Abstract
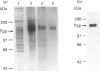
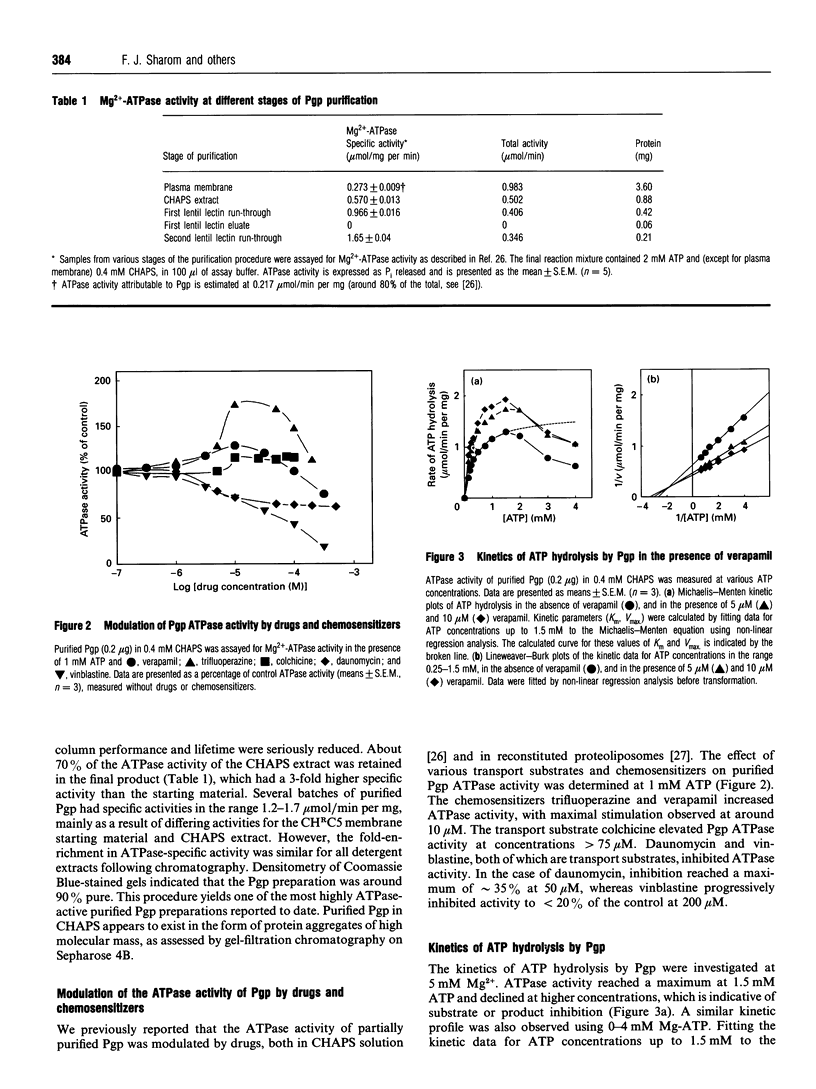
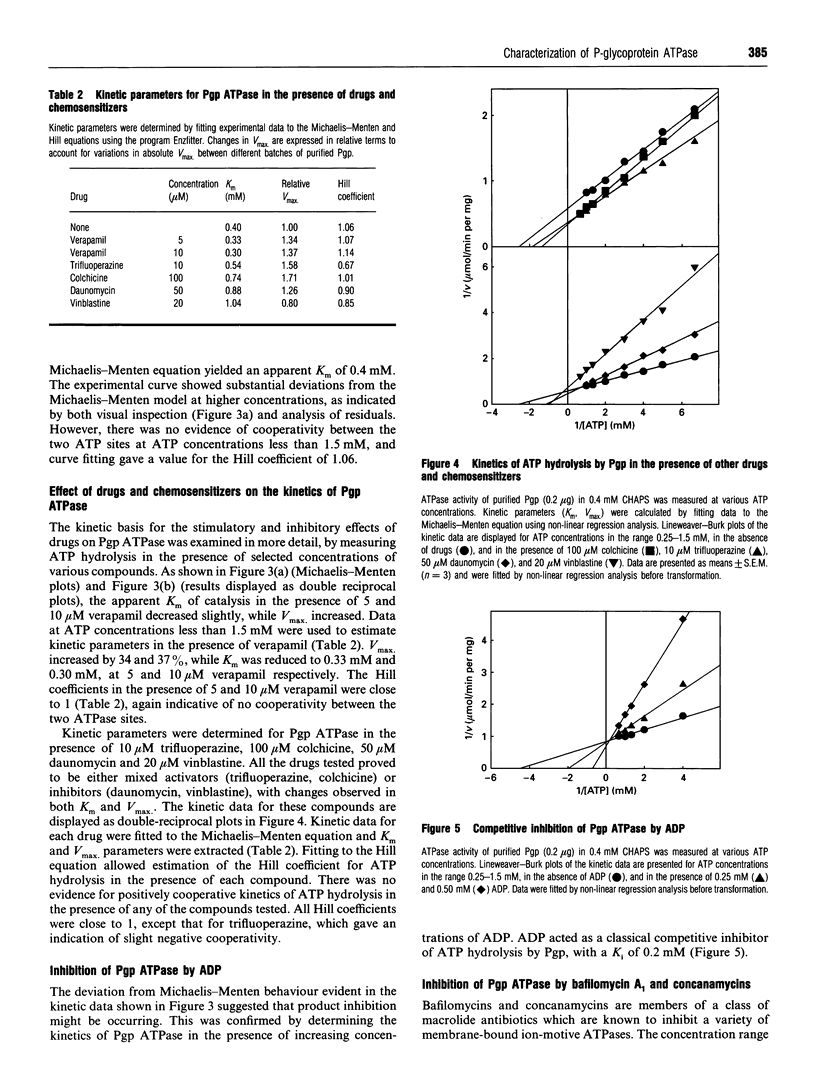
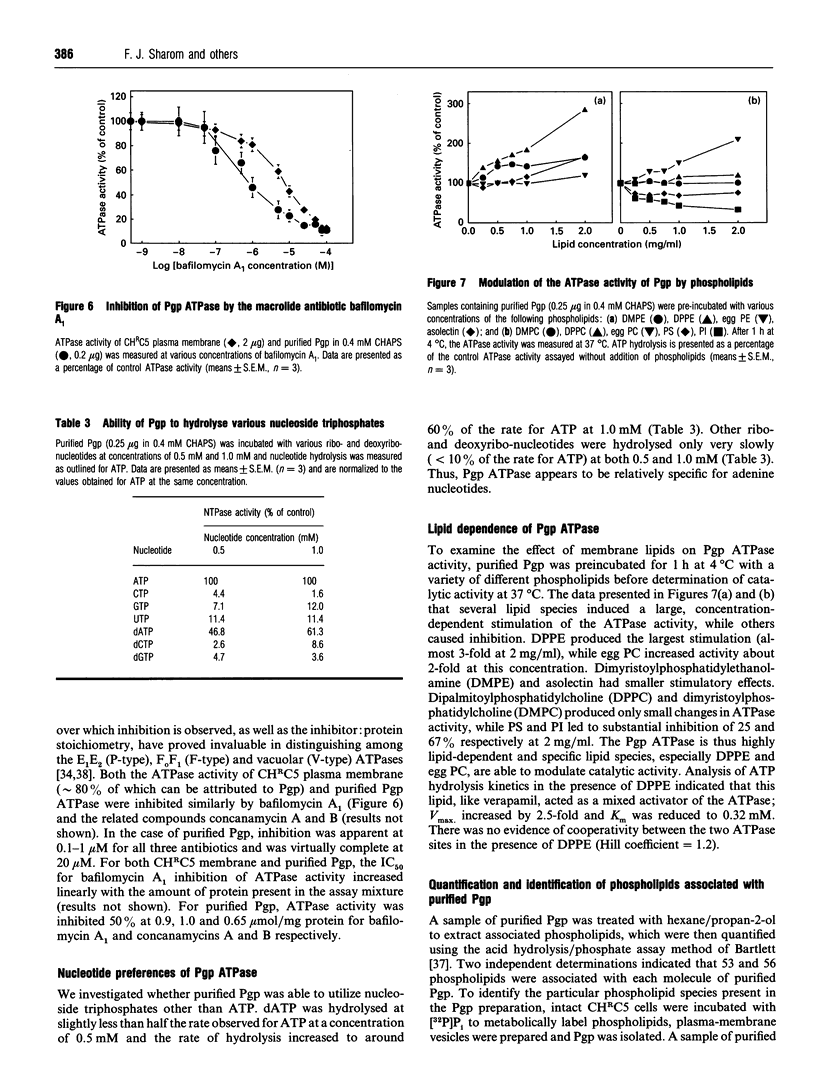
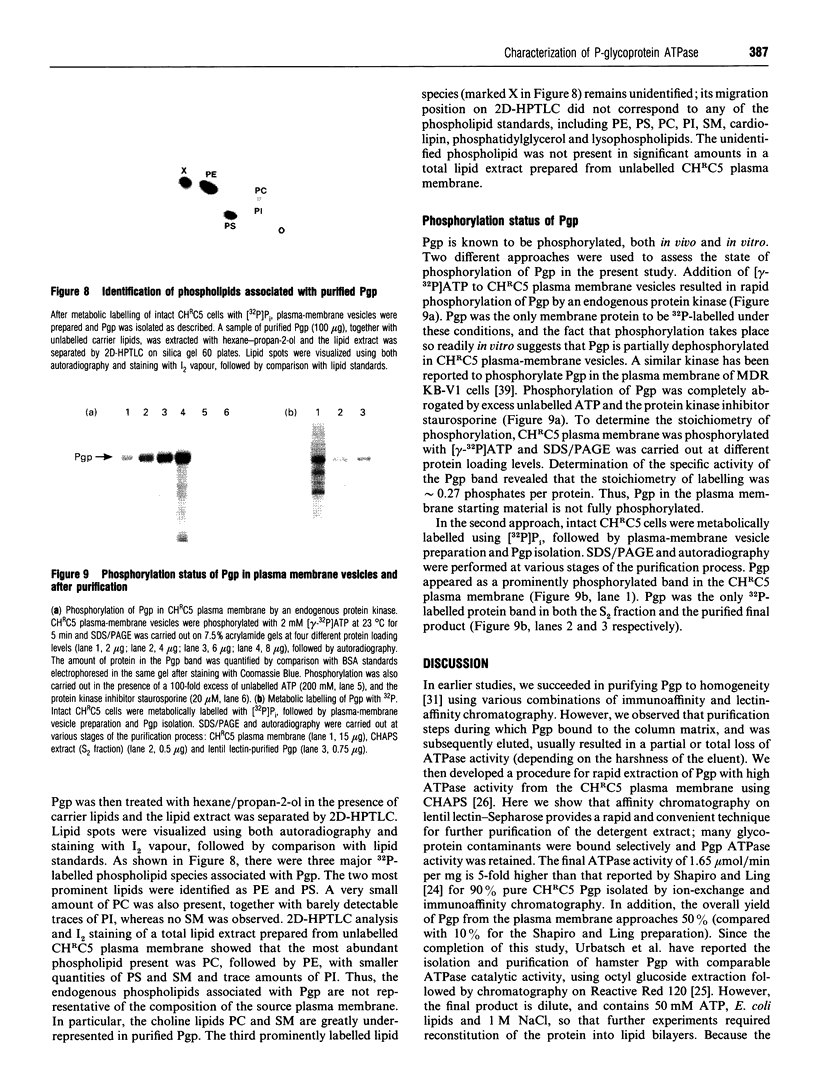
P-Glycoprotein (Pgp) was isolated from CHRC5 membranes by selective detergent extraction and further purified by lentil lectin affinity chromatography. The purified product displayed a very high basal ATPase activity (1.65 mumol/min per mg protein in the absence of added drugs or lipids) with an apparent Km for ATP of 0.4 mM. There was no evidence of cooperativity, suggesting that the two ATP sites operate independently of each other. Pgp ATPase activity was stimulated by verapamil, trifluoperazine and colchicine, and inhibited by daunomycin and vinblastine. All drugs and chemosensitizers acted as mixed activators or inhibitors, producing changes in both the Vmax of the ATPase and the Km for ATP. ADP competitively inhibited Pgp ATPase, with a Ki of 0.2 mM. The macrolide antibiotics bafilomycin A1, concanamycin A and concanamycin B, inhibited Pgp ATPase at concentrations of 0.1-10 microM, and at an inhibitor:protein stoichiometry of 0.65-1.0 mumol/mg protein, which is at the low end of the range characteristic of P-type ATPases. Pgp ATPase was relatively selective for adenine nucleotides. Several phospholipids stimulated Pgp ATPase activity in a dose-dependent manner, whereas others produced inhibition. Metabolic labelling showed that the endogenous phospholipids associated with purified Pgp consisted largely of phosphatidylethanolamine and phosphatidylserine, with only a small amount of phosphatidylcholine. 32P-Labelling studies indicated that purified Pgp was partially phosphorylated. It can be concluded that Pgp is a constitutively active, adenine nucleotide-specific ATPase whose catalytic activity can be modulated by both drugs and phospholipids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Lelong I. H., Zhang J., Cardarelli C. O., Gottesman M. M., Pastan I. Partial purification and reconstitution of the human multidrug-resistance pump: characterization of the drug-stimulatable ATP hydrolysis. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8472–8476. doi: 10.1073/pnas.89.18.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Mimura C. S., Shyamala V. Bacterial periplasmic permeases belong to a family of transport proteins operating from Escherichia coli to human: Traffic ATPases. FEMS Microbiol Rev. 1990 Aug;6(4):429–446. doi: 10.1111/j.1574-6968.1990.tb04110.x. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chambers T. C., Chalikonda I., Eilon G. Correlation of protein kinase C translocation, P-glycoprotein phosphorylation and reduced drug accumulation in multidrug resistant human KB cells. Biochem Biophys Res Commun. 1990 May 31;169(1):253–259. doi: 10.1016/0006-291x(90)91461-z. [DOI] [PubMed] [Google Scholar]
- Chambers T. C., McAvoy E. M., Jacobs J. W., Eilon G. Protein kinase C phosphorylates P-glycoprotein in multidrug resistant human KB carcinoma cells. J Biol Chem. 1990 May 5;265(13):7679–7686. [PubMed] [Google Scholar]
- Chambers T. C., Pohl J., Raynor R. L., Kuo J. F. Identification of specific sites in human P-glycoprotein phosphorylated by protein kinase C. J Biol Chem. 1993 Mar 5;268(7):4592–4595. [PubMed] [Google Scholar]
- Chambers T. C., Zheng B., Kuo J. F. Regulation by phorbol ester and protein kinase C inhibitors, and by a protein phosphatase inhibitor (okadaic acid), of P-glycoprotein phosphorylation and relationship to drug accumulation in multidrug-resistant human KB cells. Mol Pharmacol. 1992 Jun;41(6):1008–1015. [PubMed] [Google Scholar]
- Cornwell M. M., Safa A. R., Felsted R. L., Gottesman M. M., Pastan I. Membrane vesicles from multidrug-resistant human cancer cells contain a specific 150- to 170-kDa protein detected by photoaffinity labeling. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3847–3850. doi: 10.1073/pnas.83.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine S. E., Ling V., Melera P. W. Amino acid substitutions in the sixth transmembrane domain of P-glycoprotein alter multidrug resistance. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4564–4568. doi: 10.1073/pnas.89.10.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doige C. A., Sharom F. J. Strategies for the purification of P-glycoprotein from multidrug-resistant Chinese hamster ovary cells. Protein Expr Purif. 1991 Aug;2(4):256–265. doi: 10.1016/1046-5928(91)90081-s. [DOI] [PubMed] [Google Scholar]
- Doige C. A., Sharom F. J. Transport properties of P-glycoprotein in plasma membrane vesicles from multidrug-resistant Chinese hamster ovary cells. Biochim Biophys Acta. 1992 Aug 24;1109(2):161–171. doi: 10.1016/0005-2736(92)90079-2. [DOI] [PubMed] [Google Scholar]
- Doige C. A., Yu X., Sharom F. J. ATPase activity of partially purified P-glycoprotein from multidrug-resistant Chinese hamster ovary cells. Biochim Biophys Acta. 1992 Aug 24;1109(2):149–160. doi: 10.1016/0005-2736(92)90078-z. [DOI] [PubMed] [Google Scholar]
- Doige C. A., Yu X., Sharom F. J. The effects of lipids and detergents on ATPase-active P-glycoprotein. Biochim Biophys Acta. 1993 Feb 23;1146(1):65–72. doi: 10.1016/0005-2736(93)90339-2. [DOI] [PubMed] [Google Scholar]
- Dröse S., Bindseil K. U., Bowman E. J., Siebers A., Zeeck A., Altendorf K. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosinetriphosphatases. Biochemistry. 1993 Apr 20;32(15):3902–3906. doi: 10.1021/bi00066a008. [DOI] [PubMed] [Google Scholar]
- Garrigos M., Belehradek J., Jr, Mir L. M., Orlowski S. Absence of cooperativity for MgATP and verapamil effects on the ATPase activity of P-glycoprotein containing membrane vesicles. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1034–1041. doi: 10.1006/bbrc.1993.2355. [DOI] [PubMed] [Google Scholar]
- Georges E., Sharom F. J., Ling V. Multidrug resistance and chemosensitization: therapeutic implications for cancer chemotherapy. Adv Pharmacol. 1990;21:185–220. doi: 10.1016/s1054-3589(08)60343-9. [DOI] [PubMed] [Google Scholar]
- Georges E., Zhang J. T., Ling V. Modulation of ATP and drug binding by monoclonal antibodies against P-glycoprotein. J Cell Physiol. 1991 Sep;148(3):479–484. doi: 10.1002/jcp.1041480321. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gros P., Dhir R., Croop J., Talbot F. A single amino acid substitution strongly modulates the activity and substrate specificity of the mouse mdr1 and mdr3 drug efflux pumps. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7289–7293. doi: 10.1073/pnas.88.16.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Characterization of the ATPase activity of the Mr 170,000 to 180,000 membrane glycoprotein (P-glycoprotein) associated with multidrug resistance in K562/ADM cells. Cancer Res. 1988 Sep 1;48(17):4926–4932. [PubMed] [Google Scholar]
- Hamada H., Tsuruo T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. 170- to 180-kilodalton membrane glycoprotein is an ATPase. J Biol Chem. 1988 Jan 25;263(3):1454–1458. [PubMed] [Google Scholar]
- Higgins C. F., Hyde S. C., Mimmack M. M., Gileadi U., Gill D. R., Gallagher M. P. Binding protein-dependent transport systems. J Bioenerg Biomembr. 1990 Aug;22(4):571–592. doi: 10.1007/BF00762962. [DOI] [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lelong I. H., Cardarelli C. O., Gottesman M. M., Pastan I. GTP-stimulated phosphorylation of P-glycoprotein in transporting vesicles from KB-V1 multidrug resistant cells. Biochemistry. 1994 Aug 2;33(30):8921–8929. doi: 10.1021/bi00196a009. [DOI] [PubMed] [Google Scholar]
- Lelong I. H., Padmanabhan R., Lovelace E., Pastan I., Gottesman M. M. ATP and GTP as alternative energy sources for vinblastine transport by P-170 in KB-V1 plasma membrane vesicles. FEBS Lett. 1992 Jun 15;304(2-3):256–260. doi: 10.1016/0014-5793(92)80632-q. [DOI] [PubMed] [Google Scholar]
- Ling V., Kartner N., Sudo T., Siminovitch L., Riordan J. R. Multidrug-resistance phenotype in Chinese hamster ovary cells. Cancer Treat Rep. 1983 Oct;67(10):869–874. [PubMed] [Google Scholar]
- Naito M., Hamada H., Tsuruo T. ATP/Mg2+-dependent binding of vincristine to the plasma membrane of multidrug-resistant K562 cells. J Biol Chem. 1988 Aug 25;263(24):11887–11891. [PubMed] [Google Scholar]
- Orr G. A., Han E. K., Browne P. C., Nieves E., O'Connor B. M., Yang C. P., Horwitz S. B. Identification of the major phosphorylation domain of murine mdr1b P-glycoprotein. Analysis of the protein kinase A and protein kinase C phosphorylation sites. J Biol Chem. 1993 Nov 25;268(33):25054–25062. [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Vico F., Martínez-Cayuela M., Zafra M. F., García-Peregrin E., Ramírez H. A procedure for the simultaneous determination of lipid and protein in biomembranes and other biological samples. Lipids. 1991 Jan;26(1):77–80. doi: 10.1007/BF02544029. [DOI] [PubMed] [Google Scholar]
- Saeki T., Shimabuku A. M., Ueda K., Komano T. Specific drug binding by purified lipid-reconstituted P-glycoprotein: dependence on the lipid composition. Biochim Biophys Acta. 1992 Jun 11;1107(1):105–110. doi: 10.1016/0005-2736(92)90334-i. [DOI] [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Meyers M. B., Biedler J. L., Felsted R. L. Vinblastine photoaffinity labeling of a high molecular weight surface membrane glycoprotein specific for multidrug-resistant cells. J Biol Chem. 1986 May 15;261(14):6137–6140. [PubMed] [Google Scholar]
- Safa A. R., Mehta N. D., Agresti M. Photoaffinity labeling of P-glycoprotein in multidrug resistant cells with photoactive analogs of colchicine. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1402–1408. doi: 10.1016/0006-291x(89)90830-9. [DOI] [PubMed] [Google Scholar]
- Safa A. R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Price E. M., Boucher R. C., Germann U. A., Scarborough G. A. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem. 1992 Mar 5;267(7):4854–4858. [PubMed] [Google Scholar]
- Sehested M., Bindslev N., Demant E. J., Skovsgaard T., Jensen P. B. Daunorubicin and vincristine binding to plasma membrane vesicles from daunorubicin-resistant and wild type Ehrlich ascites tumor cells. Biochem Pharmacol. 1989 Sep 15;38(18):3017–3027. doi: 10.1016/0006-2952(89)90010-5. [DOI] [PubMed] [Google Scholar]
- Shapiro A. B., Ling V. ATPase activity of purified and reconstituted P-glycoprotein from Chinese hamster ovary cells. J Biol Chem. 1994 Feb 4;269(5):3745–3754. [PubMed] [Google Scholar]
- Sharom F. J., Yu X., Doige C. A. Functional reconstitution of drug transport and ATPase activity in proteoliposomes containing partially purified P-glycoprotein. J Biol Chem. 1993 Nov 15;268(32):24197–24202. [PubMed] [Google Scholar]
- Shimabuku A. M., Nishimoto T., Ueda K., Komano T. P-glycoprotein. ATP hydrolysis by the N-terminal nucleotide-binding domain. J Biol Chem. 1992 Mar 5;267(7):4308–4311. [PubMed] [Google Scholar]
- Urbatsch I. L., al-Shawi M. K., Senior A. E. Characterization of the ATPase activity of purified Chinese hamster P-glycoprotein. Biochemistry. 1994 Jun 14;33(23):7069–7076. doi: 10.1021/bi00189a008. [DOI] [PubMed] [Google Scholar]
- al-Shawi M. K., Senior A. E. Characterization of the adenosine triphosphatase activity of Chinese hamster P-glycoprotein. J Biol Chem. 1993 Feb 25;268(6):4197–4206. [PubMed] [Google Scholar]