ABSTRACT
Lignocellulosic biomass, the most abundant natural resource on earth, can be used for cellulosic ethanol production but requires a pretreatment to improve enzyme access to the polymeric sugars while obtaining value from the other components. γ‐Valerolactone (GVL) is a promising candidate for biomass pretreatment since it is renewable and bio‐based. In the present work, the effect of a pretreatment based on GVL on the enzymatic saccharification of white birch was evaluated at a laboratory scale and the importance of the washing procedure for the subsequent saccharification was demonstrated. Both the saccharification yield and the production of cellulosic ethanol were higher using a noncommercial enzyme crude from Talaromyces amestolkiae than with the commercial cocktail Cellic CTec2 from Novozymes. Furthermore, the production of extracellular cellulases by T. amestolkiae has been optimized in 2 L bioreactors, with improvements ranging from 40% to 75%. Finally, it was corroborated by isoelectric focus that optimization of cellulase secretion by T. amestolkiae did not affect the pattern production of the main β‐glucosidases and endoglucanases secreted by this fungus.
Keywords: bioethanol, cellulases, fungi, green solvent, lignocellulosic biomass
1. Introduction
The reliance on fossil fuels presents a significant challenge due to their detrimental impact on the environment, contributing to climate change and pollution [1]. As potential solutions, biomass derived from organic matter such as agricultural waste, forest residues, or dedicated energy crops offers promising alternatives, since they can be converted into biofuels and other renewable forms of energy [2]. In this context, a biorefinery is a holistic and integrated facility encompassing a series of interconnected processes aimed at efficiently converting biomass into a diverse range of valuable products, including materials, chemicals, and energy. The biorefinery paradigm operates on the principle of “catch and release,” wherein carbon from biomass is captured, transformed, and subsequently released in a manner that is environmentally advantageous while also fostering economic viability [3]. Saccharification is a crucial step in the biorefinery process that involves breaking down complex carbohydrates (polysaccharides) into simple sugars (monosaccharides), such as glucose and xylose. These simple sugars serve as the building blocks to produce various biofuels and biochemicals. Due to the complex nature of plant biomass, a pretreatment is needed to increase saccharification efficiency. Pretreatments can be physical, chemical, or biological processes that break down the rigid cell wall structure and play a crucial role by easing the efficient enzymatic or chemical hydrolysis of polysaccharides. Common pretreatment techniques include steam explosion, acid hydrolysis, and enzymatic methods [4, 5, 6]. γ‐Valerolactone (GVL) is a renewable and bio‐based solvent that can be used for biomass pretreatment, with numerous benefits. GVL solubilizes hemicellulose and lignin, allowing their valorization, and yields a virtually pure cellulosic fraction that can be used as feedstock to produce sugars [7, 8, 9]. Notably, GVL exhibits excellent thermal stability, effectively minimizing the generation of degradation products from monosaccharides [10, 11, 12]. Additionally, it can be produced from lignocellulosic materials, further enhancing its interest as a valuable and green option for various applications [10, 13]. On the other side, its presence can lead to enzyme inactivation, although GVL recycling strategies are usually designed to reduce costs and increase process sustainability since it is a relatively expensive chemical.
Summary
Lignocellulosic biomass is the most abundant renewable resource on earth.
The exploitation of cellulosic sugars necessarily goes through a saccharification stage, releasing glucose that can be metabolized by microorganisms or transformed by enzymatic or chemical means into high–value‐added compounds.
However, this material is highly recalcitrant and resistant to hydrolysis, and different pretreatment strategies are available to increase the efficiency of the whole process.
The practical application of the research developed in this article focused on the optimization of both, pretreatment and saccharification.
The biomass was pretreated by using an environmentally friendly solvent, which is recycled, and a successful saccharification process has been established using either commercial or in‐house produced enzymatic cocktails.
Regarding enzymatic saccharification, the strategy of mining for novel biocatalysts has been described as an effective approach for obtaining lignocellulolytic enzymes with exceptional properties. In this context, filamentous fungi are the main organisms targeted by these studies. Cellulose degradation requires the synergistic action of three types of glycosyl hydrolases: endoglucanases, cellobiohydrolases, and β‐glucosidases [14]. Endoglucanases randomly cleave internal β‐1,4‐O‐glycosidic bonds in amorphous regions of the cellulose, whereas cellobiohydrolases act on the free ends of the cellulose chains releasing soluble oligosaccharides that are converted into glucose by β‐glucosidases. Talaromyces amestolkiae has been described as a relevant source of cellulases (as well as of hemicellulases), some of which have been successfully applied to the saccharification of different substrates [15, 16, 17]. In this article, the influence of different GVL pretreatment stages over the saccharification efficiency of white birch (WB) chips was evaluated. This birch that grows naturally throughout extended areas in the northern hemisphere, with a preference for cool regions, is commonly used for pulpwood, paper manufacture, or fuel, among others [18]. The saccharification process was carried out with two enzyme cocktails, the commercial Cellic CTec2 from Novozymes and an enzyme crude produced by T. amestolkiae in our laboratory, and the hydrolysates were fermented to ethanol by Saccharomyces cerevisiae. In addition, the optimization of the production of extracellular cellulases by this fungus has been assayed in a 2 L bioreactor.
2. Materials and Methods
2.1. Birch Wood Pretreatment With GVL to Obtain Cellulose
White birch chips measuring approximately 4 × 2 × 0.6 cm were used as feedstock. The samples taken from different pretreatment steps are shown in Table 1. Initially, 200 g of chips and 800 g of a solution of 0.1 M sulfuric acid in GVL/water (70/30 by weight) were added to a 1‐L reactor (20% wet biomass loading). The pretreatment reactor was externally heated by autoclaving for 1 h at 130°C. At the end of the reaction, the pretreatment reactor was cooled down to approximately 80°C and the cellulose was separated from the liquid by vacuum filtration using a Grade 1 paper filter, pore size 11 µm, and washed with 1200 g of fresh hot GVL/water 70/30 (Sample A). After pretreatment, the sample was washed with 1 volume of water (Sample B), 15 volumes of water (Sample C), or 15 volumes of water including physical screening of cellulose fibers using an 800‐mesh sieve and collecting the fibers with >150 mesh (Sample D).
TABLE 1.
Composition (%) of white birch wood samples taken at different stages of GVL pretreatment.
| Arabinose | Xylose | Mannose | Glucose | Galactose | Lignin | |
|---|---|---|---|---|---|---|
| Untreated WB | 1.1 | 9.8 | 1.8 | 42.7 | 0.6 | 26.1 |
| (A) GVL Fractionation + GVL washing | 0.5 | 1.1 | 8.2 | 73.7 | 0.3 | 6.8 |
| Steps after GVL pretreatment | ||||||
| (B) 1 × H2O washing | 0.3 | 0.6 | 10.3 | 81.0 | 0.3 | 6.7 |
| (C) 15 × H2O washing | 0.3 | 1.1 | 5.6 | 85.3 | 0.0 | 4.6 |
| (D) 15 × H2O washing + screening | 0.3 | 1.1 | 5.6 | 88.4 | 0.0 | 4.6 |
2.2. Characterization of Biomass
Sugar composition and lignin content of pretreated and raw material were determined according to that described in Tappi 222 om‐02. Briefly, the material was dried to constant weight, milled, and screened through a 0.42 mm sieve, and 300 mg were treated with 3 mL 72% sulfuric acid for 1 h at 30°C. Then, 84 mL of distilled water was added, and the sample was heated for 1 h in an autoclave (110°C). The resulting material was filtered through a preweighted funnel with a sintered glass disc of medium porosity (Vidra FOC 663/3) and 1 mL of the hydrolysate was used to determine cellulose and hemicellulose content by GC (see below). The retained slurry was washed until neutral and dried to quantify Klason lignin by gravimetry.
For cellulose and hemicellulose content determination by GC in the hydrolysate, 100 µg myo‐inositol was added to 1 mL samples as internal standard. Samples were neutralized with barium carbonate and sugars were converted into alditol acetates as described in Méndez–Líter et al. [17]. The samples were injected in a 7890A‐5975C GC/MS system (Agilent, Palo Alto, CA), fitted with an HP5‐MS column (30 m × 0.25 µm × 0.25 mm, Agilent), and analyzed at 160°C (1 min) and then rising 2°C min− 1 up to 200°C.
2.3. Production of Cellulolytic Enzymes From T. Amestolkiae
T. amestolkiae is an ascomycetous fungus isolated from wheat straw residues and deposited in the IJFM culture collection at the “Centro de Investigaciones Biológicas Margarita Salas” (Madrid, Spain), under the reference A795. Enzyme production was performed as described in de Eugenio et al. [15], using Avicel as the carbon source. Briefly, a spore solution (1% NaCl with 0.1% Tween 80) was used to preinoculate 250 mL flasks containing 50 mL of CSS medium (per liter: 40 g glucose, 0.4 g FeSO4 × 7H2O, 9 g (NH4)2SO4, 4 g K2HPO4, 26.3 g corn steep solids, 7 g CaCO3, and 2.8 mL soybean oil, pH 5.6). After 5 days, 8 mL were inoculated in 1 L flasks containing 200 mL Mandels medium (per liter: 2.0 g KH2PO4, 1.3 g (NH4)2SO4, 0.3 g urea, 0.3 g MgSO4·7H2O, 0.3 g CaCl2, 5 mg FeSO4·7H2O, 1.6 mg MnSO4·H2O, 1.4 mg ZnSO4·7H2O, and 1 g Bacto Peptone) supplemented with 1% Avicel. The initial pH was adjusted at 4.5 with HCl. The cultures were incubated at 28°C and 200 rpm for 7 days, and then the supernatant was separated by centrifugation, filtered, and concentrated using a 3 kDa cutoff polysulfone membrane and an ultrafiltration cell (Amicon, Merck–Millipore, Kenilworth, NJ, USA).
2.4. Enzymatic Activity Assays
Total protein was estimated using the Bicinchoninic acid (BCA) Protein Assay (Thermo Scientific) and bovine serum albumin as standard. Endoglucanase (EG) activity was measured by determining the release of reducing sugars [19] and the standard enzymatic assay was performed in 50 mM sodium acetate buffer, pH 5.0, containing appropriately diluted crudes and 3% carboxymethyl cellulose (w/v). β‐Glucosidase activity was measured following p‐nitrophenol (pNP) release (ε410 = 15,200 M cm−1) from 0.2% p‐nitrophenyl glucoside (pNPG) in 100 mM sodium acetate buffer pH 5.0. One unit of β‐glucosidase or endoglucanase activities accounts for the amount of enzyme needed to release 1 µmol of product in 1 min. Filter paper units (FPU), for determining total cellulase activity, were calculated using Whatman No. 1. Filter paper as the substrate, following the instructions established and published by the International Union of Pure and Applied Chemistry (IUPAC) [20]. In this case, 0.1875 FPU is the quantity of enzyme activity that, when assayed according to the instructions, will produce reducing sugars equivalent to 2.0 mg of glucose.
2.5. Saccharification and Fermentation of Raw and Pretreated White Birch Wood
For saccharification assays, 0.4 g (dry weight) white birch biomass was suspended into 2.5 mL of 100 mM sodium acetate pH 5 in 50 mL polypropylene tubes. Fifteen FPU per gram biomass of each enzymatic crude was included in the reaction (detailed cellulase activities can be found in Table 2). For better homogenization, twenty 3‐mm glass beads were added to each tube and reactions were incubated at 50°C and 200 rpm. Samples were taken every 24 h for 72 h, and sugars were separated by HPLC in an Aminex HPX‐87H column and detected by RID in an Agilent 1260 series using a standard curve prepared with glucose dilutions (0.05–200 g L–1).
TABLE 2.
Activity units added for saccharification of 1 g of pretreated white birch wood. Reactions were carried out with 0.4 g in 2.5 mL final volume.
| β‐Glucosidase (U) | Endoglucanase (U) | FPU (U) | |
|---|---|---|---|
| Cellic CTec2 | 255 | 305 | 15 |
| T. amestolkiae crude | 230 | 677 | 15 |
The production of cellulosic ethanol from pretreated lignocellulose was assayed through a two‐step process. In brief, GVL‐pretreated lignocellulose was weighted and put in 100 mM sodium acetate pH 5 buffer with 15 FPU of the enzymatic cocktail per gram of biomass. The enzyme blend was maintained at 50°C and 200 rpm for 3 days. After this time, the reaction temperature was adjusted to 30°C. Before fermentation, S. cerevisiae was rehydrated in double distilled sterile water 5:1 yeast and incubated for 15 min at 35°C, and then 2.0 g L–1 of yeast were inoculated in 2 mL tubes containing 2 mL of the saccharification mixture. Samples were taken after 3 days of incubation to measure the concentration of glucose and ethanol in the supernatants by HPLC, as seen above, using glucose and ethanol dilutions (0.05–200 g L–1 and 0.125–4 M, respectively).
2.6. Improvement of Cellulase Production by T. Amestolkiae
In order to optimize standards T. amestolkiae enzyme production in Mandels medium (described in Section 2.3), different experiments were carried out to check the influence of carbon and nitrogen sources (using shaking flasks), as well as the influence of pH and agitation rate (in a 2 L stirred tank bioreactor). Table S1 summarizes the operation conditions of flasks and bioreactor assays.
For flask experiments, we used the same culture conditions reported in Section 2.3 but adding different Avicel concentrations (0.5%, 1%, 2%, and 4%). In addition, the influence of the nitrogen source was analyzed in Mandels medium with 2% Avicel (selected as the best in the first screening assays), by using 0.1% or 0.2% Bacto peptone or replacing it with 0.2% or 0.4% corn step solids (CSS), maintaining N content around 0.1% and 0.2%, respectively.
Mandels basal broth (1% Avicel and 0.1% bacto peptone) was initially used in the 2 L tank bioreactor (Biostat B Plus, Sartorius) experiments, working with 1.5 L of medium. The bioreactor was maintained at 28°C and 200 rpm and the pH was controlled at different values (4, 4.5, 5, and 5.5) by adding 2 M NaOH or 2 M HCl. In addition, the influence of the agitation rate (100, 200, 300, 400, and 500 rpm) was studied in the basal medium. Aerobic conditions were maintained by air bubbles at 0.5 vvm in all cases. Samples were withdrawn at different times to measure enzymatic activity. Preinocula were prepared as indicated in Section 2.3, using CSS medium in 75 mL (5% of bioreactor working volume).
Finally, all conditions optimized separately (controlled pH at 4.5, 400 rpm, 2% Avicel, and 0.4% CSS) were put together using a 2 L bioreactor, and cellulase secretion was compared with that of standard initial conditions (pH initially adjusted at 4.5, 200 rpm, 1% Avicel, and 0.1% peptone).
2.7. Characterization of the Enzyme Cocktails by Isoelectric Focusing and Zymograms
The composition of β‐glucosidases and endoglucanases secreted by T. amestolkiae in standard and optimized conditions was analyzed by isoelectric focusing (IEF). The isoelectric point (pI) of the desalted protein was determined in gels with 5% polyacrylamide and ampholytes of 3–10 (GE healthcare), with 1 M H3PO4, and 1 M NaOH in anode and cathode, respectively. After IEF, the pH values were determined from the gel with a contact electrode in a pH meter (Crison), to create a pI calibration curve. Proteins were stained with colloidal blue staining (Invitrogene). For β‐glucosidase activity detection, after washing the gels for 10 min with 50 mM sodium acetate at pH 5, they were incubated with 2 mM p‐methylumbelliferyl‐β‐D‐glucopyranoside (Sigma–Aldrich) prepared in the same buffer. Then, the gel was washed 5 min with the buffer and the released fluorescence was visualized under UV light by use of the Gel Doc XR + system (Bio‐Rad). Endoglucanase activity was revealed after incubation of the isoelectric focusing gels onto 0.2% CMC agarose plates, prepared in the same buffer. Clear halos of degradation of CMC were revealed by incubation with 0.1% Congo Red (15 min), washed with 1 M NaCl until bands turned visible, and washed with 1% acetic acid.
3. Results and Discussion
3.1. Composition of Natural and Pretreated White Birch Wood
The composition of untreated and GVL‐pretreated samples of WB wood analyzed in this study is gathered in Table 1. The main components of untreated WB biomass are cellulose (42.7%), lignin (26.1%), and xylan (9.8% xylose and 1.1% arabinose). Similar cellulose and lignin values have been reported in the literature, although the hemicellulose content was lower than previously described [21, 22]. These differences can be attributed to the fact that not all studies use the same variety of birch (silver vs. white birch). The GVL pretreatment comprised biomass fractionation with 70% GVL in water (v/v) followed by a washing step to recover and recycle GVL, which is a critical step for the success of the process. Additional washing and pulp screening, which consists of passing the pulp slurry through a sieve, can be used to improve cellulose quality and remove underprocessed biomass. Biomass fractionation and washing with 70% GVL (Sample A in Table 1) decreased lignin and xylan percentages to 6.8% and 1.1%, respectively, and increased glucose content to 73.7% on a dry basis. This result was expectable since GVL has been shown to solubilize lignin and pentoses [7]. The GVL final concentration in the fractionation mixture was 47% on a wet basis. In other samples, additional washing and/or screening stages were done. Sample B (Table 1) underwent the same steps described for Sample A, followed by an additional wash with 1 volume of water. This reduced GVL concentration to 3.5%, and increased cellulose content to 81.0%, while lignin quantity remained unchanged. The third sequence (Sample C, in Table 1) involved washing with 15 volumes of hot water. In this case, the pretreated biomass was slightly enriched in glucose and had less lignin. Finally, an additional step of pulp screening helped by water (Sample D in Table 1) was evaluated, resulting in a final glucose content of 88.4%. The composition of the biomass recovered from pretreatments C and D showed to be very similar, and both allowed simple fractionation of biomass components. Solids recovery after the pretreatment ranged from 41 to 43 wt% of original dry wood. Lignin and C5 sugars solubilized in GVL can be further separated since lignin can be precipitated upon water addition.
3.2. Saccharification and Fermentation of the Cellulosic Fraction
To analyze the impact of each pretreatment sequence on the release of fermentable sugars, a saccharification test was carried out. Untreated white birch and each of the pretreated samples listed in Table 1 were subjected to enzymatic hydrolysis with Cellic CTec2 (kindly supplied by Novozymes), as described in the Section 2. Total β−glucosidase, endoglucanase, and FPU added to the reaction are shown in Table 2.
The results of these experiments are presented in Figure 1. The saccharification yield of the untreated wood amounted to 25%. However, the enzymatic hydrolysis was not successfully in wood samples pretreated with GVL (A, in Figure 1), probably due to enzymes’ inhibition by the solvent. This is supported by the fact that 48% saccharification yield was determined for the biomass subjected to the first washing step (B, in Figure 1), that removed more than 90% of the GVL. The solvent was recovered by distillation to be reused, and the commercial enzyme cocktail was able of depolymerizing cellulose considerably better than in untreated samples. Further washing with hot water, followed or not by fiber screening, produced slight improvements of the saccharification yields, that reached up to 55%.
FIGURE 1.
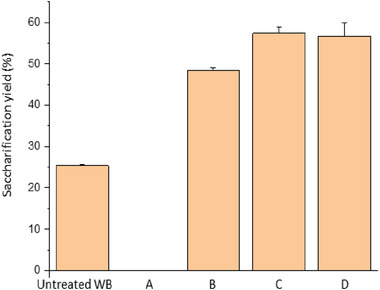
Saccharification (72 h) of untreated and GVL‐pretreated white birch wood with the commercial cocktail Cellic CTec2. The pretreatments applied to the samples were (A) GVL fractionation (there is no bar because the saccharification of this material was not successfully); (B)–(D) as treated in (A), followed by different washing steps: (B) 1 × H2O; (C) 15 × H2O; (D) 15 × H2O + screening (800 mesh).
In view of these results, different enzymatic alternatives were tested to increase the release of sugars from pretreated wood. In previous works, we described T. amestolkiae as an excellent producer of cellulases and hemicellulases when grown in the presence of cellulosic inductors [15]. Therefore, we compared the time course of saccharification catalyzed by Cellic CTec2 or by an enzyme crude from T. amestolkiae (Figure 2). Crude enzymes from T. amestolkiae were obtained using microcrystalline cellulose as the main carbon source, where cellulolytic enzyme secretion is improved [15], since the main component of the GVL‐pretreated biomass was cellulose. The doses of the cellulolytic enzymes added to the reaction are shown in Table 2. Among the wood samples listed in Table 1, we selected the one recovered after pretreatment C for giving similar saccharification yields to those determined after treatment D without the need for pulp screening.
FIGURE 2.
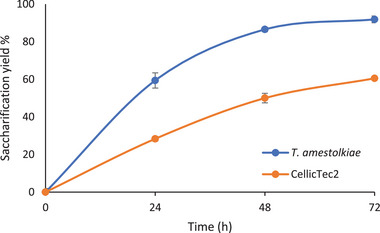
Time course of the saccharification yields of white birch wood pre‐treated with GVL and washed with Hot GVL and then with H2O, using Cellic CTec2 or a T. amestolkiae enzymatic crude as saccharification cocktails.
The efficiency demonstrated by the enzymatic crude of T. amestolkiae was far superior to that of Cellic CTec2 across the whole experiment, with 60% saccharification in 24 h and 91% in 72 h. Besides the differences related to catalytic efficiency and robustness of T. amestolkiae enzymes, this cocktail contained more endoglucanase and similar β‐glucosidase levels than Cellic CTec2, since FPU activity was selected for normalization of the dosage (Table 2).
To corroborate the interest of these results, the saccharification products from both enzymatic reactions were used as substrates in a two‐step fermentation to bioethanol, as described in Section 2. In the sample saccharified with the T. amestolkiae enzymatic crude, 65.9% of sugars were converted into ethanol in 72 h, while the yield decreased to 50.2% in the sample hydrolyzed with Cellic CTec2.
3.3. Optimization of Cellulase Production
Considering the good saccharification results obtained with the enzymatic crude from T. amestolkiae, a series of experiments were conducted in order to enhance the level of secreted cellulases.
First, a study was carried out in flasks to assess the influence of the composition of the culture broth, a crucial parameter to scale enzyme production. Different concentrations of Avicel (ranging from 0.5% to 4%) were initially assayed in Mandels basal medium (described in Section 3.3). In these conditions (Figure S1), there was a positive effect of Avicel as an inducer of endoglucanase and β‐glucosidase activities (with 1% and 2% Avicel), since these enzymes are essential in the first steps of cellulose degradation and to complete the saccharification process, respectively [23]. However, concentrations exceeding 2% Avicel had a detrimental effect on the production of T. amestolkiae β‐glucosidases, the key enzyme for saccharification, while similar levels of endoglucanase were observed in the cultures with 2% and 4% Avicel. Then, 2% Avicel concentration was selected for supplementing Mandels broth to study the effect of different nitrogen sources: 1 or 2 g L–1 of Bacto peptone, or 2 and 4 g L–1 CSS (Figure S2). These concentrations were chosen since the N content can be considered 2‐fold higher in peptone than in CSS. Higher N concentration produced higher endoglucanase and β‐glucosidase levels, with both peptone and CSS. When comparing N origin, slightly higher enzymatic levels were obtained using similar N content from CSS than from peptone. This is especially remarkable since CSS is an economic alternative to peptone.
In the following approach, the effect of pH and agitation rate were studied in a stirred tank 2 L bioreactor. Both are essential parameters because they induce morphological changes in fungi and increase enzyme secretion [24]. Regarding pH, although similar values were used in flasks and bioreactor experiments, pH was not controlled and only initially adjusted in flasks, while it was maintained constant throughout the experiment in the 2 L tank. pH ranges from 4 to 5.5 at 200 rpm were studied. The highest β‐glucosidase and endoglucanase levels were found to be at pH 4.5 (Figure S3A). Then, this pH value was selected to analyze the influence of agitation rate in the bioreactor. Figure S4 shows that enzyme secretion is favored with agitation, obtaining the highest βglucosidase levels at 400 rpm, although endoglucanase levels were similar at 200 and 400 rpm.
Finally, experiments in the 2 L bioreactor were carried out in order to compare the cellulase levels secreted by T. amestolkiae in the basal conditions described in Section 2.3 (Mandels medium, pH adjusted initially at 4.5, 200 rpm, 1% Avicel and 0.1% peptone) and in those selected from these experiments (controlled pH at 4.5, 400 rpm, 2% Avicel and 0.4% CSS). In addition to the β‐glucosidase and endoglucanase activities, FPU were also determined since this is the most common assay to determine total cellulase activity, established by the International Union of Pure and Applied Chemistry (IUPAC) [20]. Figure 3A shows that when pH is not controlled, it decreases in the first days and then stabilizes at pH 4 throughout the fermentation process. Our results agree with those reported for Trichoderma reesei, which secretes the highest cellulase levels at acidic pH, between 4 and 5.5 [25]. Also, the effect of pH in the expression of genes encoding carbohydrate‐active enzymes has been described [26]. It is remarkable that in the selected conditions, where pH is controlled at 4.5 but also carbon and nitrogen sources have been optimized (2% Avicel and 0.4% CSS) cellulase secretion by T. amestolkiae increased markedly, about 1.4‐fold for FPU (∼40%) and 1.7‐fold for β‐glucosidase and endoglucanase activities (∼75%) (Figure 3B).
FIGURE 3.
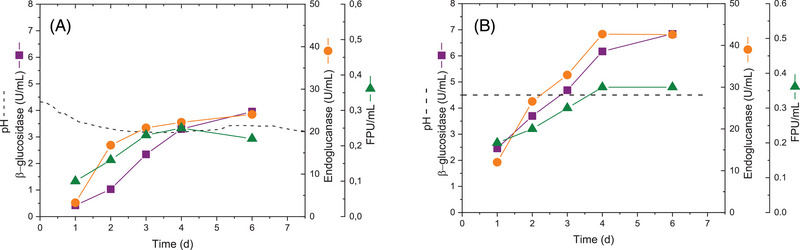
Time evolution of β‐glucosidase, endoglucanase, and FPU activities secreted by T. amestolkiae in 2 L bioreactor. (A) Before optimization (initial pH 4.5, 200 rpm, Mandels broth with 1% Avicel and 0.1% bacto peptone); (B) After optimization (pH controlled at 4.5, 400 rpm, Mandels broth modified with 2% Avicel and 0.4% CSS).
3.4. Molecular Fingerprint of β‐Glucosidase and Endoglucanase From Optimized Crudes
T. amestolkiae harbors genes that encode a large battery of cellulases and xylanases, and their secretion depends on the C and N source in the culture medium, as well as on the culture conditions. The cellulolytic system of this fungus has been extensively studied, and three β‐glucosidases (BGL‐1, BGL‐2, and BGL‐3) and two endoglucanases (EG‐1 and EG‐2) have been purified, and biochemically characterized [15, 27]. While BGL‐2 is specifically produced in the presence of cellulosic substrates, BGL‐3 is produced under starvation conditions, regardless of the carbon source used. Furthermore, the catalytic efficiencies and substrate specificities of these BGLs are quite distinct. Since the total endoglucanase and β‐glucosidase activity increased considerably (around 75%) in T. amestolkiae enzyme crudes after optimization, the production of the main cellulolytic enzymes was confirmed by isoelectric focusing (IEF) and performing zymograms. Figure S5 shows that, in both conditions, the three β‐glucosidases (pI around 7.5, 5.5, and 4.7) and two main endoglucanases (pI around 5.7 and 4.1), previously characterized [16, 17, 28, 29], were produced. Hence, these changes in the parameters used for the optimization of the extracellular cellulases secreted by T. amestolkiae did not affect the pattern production of these enzymes, which is very important in order to design different biotechnological applications.
4. Conclusions
GVL organosolv pretreatment is an effective process that allows the valorization of cellulose, hemicellulose, and lignin. As the process provides a clean stream of cellulose, it is easier to hydrolyze than raw biomass. The successful application of the T. amestolkiae enzymatic crude for saccharification of the cellulosic fraction from white birch has been proven. This cocktail produced a 91% saccharification yield (higher than the commercial Cellic CTec2 with the same FPase title), and the culture medium and conditions for its production have been optimized in a 2 L bioreactor. The enhancement of extracellular β‐glucosidase and endoglucanase levels were around 75% and 40% for FPU. However, to compete at the industrial level with Cellic CTec 2, or other commercial cocktails, is crucial to produce the T. amestolkiae cocktail at a large scale. In this sense, additional parameters, that is, inoculum size and scale up the process in higher size bioreactors, should still be optimized.
Author Contributions
Conceptualization: María Jesús Martínez. Methodology: Laura I. de Eugenio, Alicia Prieto, and David Alonso. Investigation: Laura I. de Eugenio, Isabel de la Torre, Felipe de Salas, and Francisco Vila. Resources: María Jesús Martínez and Alicia Prieto. Writing–original draft preparation: Laura I. de Eugenio, Isabel de la Torre, Felipe de Salas. Writing–review and editing: María Jesús Martínez and Alicia Prieto. Funding acquisition, María Jesús Martínez. All authors have read and agreed to the published version of the manuscript.
Supporting information
Supporting Information
Acknowledgments
The authors acknowledge BioSFerA (RIA 884208) and IBISBA1.0 (H2020 730976) projects and TransENER and SusPlast‐CSIC Interdisciplinary Platforms for their support. This research is part of the CSIC program for the Spanish Recovery, Transformation, and Resilience Plan funded by the Recovery and Resilience Facility of the European Union, established by the Regulation (EU) 2020/2094.
Funding: This research was supported by MCIN with funding from NextGenerationEU (PRTR‐C17.i1) within the Planes Complementarios con CCAA (Area of Green Hydrogen and Energy) and it has been carried out in the CSIC Interdisciplinary Thematic Platform (PTI+) Transición Energética Sostenible+ (PTI‐‐TRANSENER+).
References
- 1. Fossil Fuels and Climate Change: The Facts (n.d.) (accessed July 7, 2023), https://www.clientearth.org/latest/latest‐updates/stories/fossil‐fuels‐and‐climate‐change‐the‐facts/.
- 2. Amidon T. E. and Liu S., “Water‐Based Woody Biorefinery,” Biotechnology Advances 27 (2009): 542–550, 10.1016/j.biotechadv.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3. Igbokwe V. C., Ezugworie F. N., Onwosi C. O., Aliyu G. O., and Obi C. J., “Biochemical Biorefinery: A Low‐Cost and Non‐Waste Concept for Promoting Sustainable Circular Bioeconomy,” Journal of Environmental Management 305 (2022): 114333, 10.1016/j.jenvman.2021.114333. [DOI] [PubMed] [Google Scholar]
- 4. Kammoun M., Margellou A., Toteva V. B., et al., “The Key Role of Pretreatment for the One‐Step and Multi‐Step Conversions of European Lignocellulosic Materials Into Furan Compounds,” RSC Advances 13 (2023): 21395–21420, 10.1039/d3ra01533e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoang A. T., Nguyen X. P., Duong X. Q., et al., “Steam Explosion as Sustainable Biomass Pretreatment Technique for Biofuel Production: Characteristics and Challenges,” Bioresource Technology 385 (2023): 129398, 10.1016/j.biortech.2023.129398. [DOI] [PubMed] [Google Scholar]
- 6. Shabbirahmed A. M., Joel J., Gomez A., Patel A. K., Singhania R. R., and Haldar D., “Environment Friendly Emerging Techniques for the Treatment of Waste Biomass: A Focus on Microwave and Ultrasonication Processes,” Environmental Science and Pollution Research International 30 (2023): 79706–79723, 10.1007/s11356-023-28271-9. [DOI] [PubMed] [Google Scholar]
- 7. Alonso D. M., Hakim S. H., Zhou S., et al., “Increasing the Revenue From Lignocellulosic Biomass: Maximizing Feedstock Utilization,” Science Advances 3 (2017): e1603301, 10.1126/sciadv.1603301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lê H. Q., Ma Y., Borrega M., and Sixta H., “Wood Biorefinery Based on γ‐valerolactone/Water Fractionation,” Green Chemistry 18 (2016): 5466–5476, 10.1039/c6gc01692h. [DOI] [Google Scholar]
- 9. Shuai L., Questell‐Santiago Y. M., and Luterbacher J. S., “A Mild Biomass Pretreatment Using γ‐Valerolactone for Concentrated Sugar Production,” Green Chemistry 18 (2016): 937–943, 10.1039/C5GC02489G. [DOI] [Google Scholar]
- 10. Luterbacher J. S., Rand J. M., Alonso D. M., et al., “Nonenzymatic Sugar Production From Biomass Using Biomass‐Derived γ‐Valerolactone,” Science 343 (2014): 277–280, 10.1126/science.1246748. [DOI] [PubMed] [Google Scholar]
- 11. Gürbüz E. I., Gallo J. M. R., Alonso D. M., Wettstein S. G., Lim W. Y., and Dumesic J. A., “Conversion of Hemicellulose into Furfural Using Solid Acid Catalysts in γ‐Valerolactone,” Angewandte Chemie 125 (2013): 1308–1312, 10.1002/ange.201207334. [DOI] [PubMed] [Google Scholar]
- 12. Wettstein S., Martin Alonso D., Chong Y., and Dumesic J., “Production of Levulinic Acid and Gamma‐Valerolactone (GVL) From Cellulose Using GVL as a Solvent in Biphasic Systems,” Energy & Environmental Science 5 (2012): 8199–8203, 10.1039/C2ee22111j. [DOI] [Google Scholar]
- 13. Mellmer M. A., Sener C., Gallo J. M. R., Luterbacher J. S., Alonso D. M., and Dumesic J. A., “Solvent Effects in Acid‐catalyzed Biomass Conversion Reactions,” Angewandte Chemie (International ed in English) 53 (2014): 11872–11875, 10.1002/anie.201408359. [DOI] [PubMed] [Google Scholar]
- 14. Payne C. M., Knott B. C., Mayes H. B., et al., “Fungal Cellulases,” Chemical Reviews 115 (2015): 1308–1448, 10.1021/cr500351c. [DOI] [PubMed] [Google Scholar]
- 15. de Eugenio L. I., Méndez‐Líter J. A., Nieto‐Domínguez M., et al., “Differential β‐Glucosidase Expression as a Function of Carbon Source Availability in Talaromyces amestolkiae: A Genomic and Proteomic Approach,” Biotechnology for Biofuels 10 (2017): 1–14, 10.1186/s13068-017-0844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Méndez‐Líter J. A., Gil‐Muñoz J., Nieto‐Domínguez M., Barriuso J., De Eugenio L. I., and Martínez M. J., “A Novel, Highly Efficient β‐Glucosidase With a Cellulose‐Binding Domain: Characterization and Properties of Native and Recombinant Proteins,” Biotechnology for Biofuels 10 (2017): 256, 10.1186/s13068-017-0946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Méndez‐Líter J. A., de Eugenio L. I., Prieto A., and Martínez M. J., “The β‐Glucosidase Secreted by Talaromyces amestolkiae Under Carbon Starvation: A Versatile Catalyst for Biofuel Production From Plant and Algal Biomass,” Biotechnology for Biofuels 11 (2018): 1–14, 10.1186/s13068-018-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. USDA Plants Database (n.d.) (ccessed September 1, 2023), https://plants.usda.gov/home/plantProfile?symbol=BEPE3%E2%80%8E.
- 19. Somogyi M., “A New Reagent for the Determination of Sugars,” The Journal of Biological Chemistry 160 (1945): 61–68. [Google Scholar]
- 20. Percival Zhang Y.‐H., Himmel M. E., and Mielenz J. R., “Outlook for Cellulase Improvement: Screening and Selection Strategies,” Biotechnology Advances 24 (2006): 452–481, 10.1016/j.biotechadv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21. Monção M., Hrůzová K., Rova U., Matsakas L., and Christakopoulos P., “Organosolv Fractionation of Birch Sawdust: Establishing a Lignin‐First Biorefinery,” Molecules (Basel, Switzerland) 26 (2021): 6754, 10.3390/molecules26216754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Przybysz Buzala K., Kalinowska H., Małachowska E., Boruszewski P., Krajewski K., and Przybysz P., “The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields From the Enzymatic Hydrolysis of Cellulose,” Energies 12 (2019): 2952, 10.3390/en12152952. [DOI] [Google Scholar]
- 23. Houfani A. A., Anders N., Spiess A. C., Baldrian P., and Benallaoua S., “Insights from Enzymatic Degradation of Cellulose and Hemicellulose to Fermentable Sugars—A Review,” Biomass and Bioenergy 134 (2020): 105481, 10.1016/j.biombioe.2020.105481. [DOI] [Google Scholar]
- 24. Adav S. S., Ravindran A., Chao L. T., Tan L., Singh S., and Sze S. K., “Proteomic Analysis of pH and Strains Dependent Protein Secretion of Trichoderma reesei ,” Journal of Proteome Research 10 (2011): 4579–4596, 10.1021/pr200416t. [DOI] [PubMed] [Google Scholar]
- 25. Li C., Yang Z., Zhang R. H. C., Zhang D., Chen S., and Ma L., “Effect of pH on Cellulase Production and Morphology of Trichoderma reesei and the Application in Cellulosic Material Hydrolysis,” Journal of Biotechnology 168 (2013): 470–477, 10.1016/j.jbiotec.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 26. Häkkinen M., Arvas M., Oja M., et al., “Re‐annotation of the CAZy Genes of Trichoderma reesei and Transcription in the Presence of Lignocellulosic Substrates,” Microbial Cell Factories 11 (2012): 134, 10.1186/1475-2859-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prieto A., de Eugenio L. I., Méndez‐Líter J. A., et al., “Fungal Glycosyl Hydrolases for Sustainable Plant Biomass Valorization: Talaromyces amestolkiae as a Model Fungus,” International Microbiology 24 (2021): 545–558, 10.1007/s10123-021-00202-z. [DOI] [PubMed] [Google Scholar]
- 28. Méndez‐Líter J. A., Nieto‐Domínguez M., Fernández De Toro B., et al., “A Glucotolerant β‐Glucosidase From the Fungus Talaromyces amestolkiae and Its Conversion Into a Glycosynthase for Glycosylation of Phenolic Compounds,” Microbial Cell Factories 19 (2020): 1–13, 10.1186/s12934-020-01386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Eugenio L. I., Méndez‐Líter J., de los Ríos V., Prieto A., and Martínez M., “β‐1,4‐Endoglucanases From Talaromyces amestolkiae: Production of Glucooligosaccharides From Different β‐Glucans,” Biocatalysis and Biotransformation 36 (2017): 68–77, 10.1080/10242422.2017.1306741. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


