Abstract
Skeletal muscle has been recognized as an endocrine organ which communicates with different systems, including the brain. In conditions involving systemic low-grade chronic inflammation, the skeletal muscle can be negatively impacted, culminating in its quantity (mass) and quality (function) losses, referred to here as muscle wasting. The inflammatory milieu, as well known, also impairs the brain function, however there are some particularities involving skeletal muscle-brain crosstalk, including cognitive function and mental health impairments. Psychoneuroimmunology (PNI) is an important field of neuroendocrine-immune-behavior science and an approach between PNI, and the movement science, or kinesiology, field can enrich future research about the relationship between skeletal muscle wasting and brain health. Thus, in this short review, we present an overview about the interplay between skeletal muscle, inflammatory mediator markers, and brain function with the purpose to strengthen the ties between kinesiology and PNI research to enhance futures discoveries and advances in health sciences.
Keywords: Myokines, BDNF, Neuromuscular junction, Sarcopenia, Physical activity
1. Introduction
The psychoneuroimmunology (PNI) research has contributed to the elucidation of the dynamic interaction between biological, psychic, and social components through the study of nervous, endocrine, immune, and behavioral systems (Engel, 1977; Ader, 2001). The trajectory of PNI field started with the recognition of the integration between brain and immune system (bidirectional responses involving neuroendocrine/chemical and autonomic nervous system with immunoregulatory processes), which resulted in the recognition of the behavior influence on immune function, i.e., stress stimulation can mediate disease progression (Ader, 2001). Together, the studies in these areas have opened a broad integrative understanding of the body system. To date, PNI is rapidly growing as a multidisciplinary science, gathering different areas of research, such as lung-brain axis and skin-brain axis, which has contributed to the development of health sciences (Rummel et al., 2022; Peters et al., 2023).
Skeletal muscle has been recognized as an important endocrine organ which communicates with different systems, including the brain (Pedersen, 2019). Studies have shown that skeletal muscle contraction positively contributes to brain health directly and indirectly (Seifert et al., 2010; Agudelo et al., 2014; Lourenco et al., 2019; Liu et al., 2022). On the other hand, the benefits of muscle-brain crosstalk can be impaired in pathological conditions (Aby et al., 2021; Arosio et al., 2023). Immunometabolism dysregulation from different sources can cause skeletal muscle dysfunction, several negative consequences for the body, and compromised brain health (Szlejf et al., 2019; Arosio et al., 2023). The science of movement, or kinesiology, has explored related topics such as skeletal muscle physiology and immunology, physical exercise and brain function, and muscle wasting and nervous system. Although the scientific production regarding these topics is rising, our knowledge of the immune-muscle-brain loop remains insufficient. In this context, PNI can support and enrich the development of future studies involving the role of skeletal muscle contraction and improvement of immune-brain responses.
Therefore, in this short review, we present an overview about the interplay between skeletal muscle, inflammatory mediator markers, and brain function with the purpose of strengthen the ties between kinesiology and PNI research to enhance futures discoveries and advances in health sciences.
2. Skeletal muscle: an overview
Skeletal muscle is considered the largest body's tissue (Pedersen and Febbraio, 2008), comprising around 40% of total body mass (Rolfe and Brown, 1997). Its primary functions include movement, breathing, postural control, and heat production during cold stress (Powers et al., 2021). Adequate skeletal muscle function depends on several other body systems.
Morphologically, skeletal muscle is connected to bones and connective tissue, which allows movement. Physiologically, skeletal muscle shares the similar structures of other tissues (cell, nerves, blood vessels, immune resident cells) however with some peculiarities, such as, skeletal muscle cells, also referred as myocytes or muscle fibers, have a cylindric shape, multiple nuclei, the presence of satellite cells (related to muscle repair, hypertrophy, and strength gain), and sarcoplasmic reticulum (Powers et al., 2021). Metabolically, active skeletal muscle relies on a large and diverse energy supply. The source of energy supply varies according to the size of muscle being recruited, duration (time) and intensity (power) of movement execution (Robinson et al., 2015).
Skeletal muscles can work anaerobically or aerobically, and each metabolism trigger different signaling pathways involved in the skeletal muscle remodeling process. Anaerobic metabolism activates molecular mechanisms closely related to skeletal muscle hypertrophy and strength gain, including the mechanistic target of rapamycin (mTOR) signaling pathway (Powers et al., 2021). Aerobic metabolism, on the other hand, activates signaling pathways related to oxidative processes, including peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α), an important coactivator of transcription factor regulator of energy metabolism, e.g., mitochondrial biogenesis that improves the oxidative capacity of skeletal muscle (Liang and Ward, 2006).
A little over two decades ago, the skeletal muscle was recognized as an endocrine organ through the identification of interleukine-6 (IL-6) production and release by myocytes during muscle contraction in aerobic exercise (Ostrowski et al., 1998; Steensberg et al., 2000). This finding is one of the most important advances in skeletal muscle research and exercise science, allowing the expansion of the study of metabolism into the context of immunometabolism in physical exercise and health sciences. To date, hundreds of myokines have been described, however less is known about their biological function and regulation (Peake et al., 2015; Bay and Pedersen, 2020). The relationship between skeletal muscle and immune system is evidenced during physical exercise, when leukocyte traffic increases in bloodstream, and the type of immune cells varies according to the volume and intensity of the exercise bout (Peake et al., 2015; Bay and Pedersen, 2020).
Skeletal muscle and brain can communicate directly or indirectly. The skeletal muscle is directly connected to nervous system by innervation. The point of connection between the muscle fiber and the motor neuron of the peripheral nervous system is denominated neuromuscular junction (NMJ). Besides its role to fire the muscle contraction, the NMJ is also involved in mTOR signaling pathway that culminates into skeletal muscle hypertrophy and force generation. An efficient performance of skeletal muscle function, at least in parts, depends on a healthy NMJ (Arosio et al., 2023). Indirectly, the crosstalk between skeletal muscle and brain occurs by endocrine pathway, specially by exerkines released during physical activity (Chow et al., 2022). Exerkines will be discussed in more detail later in this review.
Although the skeletal muscle-brain crosstalk can be disturbed by several conditions, this short-review has limited its scope to the context of low-grade chronic inflammation.
3. Inflammation and its impact on skeletal muscle-brain crosstalk
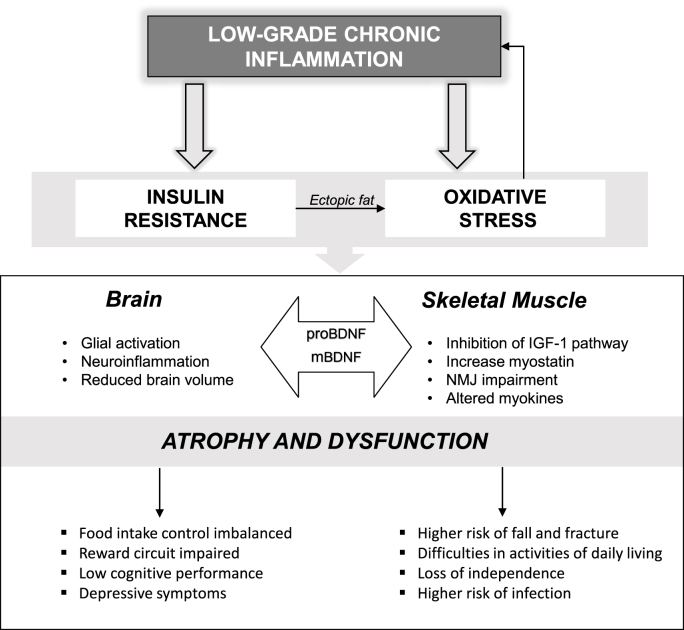
Inflammation has been a hot topic in health science as it has been recognized to be related to the emergence and progression of pathophysiological conditions, such as atherosclerosis (Ros, 1999), obesity (Hotamisligil et al., 1993), chronic obstructive pulmonary disease (King, 2015), aging, and Alzheimer disease (Ogawa et al., 2018). In this context, unlike what occurs in the presence of infection or injuries, there is a low-grade inflammation which results from a prolonged state of tissue malfunction (Medzhitov, 2008). When this low-grade chronic inflammation becomes systemic, it can affect all functions of the body, including the brain and skeletal muscles (Fig. 1).
Fig. 1.
Low-Grade Chronic Inflammation, Skeletal Muscle Wasting, and Brain Impairments. The systemic inflammation triggers a disruptive cascade that impacts brain and skeletal muscle physiology and morphology and impairing behavior. IGF-1: Insulin like growth factor-1 mBDNF: mature brain-derived neurotrophic factor isoform; proBDNF: pro-brain-derived neurotrophic factor isoform; NMJ: neuromuscular junction.
The interplay between low-grade chronic inflammation and cognitive function have been well documented in pathological conditions such as cardiometabolic diseases, in which the inflammation-insulin resistance state is one of the hallmarks of body system disorders, in which pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), impair the insulin signaling pathway and adiponectin, a potent insulin-sensitizing hormone (O'Brien et al., 2017; Inoue et al., 2020a, 2021). Indeed, both peripheral inflammation and systemic insulin resistance disrupt both blood-nerve and the blood-brain barrier (BBB), increasing the release of circulating inflammatory mediators and immune cell infiltration into central nervous system. This results in a state of neuroinflammation and at the same time contributes to the installation of brain insulin resistance that impairs important neural signaling pathways related to behavior, such as food intake control and reward circuit (Obermeier et al., 2013; Kullmann et al., 2016; O'Brien et al., 2017, Liu et al., 2022). Moreover, studies have shown the impact of inflammation-insulin resistance state on brain size (Yau et al., 2012; Tsai et al., 2019; Liu et al., 2022), cognitive performance (Yau et al., 2012; Schwartz et al., 2013), and depressive symptoms (Dantzer et al., 2008; Dowlati et al., 2010).
Skeletal muscle is also affected by low-grade chronic inflammation. Excess pro-inflammatory mediators cause insulin resistance in skeletal muscle, which leads to several metabolic disorders, including the mitochondrial dysfunction, reduced oxidative capacity (Trouwnorst et al., 2023), and loss of skeletal muscle mass and function (Nishikawa et al., 2021; Jung et al., 2023), referred to here as muscle wasting. Skeletal muscle wasting is characterized by a decrease in muscle protein synthesis, an increase in protein degradation, or both (Gomes et al., 2017). Evidence have shown that low-grade chronic inflammation reduces the anabolic drive, which is explained, in part, by increased availability of myostatin (a negative regulator of muscle growth) and suppression of the insulin like growth factor −1 (IGF-1) axis, which promotes protein synthesis (Nishikawa et al., 2021). Additionally, inflammatory process induces oxidative stress which, in addition to being able to impair protein synthesis, can also trigger muscle protein degradation, through activation of two major proteolytic systems (Ubiquitin–Proteasome System and the Autophagy–Lysosomal Pathway) (Agrawal et al., 2023). Indeed, there is a positive feedback loop between oxidative stress and inflammation with oxidative stress driving inflammation, and inflammation, in turn, promoting oxidative stress. In this context, the nuclear factor erythroid 2-related factor 2 (Nrf2), an antioxidant key molecule, and transcription factor nuclear factor-κB (NF-κB), a critical inflammatory driver, regulate cellular responses to oxidative stress and inflammation (Gao et al., 2022).
The state of inflammation-insulin resistance resulting from dysregulated inflammatory signaling in both skeletal muscle and brain also affects the NMJ (O'Brien et al., 2017; Arosio et al., 2023). This inflamed microenvironment disturbs the expression of important neurotrophic factors, such as brain-derived neurotrophic factor (BDNF). It is well established that BDNF is a pleiotropic protein expressed in different tissues. In both nervous and skeletal muscle systems, the mature-BDNF (mBDNF) isoform is involved with cellular genesis, growth, and regeneration, while the pro-BDNF isoform is related to cellular apoptosis and atrophy (Marosi and Mattson, 2014; Arosio et al., 2023). In fact, mBDNF supports motor neuron at the NMJ by increasing its viability, acetylcholine release, and postsynaptic maintenance (Arosio et al., 2023). On the other hand, the pro-BDNF isoform triggers signaling pathways related to pro-inflammatory responses and apoptosis (Aby et al., 2021), and, in this context, synaptic transmission is compromised, and the maintenance of muscle mass and function is impaired.
Ultimately, long-term skeletal muscle wasting can culminate in increased risk of falls and fractures, decreased performance in activities of daily living, loss of independence (Cruz-Jentoft et al., 2019) and an increased risk for infections (Nelke et al., 2019). Moreover, studies have shown an association between sarcopenia and impaired brain function (Ogawa et al., 2018; Peng et al., 2020). A meta-analysis by Peng et al. (2020) showed that patients with sarcopenia had twice the risk to mild impairment of brain function. In addition, a large study by Szlejf et al. (2019) found that depression was also associated with sarcopenia. These data reveal the involvement of skeletal muscle-brain crosstalk and low-grade chronic inflammation in the skeletal muscle wasting and the impairment of brain function. Nevertheless, the mechanisms involved in these health disorders are not yet completely understood.
As mentioned above, skeletal muscle produce myokines at rest and during muscle contractions; however, only during the immunometabolic challenges of physical exercise (when there is a counterbalance of time and intensity of training) that its ability to produce anti-inflammatory responses is enhanced. Thus, kinesiology is an emerging field for connecting low-grade chronic inflammation, skeletal muscle wasting and brain impairments.
4. The power of skeletal muscle contraction: a tool to improve health and new horizons to explore
If, on the one hand, low-grade chronic inflammation leads to skeletal muscle wasting, on the other hand, skeletal muscle contraction holds great promise as the key to overcome this condition, given the well-established anti-inflammatory role of exercise-induced muscle contraction. Skeletal muscle contraction during physical activities is a powerful stimulant and producer of several signaling molecules, called exerkines (Table 1). Exerkines can subsequently act on the cell itself (autocrine signaling) or interact with neighboring (paracrine signaling) and distant (endocrine signaling) cells, providing not only local but also systemic benefits (Chow et al., 2022). In a previous study, we demonstrated that combined aerobic and strength exercise altered exerkines that are associated with food intake circuit (Inoue et al., 2018). Exerkines are produced by different sources such as cardiovascular, endocrine, immune systems, bone, gut, liver, adipose tissue and, finally, skeletal muscle and nervous systems (Chow et al., 2022).
Table 1.
Summary of major molecular mechanisms induced by physical activity and skeletal muscle contraction that reduce the inflammation-insulin resistance state, improving brain and skeletal muscle health.
| SKELETAL MUSCLE CONTRACTION | |
|---|---|
| Physiological response | Effect |
| Production of IL-1ra, IL-6, IL-10 | Reduced inflammation |
| Production of Adiponectin | Reduced Insulin Resistance |
| Activation of Nrf2 | Reduced Oxidative Stress |
| Increased energy expenditure | Reduced Ectopic Fat |
| REGULAR PHYSICAL ACTIVITY | ||
|---|---|---|
| Physiological adaptation | Mediator | Major affected tissue |
| Production of kynurenine aminotransferase | PGC-1 α | Brain |
| Production of irisin | PGC-1 α | Brain/Skeletal Muscle |
| Mitochondrial biogenesis | PGC-1 α | Brain/Skeletal Muscle |
| Improved NMJ function | mBDNF | Skeletal Muscle |
| Hypertrophy | IGF-1, mTORC1 | Skeletal Muscle |
IGF-1: Insulin like growth factor-1; IL-10: Interleukine-10; IL-1ra: Interleukine-1 receptor antagonist; mBDNF: mature brain-derived neurotrophic factor isoform; mTOR: mammalian target of rapamycin; Nrf2: Nuclear factor erythroid 2-related factor 2; PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator-1alpha; NMJ: neuromuscular junction.
In general, it is well documented that physical exercise increases circulating anti-inflammatory and insulin-sensitizing markers, such as interleukine-10 (IL-10), interleukine-1 receptor antagonist (IL-1ra) (Pedersen and Febbraio, 2008), and adiponectin (Boassida et al., 2010), mitigating the systemic inflammation-insulin resistance state (Inoue et al., 2015). Moreover, long-term regular physical activity reduces the pro-inflammatory vicious cycle by decreasing visceral fat storage and other ectopic fat deposits (due higher energy expenditure), which are relate to oxidative stress and upregulation of inflammatory processes (Wei et al., 2008; Loher et al., 2016). This exercise-induced anti-inflammatory environment reinforces the role of exercise in building brain health (Cotman et al., 2007; Mattson, 2012), stimulating brain plasticity (Ding et al., 2006; Batouli and Saba, 2017), and mediating resilience to depression (Agudelo et al., 2014).
More recently, the skeletal muscle contraction-brain health crosstalk has received important interest but our current knowledge of the mechanisms by which skeletal muscle contraction improves or maintains brain health is insufficient. For example, mBDNF is known to be upregulated by physical exercise in both skeletal muscle and nervous system. Thus, exercise may mitigate the impairments of low-grade chronic inflammation on muscle atrophy and dysfunction and nervous systems by modulating mBDNF concentration (Marosi and Mattson, 2014; Pedersen, 2019; Arosio et al., 2023). BDNF expression in the brain seems to be mediated by skeletal muscle-derived exerkines, or myokines, such as cathepsin B, irisin, β-hydroxybutyrate and β-adrenergic stimulus, but the mechanisms involved still need further clarification (Pedersen, 2019). Conversely, little is known about the impact of physical exercise on pro-BDNF responses, as well as the molecular mechanisms involved in these tissues, including in NMJ site. A better understanding of the modulation of BDNF isoforms by exercise-induced signaling pathways may contribute to mitigate the negative consequences of the low-grade chronic inflammation, skeletal muscle wasting, and brain dysfunction vicious cycle. In a previous study (Inoue et al., 2020b), we showed that different intensities of short-term aerobic training increased cognitive performance and serum mBDNF acutely in obese adults, without changes in serum pro-BDNF levels. Further studies are needed to clarify the cellular pathways involved, especially at the tissue level.
Furthermore, the benefits of skeletal muscle contraction and brain health seems to be directly related to the PGC-1α signaling pathway. Although PGC-1α is downregulated during an inflammation-insulin resistance state, short-term exercise increases its production (Liang and Ward, 2006). Consequently, higher levels of PGC-1α improve muscle mass and function by increasing mitochondrial biogenesis and oxidative myosin heavy chain expression (Liang and Ward, 2006), and preventing skeletal muscle wasting. Exercise may also upregulate irisin (Marosi and Mattson, 2014) and kynurenic acid (Agudelo et al., 2014) production.
Activation of the PGC-1α complex induced by skeletal muscle contraction leads to an increase in the expression of the exerkine fibronectin Type III exerkine domain containing 5 (FNDC5), a transmembrane protein precursor to the exerkine irisin. (Wrann, 2015). Studies suggest that irisin can cross BBB and trigger the expression of BDNF expression, for example, in hippocampus (Wrann et al., 2013), contributing to improved cognitive function. Another signaling pathway activated by skeletal muscle contraction via PGC-1α is the production of the enzyme kynurenine aminotransferase. This enzyme interacts with kynurenine, a molecule derived from tryptophan metabolism capable of crossing the BBB and causing depression. Kynurenine aminotransferase interacts with kynurenine, converting it into kynurenic acid, which is not capable of crossing the BBB, thus preventing the onset of depression (Agudelo et al., 2014). However, the molecular mechanisms described above still need to be better understood in the context of different types and intensities of physical activities.
5. Final considerations
Recent studies have shown the deleterious effects of the vicious cycle between low-grade chronic inflammation, skeletal muscle wasting and brain impairments. Systemic inflammation-insulin resistance state impairs the integrity of BBB and blood-nerve barrier contributing to neuroinflammation microenvironment, which is associated with cognitive decline and depressive symptoms. In addition, systemic inflammation-insulin resistance state also impairs skeletal muscle health, disturbing directly (through NMJ) and indirectly (through endocrine action) its communication with the brain. At the same time, an ill skeletal muscle is associated with cognitive impairment and depression, skeletal muscle's ability of contraction is a key factor to achieve homeostasis or, at least in part, mitigate the effects of the low-grade chronic inflammation, skeletal muscle wasting and brain dysfunction loop. Despite growing evidence, more research is needed to clarify the molecular mechanisms by which the skeletal muscle contraction-brain crosstalk counteracts low-grade chronic inflammation. In this context, we believe that a close integration of the fields of kinesiology and PNI will support and enhance new advances in health sciences.
CRediT authorship contribution statement
Daniela Sayuri Inoue: Writing – original draft, Visualization, Project administration, Conceptualization. Mariana Janini Gomes: Writing – review & editing, Supervision.
Declaration of competing interest
We declare that there are no conflicts of interest (including financial and other relationships) for each author.
Data availability
No data was used for the research described in the article.
References
- Aby K., Antony R., Eichholz M., Srinivasan R., Li Y. Enhanced pro-BDNF-p75NTR pathway activity in denervated skeletal muscle. Life Sci. 2021;286 doi: 10.1016/j.lfs.2021.120067. Epub 2021 Oct 19. PMID: 34678261; PMCID: PMC8595791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ader R. International Encyclopedia of the Social & Behavioral Sciences. 2001. [DOI] [Google Scholar]
- Agrawal S., Chakole S., Shetty N., Prasad R., Lohakare T., Wanjari M. Exploring the role of oxidative stress in skeletal muscle atrophy: mechanisms and implications. Cureus. 2023;15(7) doi: 10.7759/cureus.42178. PMID: 37602126; PMCID: PMC10439769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo L.Z., Femenía T., Orhan F., Porsmyr-Palmertz M., Goiny M., Martinez-Redondo V., Correia J.C., Izadi M., Bhat M., Schuppe-Koistinen I., Pettersson A.T., Ferreira D.M.S., Krook A., Barres R., Zierath J.R., Erhardt S., Lindskog M., Ruas J.L. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159(1):33–45. doi: 10.1016/j.cell.2014.07.051. PMID: 25259918. [DOI] [PubMed] [Google Scholar]
- Arosio B., Calvani R., Ferri E., Coelho-Junior H.J., Carandina A., Campanelli F., Ghiglieri V., Marzetti E., Picca A. Sarcopenia and cognitive decline in older adults: targeting the muscle-brain Axis. Nutrients. 2023;15(8):1853. doi: 10.3390/nu15081853. PMID: 37111070; PMCID: PMC10142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batouli S.A.H., Saba V. At least eighty percent of brain grey matter is modifiable by physical activity: a review study. Behav. Brain Res. 2017;332:204–217. doi: 10.1016/j.bbr.2017.06.002. Epub 2017 Jun 7. PMID: 28600001. [DOI] [PubMed] [Google Scholar]
- Bay M.L., Pedersen B.K. Muscle-organ crosstalk: focus on immunometabolism. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.567881. PMID: 33013484; PMCID: PMC7509178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouassida A., Chamari K., Zaouali M., Feki Y., Zbidi A., Tabka Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 2010;44(9):620–630. doi: 10.1136/bjsm.2008.046151. Epub 2008 Oct 16. PMID: 18927166. [DOI] [PubMed] [Google Scholar]
- Chow L.S., Gerszten R.E., Taylor J.M., Pedersen B.K., van Praag H., Trappe S., Febbraio M.A., Galis Z.S., Gao Y., Haus J.M., Lanza I.R., Lavie C.J., Lee C.H., Lucia A., Moro C., Pandey A., Robbins J.M., Stanford K.I., Thackray A.E., Villeda S., Watt M.J., Xia A., Zierath J.R., Goodpaster B.H., Snyder M.P. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022;18(5):273–289. doi: 10.1038/s41574-022-00641-2. Epub 2022 Mar 18. PMID: 35304603; PMCID: PMC9554896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C.W., Berchtold N.C., Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. doi: 10.1016/j.tins.2007.06.011. Epub 2007 Aug 31. Erratum in: Trends Neurosci. 2007 Oct;30(10):489. PMID: 17765329. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., Schneider S.M., Sieber C.C., Topinkova E., Vandewoude M., Visser M., Zamboni M. Writing group for the European working group on sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. Erratum in: Age Ageing. 2019 Jul 1;48(4):601. PMID: 30312372; PMCID: PMC6322506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. PMID: 18073775; PMCID: PMC2919277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q., Vaynman S., Akhavan M., Ying Z., Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140(3):823–833. doi: 10.1016/j.neuroscience.2006.02.084. Epub 2006 May 2. PMID: 16650607. [DOI] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatr. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. Epub 2009 Dec 16. PMID: 20015486. [DOI] [PubMed] [Google Scholar]
- Engel G.L. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–136. doi: 10.1126/science.847460. PMID: 847460. [DOI] [PubMed] [Google Scholar]
- Gao W., Guo L., Yang Y., Wang Y., Xia S., Gong H., Zhang B.K., Yan M. Dissecting the crosstalk between Nrf2 and NF-κB response pathways in drug-induced toxicity. Front. Cell Dev. Biol. 2022;9 doi: 10.3389/fcell.2021.809952. PMID: 35186957; PMCID: PMC8847224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes M.J., Martinez P.F., Pagan L.U., Damatto R.L., Cezar M.D.M., Lima A.R.R., Okoshi K., Okoshi M.P. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8(12):20428–20440. doi: 10.18632/oncotarget.14670. PMID: 28099900; PMCID: PMC5386774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. PMID: 7678183. [DOI] [PubMed] [Google Scholar]
- Inoue D.S., De Mello M.T., Foschini D., Lira F.S., De Piano Ganen A., Da Silveira Campos R.M., De Lima Sanches P., Silva P.L., Corgosinho F.C., Rossi F.E., Tufik S., Dâmaso A.R. Linear and undulating periodized strength plus aerobic training promote similar benefits and lead to improvement of insulin resistance on obese adolescents. J. Diabet. Complicat. 2015;29(2):258–264. doi: 10.1016/j.jdiacomp.2014.11.002. Epub 2014 Nov 13. PMID: 25441178. [DOI] [PubMed] [Google Scholar]
- Inoue D.S., Panissa V.L., Antunes B.M., Oliveira F.P., Malta R.B., Caldeira R.S., Campos E.Z., Pimentel G.D., Franchini E., Lira F.S. Reduced leptin level is independent of fat mass changes and hunger scores from high-intensity intermittent plus strength training. J. Sports Med. Phys. Fit. 2018;58(7–8):1045–1051. doi: 10.23736/S0022-4707.17.07370-4. Epub 2017 May 9. PMID: 28488831. [DOI] [PubMed] [Google Scholar]
- Inoue D.S., Antunes B.M., Maideen M.F.B., Lira F.S. Pathophysiological features of obesity and its impact on cognition: exercise training as a non-pharmacological approach. Curr. Pharmaceut. Des. 2020;26(9):916–931. doi: 10.2174/1381612826666200114102524. PMID: 31942854. [DOI] [PubMed] [Google Scholar]
- Inoue D.S., Monteiro P.A., Gerosa-Neto J., Santana P.R., Peres F.P., Edwards K.M., Lira F.S. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-70326-1. PMID: 32778721; PMCID: PMC7417991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D.S., Bin Maideen M.F., Jiménez-Maldonado A., Lira F.S. Role of neuronal guidance cues in the pathophysiology of obesity: a peripheral and central overview. Curr. Pharmaceut. Des. 2021;27(21):2512–2521. doi: 10.2174/1381612824666210316094659. . PMID: 33726646. [DOI] [PubMed] [Google Scholar]
- Jung H.N., Jung C.H., Hwang Y.C. Sarcopenia in youth. Metabolism. 2023;144 doi: 10.1016/j.metabol.2023.155557. Epub 2023 Apr 18. PMID: 37080353. [DOI] [PubMed] [Google Scholar]
- King P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015;4(1):68. doi: 10.1186/s40169-015-0068-z. Epub 2015 Jul 29. PMID: 26220864; PMCID: PMC4518022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S., Heni M., Hallschmid M., Fritsche A., Preissl H., Häring H.U. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol. Rev. 2016;96(4):1169–1209. doi: 10.1152/physrev.00032.2015. Epub 2016 Aug 3. PMID: 27489306. [DOI] [PubMed] [Google Scholar]
- Liang H., Ward W.F. PGC-1alpha: a key regulator of energy metabolism. Adv. Physiol. Educ. 2006;30(4):145–151. doi: 10.1152/advan.00052.2006. PMID: 17108241. [DOI] [PubMed] [Google Scholar]
- Liu C., Wong P.Y., Chow S.K.H., Cheung W.H., Wong R.M.Y. Does the regulation of skeletal muscle influence cognitive function? A scoping review of pre-clinical evidence. J Orthop Translat. 2022;38:76–83. doi: 10.1016/j.jot.2022.10.001. PMID: 36381246; PMCID: PMC9619139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loher H., Kreis R., Boesch C., Christ E. The flexibility of ectopic lipids. Int. J. Mol. Sci. 2016;17(9):1554. doi: 10.3390/ijms17091554. PMID: 27649157; PMCID: PMC5037826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco M.V., Frozza R.L., de Freitas G.B., Zhang H., Kincheski G.C., Ribeiro F.C., Gonçalves R.A., Clarke J.R., Beckman D., Staniszewski A., Berman H., Guerra L.A., Forny-Germano L., Meier S., Wilcock D.M., de Souza J.M., Alves-Leon S., Prado V.F., Prado M.A.M., Abisambra J.F., Tovar-Moll F., Mattos P., Arancio O., Ferreira S.T., De Felice F.G. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25(1):165–175. doi: 10.1038/s41591-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K., Mattson M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metabol. 2014;25(2):89–98. doi: 10.1016/j.tem.2013.10.006. Epub 2013 Dec 19. PMID: 24361004; PMCID: PMC3915771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6) doi: 10.1016/j.cmet.2012.08.012. 706-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. PMID: 18650913. [DOI] [PubMed] [Google Scholar]
- Nelke C., Dziewas R., Minnerup J., Meuth S.G., Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. Epub 2019 Oct 26. PMID: 31662290; PMCID: PMC6945275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H., Asai A., Fukunishi S., Nishiguchi S., Higuchi K. Metabolic syndrome and sarcopenia. Nutrients. 2021;13(10):3519. doi: 10.3390/nu13103519. PMID: 34684520; PMCID: PMC8541622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien P.D., Hinder L.M., Callaghan B.C., Feldman E.L. Neurological consequences of obesity. Lancet Neurol. 2017;16(6):465–477. doi: 10.1016/S1474-4422(17)30084-4. PMID: 28504110; PMCID: PMC5657398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. Epub 2013 Dec 5. PMID: 24309662; PMCID: PMC4080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y., Kaneko Y., Sato T., Shimizu S., Kanetaka H., Hanyu H. Sarcopenia and muscle functions at various stages of alzheimer disease. Front. Neurol. 2018;9:710. doi: 10.3389/fneur.2018.00710. PMID: 30210435; PMCID: PMC6121095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K., Rohde T., Zacho M., Asp S., Pedersen B.K. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. 1998;508(Pt 3):949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. PMID: 9518745; PMCID: PMC2230908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake J.M., Della Gatta P., Suzuki K., Nieman D.C. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc. Immunol. Rev. 2015;21:8–25. PMID: 25826432. [PubMed] [Google Scholar]
- Pedersen B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019;15(7):383–392. doi: 10.1038/s41574-019-0174-x. PMID: 30837717. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. PMID: 18923185. [DOI] [PubMed] [Google Scholar]
- Peng T.C., Chen W.L., Wu L.W., Chang Y.W., Kao T.W. Sarcopenia and cognitive impairment: a systematic review and meta-analysis. Clin. Nutr. 2020;39(9):2695–2701. doi: 10.1016/j.clnu.2019.12.014. Epub 2019 Dec 17. PMID: 31917049. [DOI] [PubMed] [Google Scholar]
- Peters E., Del Rey A., Krüger K., Rummel C. 2nd European psychoneuroimmunology network autumn school: the skin-brain Axis and the breaking of barriers. Neuroimmunomodulation. 2023;30(1):3–7. doi: 10.1159/000533611. Epub 2023 Aug 21. PMID: 37604137; PMCID: PMC10627488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers S.K., Howley E.T., Quindry J., editors. Exercise Physiology: Theory and Application to Fitness and Performance. 11e. McGraw Hill; 2021. https://accessphysiotherapy.mhmedical.com/content.aspx?bookid=3100§ionid=259267162 [Google Scholar]
- Robinson S.L., Hattersley J., Frost G.S., Chambers E.S., Wallis G.A. Maximal fat oxidation during exercise is positively associated with 24-hour fat oxidation and insulin sensitivity in young, healthy men. J. Appl. Physiol. 2015;118(11):1415–1422. doi: 10.1152/japplphysiol.00058.2015. Epub 2015 Mar 26. PMID: 25814634. [DOI] [PubMed] [Google Scholar]
- Rolfe D.F., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997;77(3):731–758. doi: 10.1152/physrev.1997.77.3.731. PMID: 9234964. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. PMID: 9887164. [DOI] [PubMed] [Google Scholar]
- Rummel C., Del Rey A., Bähr L., Krüger K., Peters E. 1st European psychoneuroimmunology network (EPN) autumn school: lung-brain Axis in health and disease. Neuroimmunomodulation. 2022;29(Suppl. 2):3–8. doi: 10.1159/000526565. Epub 2022 Sep 1. PMID: 36049468; PMCID: PMC9677835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.H., Leonard G., Perron M., Richer L., Syme C., Veillette S., Pausova Z., Paus T. Visceral fat is associated with lower executive functioning in adolescents. Int. J. Obes. 2013;37(10):1336–1343. doi: 10.1038/ijo.2013.104. Epub 2013 Jun 5. PMID: 23797144; PMCID: PMC5061567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert T., Brassard P., Wissenberg M., Rasmussen P., Nordby P., Stallknecht B., Adser H., Jakobsen A.H., Pilegaard H., Nielsen H.B., Secher N.H. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298(2):R372–R377. doi: 10.1152/ajpregu.00525.2009. Epub 2009 Nov 18. PMID: 19923361. [DOI] [PubMed] [Google Scholar]
- Steensberg A., van Hall G., Osada T., Sacchetti M., Saltin B., Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000;529(Pt 1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. PMID: 11080265; PMCID: PMC2270169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szlejf C., Suemoto C.K., Brunoni A.R., Viana M.C., Moreno A.B., Matos S.M.A., Lotufo P.A., Benseñor I.M. Depression is associated with sarcopenia due to low muscle strength: results from the ELSA-brasil study. J. Am. Med. Dir. Assoc. 2019;20(12):1641–1646. doi: 10.1016/j.jamda.2018.09.020. Epub 2018 Nov 5. PMID: 30409492. [DOI] [PubMed] [Google Scholar]
- Trouwborst I., Wouters K., Jocken J.W., Jardon K.M., Gijbels A., Dagnelie P.C., van Greevenbroek M.M.J., van der Kallen C.J., Stehouwer C.D.A., Schalkwijk C.G., Richard N., Bendik I., Afman L.A., Blaak E.E., Goossens G.H. Circulating and adipose tissue immune cells in tissue-specific insulin resistance in humans with overweight and obesity. Obesity. 2023;31(5):1326–1337. doi: 10.1002/oby.23714. Epub 2023 Mar 30. PMID: 36998153. [DOI] [PubMed] [Google Scholar]
- Tsai S.Y., Gildengers A.G., Hsu J.L., Chung K.H., Chen P.H., Huang Y.J. Inflammation associated with volume reduction in the gray matter and hippocampus of older patients with bipolar disorder. J. Affect. Disord. 2019;244:60–66. doi: 10.1016/j.jad.2018.10.093. Epub 2018 Oct 6. PMID: 30317016. [DOI] [PubMed] [Google Scholar]
- Wei Y., Chen K., Whaley-Connell A.T., Stump C.S., Ibdah J.A., Sowers J.R. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294(3):R673–R680. doi: 10.1152/ajpregu.00561.2007. Epub 2007 Dec 19. PMID: 18094066. [DOI] [PubMed] [Google Scholar]
- Wrann C.D. FNDC5/irisin - their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Brain Plast. 2015;1(1):55–61. doi: 10.3233/BPL-150019. PMID: 28480165; PMCID: PMC5419585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D., Lin J.D., Greenberg M.E., Spiegelman B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau P.L., Castro M.G., Tagani A., Tsui W.H., Convit A. Obesity and metabolic syndrome and functional and structural brain impairments in adolescence. Pediatrics. 2012;130(4):e856–e864. doi: 10.1542/peds.2012-0324. Epub 2012 Sep 3. PMID: 22945407; PMCID: PMC3457620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.