most CTO lesions, the antegrade approach, including AWE and ADR, is often adopted as the first‐choice crossing strategy, and successful recanalization can be achieved in most situations. In some circumstances however, especially in prior‐failed‐attempt CTO‐PCI, a retrograde approach may be considered (including RWE and RDR); this approach can improve the procedure's success rate. 6 , 18 , 19 , 20 In addition, it has been reported that the use of dedicated CTO devices, such as the CrossBoss, or the Stingray catheter is associated with an increased successful CTO‐PCI rates. 11 , 21 However, during the study period, CrossBoss and the Stingray catheter were unavailable in our center.
The incidence of major procedural complications was comparable between patients who underwent prior‐failed‐attempt CTO‐PCI and initial‐attempt CTO‐PCI in our study, which is consistent with the latest report. 18 This implies that the use of more complex techniques and devices by experienced operators in re‐attempting CTO‐PCI does not necessarily translate to an increased incidence of adverse events. There was no significant effect of a prior attempt on the MACE rate of the subsequent CTO‐PCI in our study. There are several potential explanations for this lack of effect. First, despite the fact that aggressive techniques and dedicated dissection and reentry devices, such as the retrograde approach, the CrossBoss, or the Stingray catheter, were more frequently used in the reattempt CTO‐PCI; the procedures for the patients with prior‐failed‐attempt CTO‐PCI were often well prepared and performed by experienced operators. Timely termination of the procedure is important in cases of major procedural complications. Second, although lower optimal success rates were observed in the prior‐failed‐attempt group than in the initial‐attempt group, during the reattempt procedure, the so‐called subintimal plaque modification or “investment” procedures might predispose to subsequent success with a favorable outcome. 14 , 22 Third, even if successful CTO revascularization in the initial attempt could be achieved more often, a trend of a higher TVR rate, mainly resulting from stent restenosis, might contribute to an unsatisfactory outcome.
4.1. Study limitations
Our study has some limitations, owing to a few reasons. This was a small observational study conducted at a single center, and the decision to perform CTO‐PCI was made at the discretion of the interventionist, which would result in selection bias. Furthermore, the number of patients in the prior‐failed‐attempt group was relatively small, and the two groups were not balanced in size. Although we performed a propensity score‐matched analysis to correct for these potential confounding factors, it was difficult to adjust for all unmeasured variables. Due to the gradual accumulation of expertise in CTO‐PCI across the study period, CTO intervention techniques have undergone a sustained improvement. Thus, the results of our study should be interpreted with caution. In addition to these, the success rate of CTO‐PCI in our study was lower than those reported in the literature (76% vs. 83%). 13 , 23 Experience with percutaneous treatment of CTO lesions is more variable in diverse centers; therefore, the results of this study should only be interpreted in certain settings. Nonetheless, our results are representative of real‐world practice. Third, angiographic evaluation during follow‐up was not mandatory; only patients presenting with recurrence of angina and/or new emerging ischemic evidence underwent repeat angiography, followed by subsequent TVR when indicated. This might have resulted in an underestimation of the TVR rate driven by target vessel failure.
5. CONCLUSIONS
Patients who undergo prior‐failed‐attempt CTO‐PCI constitute a noticeable subset of all patients undergoing CTO‐PCI. Prior‐failed‐attempt CTO‐PCI appears even harder, which is associated with a lower successful CTO revascularization rate. Prior failed attempt, J‐CTO score ≥2, and IVUS procedure are the determining factors for predicting successful CTO revascularization. Despite the lower optimal success rate in the patients with a prior failed attempt, no significantly unfavorable outcomes in terms of major procedural complications and MACE are observed. Special considerations should be taken regarding the strategy and algorithm of recanalization of CTO in future practice; these include the utilization of modern imaging techniques, such as coronary computed tomography angiogram before re‐attempt procedures and IVUS use during the procedure.
AUTHOR CONTRIBUTIONS
H.P.Z. and N.X.Z.: Study conception and design. H.P.Z., X.P., L.L., and H.A.: Acquisition of data. N.X.Z., X.P., H.P.Z., and H.A.: Analysis and interpretation of data (e.g., statistical and computational analyses). N.X.Z. and H.P.Z.: Writing, review, and/or revision of the manuscript. H.P.Z.: Study supervision.
FUNDING INFORMATION
This study was supported by the National High Level Hospital Clinical Research Funding (BJ‐2018‐201).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
This study was approved by the institutional Ethics Committee (approval no. 2019BJYYEC‐021‐02).
CONSENT
All patients provided informed consent to participate in this study and underwent the intervention procedure.
Zheng N‐X, Ai H, Zhao Y, et al. Effects of a prior failed attempt on the outcomes of subsequent chronic total occlusion‐percutaneous coronary intervention. Aging Med. 2024;7:463‐471. doi: 10.1002/agm2.12350
DATA AVAILABILITY STATEMENT
The datasets analyzed in the current study are available from the corresponding author upon request.
REFERENCES
- 1. George S, Cockburn J, Clayton TC, et al. Long‐term follow‐up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. central cardiac audit database. J Am Coll Cardiol. 2014;64(24):235‐243. doi: 10.1016/j.jacc.2014.04.040 [DOI] [PubMed] [Google Scholar]
- 2. Wilson WM, Walsh SJ, Bagnall A, et al. One‐year outcomes after successful chronic total occlusion percutaneous coronary intervention: the impact of dissection re‐entry techniques. Catheter Cardiovasc Interv. 2017;90(5):703‐712. doi: 10.1002/ccd.26980 [DOI] [PubMed] [Google Scholar]
- 3. Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta‐analysis. Am Heart J. 2010;160(1):179‐187. doi: 10.1016/j.ahj.2010.04.015 [DOI] [PubMed] [Google Scholar]
- 4. Christakopoulos GE, Christopoulos G, Carlino M, et al. Meta‐analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. 2015;115(10):1367‐1375. doi: 10.1016/j.amjcard.2015.02.038 [DOI] [PubMed] [Google Scholar]
- 5. Maeremans J, Walsh S, Knaapen P, et al. The hybrid algorithm for treating chronic total occlusions in Europe: the RECHARGE registry. J Am Coll Cardiol. 2016;68(18):1958‐1970. doi: 10.1016/j.jacc.2016.08.034 [DOI] [PubMed] [Google Scholar]
- 6. Megaly M, Ali A, Saad M, et al. Outcomes with retrograde versus antegrade chronic total occlusion revascularization. Catheter Cardiovasc Interv. 2020;96(5):1037‐1043. doi: 10.1002/ccd.28616 [DOI] [PubMed] [Google Scholar]
- 7. Morino Y, Abe M, Morimoto T, et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J‐CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4(2):213‐221. doi: 10.1016/j.jcin.2010.09.024 [DOI] [PubMed] [Google Scholar]
- 8. Serruys PW, Morice M‐C, Kappetein P, et al. Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease (the SYNTAX trial). N Engl J Med. 2009;360(10):961‐972. doi: 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 9. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5(3):587‐592. doi: 10.1016/s0735-1097(85)80380-6 [DOI] [PubMed] [Google Scholar]
- 10. Hannan EL, Racz M, Holmes DR, et al. Impact of completeness of percutaneous coronary intervention revascularization on long‐term outcomes in the stent era. Circulation. 2006;113(20):2406‐2412. doi: 10.1161/CIRCULATIONAHA.106.612267 [DOI] [PubMed] [Google Scholar]
- 11. Karacsonyi J, Tajti P, Rangan BV, et al. Randomized comparison of a CrossBoss first versus standard wire escalation strategy for crossing coronary chronic total occlusions. JACC Cardiovasc Interv. 2018;11(3):225‐233. doi: 10.1016/j.jcin.2017.10.023 [DOI] [PubMed] [Google Scholar]
- 12. Karacsonyi J, Karatasakis A, Karmpaliotis D, et al. Effect of previous failure on subsequent procedural outcomes of chronic total occlusion percutaneous coronary intervention (from a contemporary multicenter registry). Am J Cardiol. 2016;117(8):1267‐1271. doi: 10.1016/j.amjcard.2016.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee SH, Cho JY, Kim JS, et al. A comparison of procedural success rate and long‐term clinical outcomes between in‐stent restenosis chronic total occlusion and de novo chronic total occlusion using multicenter registry data. Clin Res Cardiol. 2020;109(5):628‐637. doi: 10.1007/s00392-019-01550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li W, Wu Z, Liu T, Wu X, Liu J. Long term clinical outcome after success re‐attempt percutaneous coronary intervention of chronic total occlusion. BMC Cardiovasc Disord. 2023;23(1):23. doi: 10.1186/s12872-023-03045-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanabe M, Kodama K, Asada K, Kunitomo T. Lesion characteristics and procedural outcomes of re‐attempted percutaneous coronary interventions for chronic total occlusion. Heart Vessel. 2018;33(6):573‐582. doi: 10.1007/s00380-017-1091-3 [DOI] [PubMed] [Google Scholar]
- 16. Christopoulos G, Wyman RM, Alaswad K, et al. Clinical utility of the Japan‐chronic total occlusion score in coronary chronic total occlusion interventions: results from a multicenter registry. Circ Cardiovasc Interv. 2015;8(7):e002171. doi: 10.1161/CIRCINTERVENTIONS.114.002171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong SJ, Kim BK, Shin DH, et al. Usefulness of intravascular ultrasound guidance in percutaneous coronary intervention with second‐generation drug‐eluting stents for chronic total occlusions (from the Multicenter Korean‐Chronic total Occlusion Registry). Am J Cardiol. 2014;114(4):534‐540. doi: 10.1016/j.amjcard.2014.05.027 [DOI] [PubMed] [Google Scholar]
- 18. Guelker JE, Blockhaus C, Bufe A, et al. In‐hospital outcome of re‐attempted percutaneous coronary interventions for chronic total occlusion. Cardiol J. 2023;30(1):44‐50. doi: 10.5603/CJ.a2021.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohandes M, Moreno C, Fuertes M, et al. Angiographic characteristics and outcomes of percutaneous coronary intervention of reattempted chronic total occlusion: potential contributing factors to procedural success. J Clin Med. 2021;10(23):5661. doi: 10.3390/jcm10235661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karmpaliotis D, Karatasakis A, Alaswad K, et al. Outcomes with the use of the retrograde approach for coronary chronic total occlusion interventions in a contemporary multicenter US registry. Circ Cardiovasc Interv. 2016;9(6):e003434. doi: 10.1161/CIRCINTERVENTIONS.115.003434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson WM, Walsh S, Hanratty C, et al. A novel approach to the management of occlusive in‐stent restenosis (ISR). EuroIntervention. 2014;9:1285‐1293. doi: 10.4244/EIJV9I11A218 [DOI] [PubMed] [Google Scholar]
- 22. Wilson WM, Walsh SJ, Yan AT, et al. Hybrid approach improves success of chronic total occlusion angioplasty. Heart. 2016;102(18):1486‐1493. doi: 10.1136/heartjnl-2015-308,891 [DOI] [PubMed] [Google Scholar]
- 23. Xenogiannis I, Choi JW, Alaswad K, et al. Outcomes of subintimal plaque modification in chronic total occlusion percutaneous coronary intervention. Catheter Cardiovasc Interv. 2020;96(5):1029‐1035. doi: 10.1002/ccd.28614 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the current study are available from the corresponding author upon request.


